Subjective Cognitive Decline Among Middle-Aged and Older Adults in Hong Kong: A Cross-Sectional Study
Abstract
Background: Subjective cognitive decline (SCD) is a self-reported perception of cognitive deterioration in individuals who are cognitively normal. Cognitive functions keep steady during adulthood up until around age 40; thereafter, individuals are more likely to experience cognitive decline. SCD is viewed as a possible early sign of Alzheimer’s disease and other forms of dementia. Early detection and intervention addressing SCD could delay the onset of mild cognitive impairment and dementia. Several tools have been developed and evaluated for the phenomenon of SCD in different countries; however, limited research findings can be found in Hong Kong.
Objective: To investigate the prevalence of SCD among middle-aged and older adults in Hong Kong and identify the related factors contributing to its occurrence.
Methods: A cross-sectional survey was undertaken from December 2023 to January 2024 to 200 individuals living in Hong Kong aged 50 years or older. The respondents filled out a questionnaire that collected demographic information, including gender, age, education level and health status. They also completed the Subjective Cognitive Decline Questionnaire-21. Descriptive analysis, logistic analysis and factor analysis were conducted in this study.
Results: A total of 200 samples were collected, of which 122 were classified as having SCD, leading to a prevalence rate of 61% that exceeds that of neighbouring countries. SCD was correlated with the self-rated health score. The Cronbach’s alpha was 0.905. The findings demonstrated significant differences in response to patterns between the two scoring groups for all questions. The results of the factor analysis confirmed the reliability of the four-factor structure, reinforcing the strength of the scale.
Conclusion: The study analysed the prevalence of SCD among middle-aged and older adults in Hong Kong as well as explored the relationship between various factors and SCD. The initial insights gained from the questionnaire will inform the future development of more comprehensive and effective solutions to address cognitive decline in older adults in Hong Kong.
1. Introduction
Subjective cognitive decline (SCD) is a condition that encompasses the self-perceived deterioration of memory and frequent occurrences of confusion that individuals with normal cognitive function have experienced over the past 12 months [1]. SCD is not only limited to older adults but can also affect younger individuals. Previous study has indicated that cognitive function often reaches its highest point between the ages of 30 and 40, after which it starts to deteriorate, in which older adults have a higher tendency of experiencing SCD [2]. Despite the absence of objective evidence, individuals with SCD are often concerned about their memory and cognitive function, which can impact their quality of life and daily functioning [3, 4]. SCD is viewed as a possible early sign of Alzheimer’s disease and other forms of dementia. Multiple studies have shown that individuals with SCD may have an increased likelihood of advancing cognitive decline, leading to mild cognitive impairment and/or dementia compounded by facing challenges in daily activities and a heightened risk of memory-related functional problems [5, 6]. A study which included over 1000 older adults found that 20%–25% of those with SCD acquired mild cognitive impairment or dementia within 2–4 years, compared with just 5%–10% of those without SCD [7]. The cause of SCD is intricate and involves several factors. For example, psychological factors, particularly mood disorders such as anxiety and depression, substantially influence the experience and reporting of SCD. Various studies have demonstrated a significant correlation between mood disorders and SCD, indicating that psychological distress might worsen the sense of cognitive decline. People with depression are prone to reporting cognitive issues, even in cases when objective cognitive examinations do not show notable deficits [8]. The occurrence of these phenomena can be ascribed to the cognitive biases that are present in mood disorders. These biases result in negative thinking patterns and an intensified self-focus, which in turn lead to a heightened awareness and reporting of cognitive failures. Moreover, anxiety can intensify the focus on cognitive failures and memory lapses, leading to a cycle that strengthens the belief of cognitive decline [9].
Individuals afflicted with chronic illnesses such as diabetes, heart disease and renal disease have shown a significant association with SCD as a result of the direct and indirect impact on brain health and the entirety of cognitive performance, as evidenced in numerous studies [10–12]. Compared with those without SCD, individual with SCD had a prevalence of certain chronic conditions that was two to three times higher [12]. Different chronic conditions have distinct aetiologies for cognitive decline. For instance, atherosclerosis, inflammation, oxidative stress, heart disease and reduced cerebral blood flow are a few examples of the factors that directly contribute to the link between heart disease and decreased cognitive function [13–15].
It is crucial to point out that despite SCD is linked to more serious and progressive cognitive decline, such as dementia and mild cognitive impairment, it does not mean that persons with SCD will necessarily develop dementia in their later years [1]. Previous studies have indicated that prompt identification and early intervention with individuals who may be at risk of developing SCD may efficiently delay the onset of dementia [1, 16, 17]. Several tools have been developed to evaluate SCD, such as the Subjective Cognitive Decline Questionnaire (SCD-Q) and Subjective Cognitive Decline in Ageing (SCDA) scale [18, 19]. Routine use of these screening tools can help track changes and pinpoint individuals at high risk who need further clinical evaluation and follow-up.
Hong Kong has the highest life expectancy in the world. It is projected that the older population (i.e., those aged over 65) in Hong Kong will increase from 1.16 million in 2016 to 2.74 million by 2046 (Census and Statistics Department of Hong Kong Special Administrative Region [20]). As advanced age is the strongest risk factor for dementia [21], an ageing society like Hong Kong means that the city will likely face a growing dementia epidemic. It is expected that in 2039, over 300 thousand older people will be living with dementia in Hong Kong [22]. The Hong Kong government has consistently allocated substantial resources on services for older peoples’ health. With the ageing population, there is a rise in expenditures in this field. It has increased from 1529 million in 2012–2013 to about 10,235 million in 2019-2020 [23]. It is evident that ageing-related health issues will persist, ultimately exacerbating the economic burden on the government. Hence, timely detection of individuals with possible SCD might diminish the likelihood of acquiring dementia and hence will decrease health and social care expenses. Our research argues that early intervention of cognitive impairment as early as the SCD stage is crucial and may result in beneficial outcomes; yet most studies on the association between SCD and other cognitive outcomes have mostly been undertaken in Western countries. Therefore, the purpose of this study is to investigate the prevalence of SCD in older individuals in Hong Kong and identify the underlying factors contributing to its occurrence.
2. Method
2.1. Study Design, Study Population and Procedures
A cross-sectional study was carried out among older adults who live in the community. The target participants were required to fulfil the following criteria: (1) over the age of 50 and above, (2) Hong Kong resident and (3) possess the ability to read and comprehend Chinese. Data for the study were gathered using the online survey platform, the Google Forms. The online survey was sent out by means of email to a membership database of older adults affiliated with a research centre at the host university from December 19, 2023, to January 11, 2024. All participants completed a survey comprising two sections. The first part contained demographic information and other enquiries about health issues, while another section consisted of 21 questions pertaining to SCD, the subjective cognitive decline questionnaire-21 (SCD-Q21). Two hundred samples were taken from 151 women and 49 men whose ages varied from 50 to 86.
2.2. Measures
2.2.1. Demographic Characteristics
Participants were required to provide demographic information, including age, gender and any diagnoses of common health conditions such as hypertension, high cholesterol, diabetes, gout, hearing issues, vision problems, kidney disorders or other conditions diagnosed by medical practitioners. Also, they were requested to evaluate their health on a scale from 1 (extremely poor) to 10 (extremely excellent).
2.2.2. SCD-Q21
SCD was measured using the SCD-Q21 [24]. The questionnaire is utilized to assess the perceived subjective decline in three main areas: memory, language, and executive functions [25]. Participants are requested to evaluate their perceived changes in these domains during a specified timeframe. The questionnaire consists of 21 items, including 15 binary questions and 6 three-choice questions, yielding a maximum score of 21. For binary questions, participants are required to respond with either “yes” or “no” or “good” or “bad,” such as “Do you believe you have memory issues?” They will receive 1 mark if they respond “yes” and 0 marks if they respond “no.” However, two of the queries are reversible, i.e., Q11: “Overall, do you think your memory can remember things you used to?” and Q19: “Overall, do you think your memory is good or poor?” In question 11, participants who respond “yes” will receive 0 marks while those who respond “no” will be awarded 1 mark. Similarly, in Question 19, participants who answer “good” get 0 mark whereas those who answer “poor” will receive 1 mark.
For the three-choice questions, participants are required to respond with “Never” or “Often” or “Always” (e.g., “How often is the following a problem for you: Personal dates (e.g., birthday)?”). The participant will receive 0 marks if they choose “Never,” 0.5 marks for “Often,” and 1 mark for “Always.” Consequently, a higher score corresponds to a greater likelihood of mild cognitive impairment [24].
2.3. Statistical Analysis
Statistical analysis was conducted using IBM SPSS Version 26, with a p value of 0.05 or lower deemed statistically significant. Descriptive statistics were utilised for demographic data and SCD-Q21 for each item to compute their frequency and percentage. For the SCD-Q21, it also indicated the p value between two groups (low SCD and high SCD). Continuous variables were presented as the mean ± standard deviation. Logistic regression was conducted to investigate the relationship between demographic variables including age, gender, education level, medical condition and subjective health score and SCD score. The p value, 95% confidence intervals (CIs) and odds ratio (OR) were reported for each explanatory variable. Internal consistency of the factor scores were utilised by Cronbach’s alpha. The scree plot was also generated to exact common components. Factor analysis was performed with principal component analysis to establish robust interitem correlations.
2.4. Ethical Approval and Informed Consent Statements
This study received ethical approval from the Institutional Review Board at (BLINDED For REVIEW) (approval #HSEARS20231011007). Participants were provided with a Participant Information Sheet (PIS) containing specific details of the study and informed consent was obtained.
3. Result
3.1. Descriptive Statistics
A total of 200 samples were collected and analysed, comprising 49 (24.5%) males and 151 (75.5%) females. The participants’ ages varied from 50 to 86, yielding a mean age of 65.04 ± 5.75 years. Over half of the respondents (n = 102) possessed a high level of education, defined as postsecondary education or higher, while 98 respondents (49%) were secondary education or lower. Among the respondents, 62 (31%) were not diagnosed with any medical conditions, while 66 (33%) had one condition and 72 (36%) had two or more conditions. A total of 138 respondents have reported that they have experienced at least one medical condition. Hyperlipidaemia (n = 70), hypertension (n = 62) and visual impairment (n = 41) were the top three medical conditions reported. In addition, this study required respondents to subjectively rank their health on a scale of 1–10. Approximately 80% of the participants (n = 159) have given themselves scores between 6 and 10 whereas 20.5% (n = 41) given scores between 1 and 5. The mean SCD-Q21 score among the responders was 10.73 ± 5.089 marks. A detailed description of the demographic characteristics of the study’s population is presented in Table 1.
| Characteristics | N = 200 |
|---|---|
| Age, mean ± S.D., (year) | 65.04 ± 5.75 |
| Middle age (50–64), n (%) | 92 (46) |
| Old age (65 or above), n (%) | 108 (54) |
| Gender, n (%) | |
| Male | 49 (24.5) |
| Female | 151 (75.5) |
| Education level, n (%) | |
| Low education | 98 (49) |
| High education | 102 (51) |
| Medical condition, n (%) | |
| 0 | 62 (31) |
| 1 | 66 (33) |
| 2 or more | 72 (36) |
| Common chronic disease diagnosed, n (%) | |
| Diabetes | 20 (10) |
| Hypertension | 62 (31) |
| Hyperlipidaemia | 70 (35) |
| Auditory impairments | 14 (7) |
| Visual impairments | 41 (20.5) |
| Gout | 5 (2.5) |
| Renal disorders | 8 (4) |
| Self-rated health score, n (%) | |
| 1–5 | 41 (20.5) |
| 6–10 | 159 (79.5) |
| SCD score, mean ± S.D. | 10.73 ± 5.089 |
| Score 0–9.25, n (%) | 78 (39) |
| Score 9.25–21 n (%) | 122 (61) |
- Note: Age categories: middle age refers to individuals aged 50–64 years, while old age refers to those aged 65 years or above. Education level: low education includes individuals with primary or lower secondary education, while high education includes those with upper secondary education or higher. Self-rated health score: participants rated their overall health on a scale of 1–10, with scores grouped into 1–5 (lower perceived health) and 6–10 (higher perceived health). SCD score refers to the subjective cognitive decline (SCD) score.
- Abbreviation: SD = standard deviation.
3.2. The Relationship Between Demographic Factors and SCD Score
The study also conducted a logistic regression analysis to investigate the relationship between demographic factors and SCD score, as presented in Table 2. The p value, 95% CIs and OR were reported. The results indicated that most variables, including age, gender, education level and medical condition, were not significantly associated with the SCD score as indicated by p values greater than 0.05. In contrast, self-rated health scores demonstrated a significant association with the SCD score. Individuals who reported higher self-rated health (scores of 6–10) exhibited a significantly lower likelihood of higher SCD scores in comparison to those with lower self-rated health (scores of 0–5), as evidenced by the negative OR (OR = −1.511, p = 0.002 and CI = 0.086–0.565). Therefore, this finding pointed out the potential impact of self-perceived health on the SCD score.
| Regression | OR | p value | 95% CI | |
|---|---|---|---|---|
| Gender | ||||
| Male | Ref | … | … | |
| Female | 0.118 | 0.707 | 0.609 | 2.076 |
| Age | ||||
| Middle age | Ref | … | … | |
| Old age | −0.331 | 0.369 | 0.349 | 1.479 |
| Education level | ||||
| Low education | Ref | |||
| High education | −0.382 | 0.217 | 0.372 | 1.252 |
| Medical condition | ||||
| 0 | Ref | … | … | |
| 1 | −0.296 | 0.43 | 0.356 | 1.551 |
| 2 or more | 0.166 | 0.669 | 0.551 | 2.534 |
| Self-rated health score | ||||
| 0 to 5 | Ref | … | … | |
| 6 to 10 | −1.511 | 0.002 | 0.086 | 0.565 |
- Note: Ref: reference category.
- Abbreviations: CI = confidence interval, OR = odds ratio.
3.3. Identification of Four Components of the SCD by Principal Component Analysis
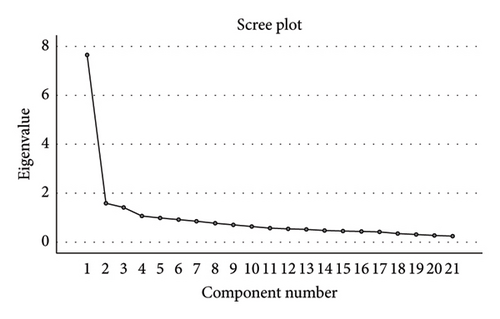
The reliability and validity were conducted in this study. The SCD-Q21 reported a Cronbach’s alpha of 0.905, which indicated the scale had excellent internal consistency [26]. The computed Cronbach’s alpha coefficients for each item of SCD-Q21 varied from 0.895 to 0.905. The Kaiser–Meyer–Olkin (KMO) test for construct validity was 0.901, and the Bartlett test for sphericity generated the chi-square value of 1681.739 with p ≤ 0.001. Previous literature has indicated that a KMO value above 0.60 implies that it is suitable of conducting factor analysis [27]. After our team conducted factor analysis, four components were identified from the questionnaire, showing eigenvalues of 7.644, 1.582, 1.410 and 1.062, respectively. The cumulative contribution rate for these components was 55.706%. Comprehensive information for eigenvalue, variance contribution rate and cumulative variance contribution rate are shown in Table 3 and Figure 1. Based on the factor loadings, it supports four components. The first component comprised Q1, Q2, Q3, Q6, Q12, Q13, Q14, Q15 and Q19. These questions pertained to general memory concerns and perceptions, emphasising overall assessments of memory capability, alterations in memory and general memory-related complaints over time. The second component comprises Q4, Q5, Q16, Q17, Q18 and Q21, which pertain to specific memory challenges. These questions focus on issues related to memory retrieval, including the recall of names, phone numbers, personal dates, and the observation of repetitive behaviours or misplacing objects. The third component comprises Q8, Q9 and Q11, which address perceived memory decline over time, specifically focussing on the perception of memory deterioration and challenges in recalling the locations of objects. The final component comprised questions Q7, Q10 and Q20, which pertain to common everyday memory lapses, including forgetting conversations, information conveyed by others, or items to purchase at the store. A detailed overview of factor analysis and internal consistency of SCD-Q21 is presented in Table 4.
| Components (SCD questions) | Initial eigenvalues | Extraction sums of squared loadings | Rotation sums of squared loadings | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | % of variance | Cumulative (%) | Total | % of variance | Cumulative (%) | Total | % of variance | Cumulative (%) | |
| Q1 | 7.644 | 36.041 | 36.041 | 7.644 | 36.041 | 36.401 | 3.989 | 18.995 | 18.995 |
| Q2 | 1.582 | 7.531 | 43.932 | 1.582 | 7.531 | 43.932 | 2.768 | 13.183 | 32.178 |
| Q3 | 1.410 | 6.715 | 50.646 | 1.410 | 6.715 | 50.646 | 2.736 | 13.030 | 45.208 |
| Q4 | 1.062 | 5.059 | 55.706 | 1.062 | 5.059 | 55.706 | 2.205 | 10.498 | 55.706 |
| Q5 | 0.992 | 4.723 | 60.429 | ||||||
| Q6 | 0.914 | 4.350 | 64.779 | ||||||
| Q7 | 0.857 | 4.079 | 68.858 | ||||||
| Q8 | 0.761 | 3.622 | 72.479 | ||||||
| Q9 | 0.693 | 3.298 | 75.777 | ||||||
| Q10 | 0.623 | 2.965 | 78.743 | ||||||
| Q11 | 0.561 | 2.669 | 81.412 | ||||||
| Q12 | 0.531 | 2.529 | 83.941 | ||||||
| Q13 | 0.499 | 2.378 | 86.319 | ||||||
| Q14 | 0.480 | 2.288 | 88.607 | ||||||
| Q15 | 0.442 | 2.107 | 90.714 | ||||||
| Q16 | 0.414 | 1.971 | 92.685 | ||||||
| Q17 | 0.398 | 1.895 | 94.580 | ||||||
| Q18 | 0.340 | 1.617 | 96.197 | ||||||
| Q19 | 0.303 | 1.442 | 97.639 | ||||||
| Q20 | 0.263 | 1.252 | 98.891 | ||||||
| Q21 | 0.233 | 1.109 | 100.000 | ||||||
| Factors | Corrected item: total correlation | Cronbach’s alpha if item deleted | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Scale Cronbach’s alpha | 0.905 | ||||||
| % of variance (rotation sum of squared loadings) | 36.401 | 7.531 | 6.715 | 5.059 | |||
| Q13 | Better memory than others | 0.728 | 0.391 | 0.904 | |||
| Q19 | Good memory | 0.698 | 0.643 | 0.898 | |||
| Q6 | Ease in remembering tasks | 0.664 | 0.694 | 0.896 | |||
| Q1 | No memory problems | 0.636 | 0.725 | 0.895 | |||
| Q2 | Easily recall recent conversations | 0.599 | 0.613 | 0.899 | |||
| Q12 | Memory remained stable | 0.579 | 0.694 | 0.896 | |||
| Q14 | Memory issues do not affect task completion | 0.560 | 0.662 | 0.897 | |||
| Q15 | Easily remember recent events | 0.544 | 0.539 | 0.901 | |||
| Q3 | No memory complaints in 2 years | 0.463 | 0.591 | 0.899 | |||
| Q18 | Remember friends’ names | 0.701 | 0.436 | 0.903 | |||
| Q5 | Recall easily phone numbers | 0.617 | 0.373 | 0.904 | |||
| Q4 | Always remember dates | 0.591 | 0.479 | 0.903 | |||
| Q16 | Not repeat questions or stories | 0.556 | 0.394 | 0.905 | |||
| Q17 | Rarely lose items | 0.524 | 0.520 | 0.901 | |||
| Q21 | Never forget words | 0.444 | 0.468 | 0.903 | |||
| Q8 | Memory better/remain unchanged than 5 years ago | 0.829 | 0.505 | 0.902 | |||
| Q11 | Remember as well as before | 0.770 | 0.432 | 0.903 | |||
| Q9 | Remember where you place things | 0.522 | 0.663 | 0.897 | |||
| Q10 | Always remember if you have shared something | 0.804 | 0.510 | 0.903 | |||
| Q20 | Never forget what people tell you | 0.718 | 0.510 | 0.903 | |||
| Q7 | Never forget the shopping list | 0.631 | 0.497 | 0.902 | |||
- Note: Extraction: principal component analysis; rotation: Varimax with Kaiser normalization. 1. Do you think you have problems with your memory? 2. Do you have difficulty remembering a conversation from a few days ago? 3. Do you have complaints about your memory in the last 2 years? 4. How often is the following a problem for you: Personal dates (e.g., birthdays)? 5. How often is the following a problem for you: Phone numbers you use frequently? 6. On a whole, do you think that you have problems remembering things that you want to do or say? 7. How often is the following a problem for you: going to the store and forgetting what you wanted to buy? 8. Do you think that your memory is worse than 5 years ago? 9. Do you feel you are forgetting where things were placed? 11. Overall, do you think you can remember things you used to? 12. Has your memory changed significantly? 13. Do you feel that you have more memory problems than most? 14. Do memory problems make it harder to complete tasks that used to be easy? 15. Do you have more trouble remembering things that have happened recently? 16. Do you notice yourself repeating the same question or story? 17. Do you lose objects more often than you did previously? 18. Do you feel you are unable to recall the names of good friends? 19. Overall, do you think your memory is good or poor? 20. How often is the following a problem for you: things people tell you? 21. How often is the following a problem for you: words?
3.4. Responses Frequencies in SCD-21 Questions Between Two Cognitive Levels
The frequency distribution of responses, categorised by scoring groups (low SCD score: scores 0–9.25 and high SCD score: scores 9.25–21), across all 21 questions in the SCD-Q21, along with the associated p values, is presented in Table 5. The findings demonstrated significant differences in response to patterns between the two scoring groups for all questions, as shown by p ≤ 0.001, demonstrating strong differences between low and high SCD groups.
| Factor | SCD group, n (%) | p value | |
|---|---|---|---|
| Low SCD (score 0–9.25) | High SCD (score 9.25–21) | ||
| Q1 | ≤ 0.001 | ||
| No | 62 (31) | 9 (4.5) | |
| Yes | 16 (8) | 113 (56.5) | |
| Q2 | ≤ 0.001 | ||
| No | 68 (34) | 33 (16.5) | |
| Yes | 10 (5) | 89 (44.5) | |
| Q3 | ≤ 0.001 | ||
| No | 44 (22) | 9 (4.5) | |
| Yes | 34 (17) | 113 (56.5) | |
| Q4 | ≤ 0.001 | ||
| Never | 42 (21) | 18 (9) | |
| Sometimes | 36 (18) | 96 (48) | |
| Always | 0 (0) | 8 (4) | |
| Q5 | ≤ 0.001 | ||
| Never | 45 (22.5) | 37 (18.5) | |
| Sometimes | 33 (16.5) | 77 (38.5) | |
| Always | 0 (0) | 8 (4) | |
| Q6 | ≤ 0.001 | ||
| No | 74 (37) | 27 (13.5) | |
| Yes | 4 (2) | 95 (47.5) | |
| Q7 | ≤ 0.001 | ||
| Never | 29 (14.5) | 9 (4.5) | |
| Sometimes | 49 (24.5) | 102 (51) | |
| Always | 0 (0) | 11 (5.5) | |
| Q8 | ≤ 0.001 | ||
| No | 23 (11.5) | 1 (0.5) | |
| Yes | 55 (27.5) | 121 (60.5) | |
| Q9 | ≤ 0.001 | ||
| No | 56 (28) | 11 (5.5) | |
| Yes | 22 (11) | 111 (55.5) | |
| Q10 | ≤ 0.001 | ||
| Never | 24 (12) | 5 (2.5) | |
| Sometimes | 54 (27) | 107 (53.5) | |
| Always | 0 (0) | 10 (5) | |
| Q11 | ≤ 0.001 | ||
| Yes | 23 (11.5) | 4 (2) | |
| No | 55 (27.5) | 118 (59) | |
| Q12 | ≤ 0.001 | ||
| No | 58 (29) | 10 (5) | |
| Yes | 20 (10) | 112 (56) | |
| Q13 | ≤ 0.001 | ||
| No | 73 (36.5) | 75 (37.5) | |
| Yes | 5 (2.5) | 47 (23.5) | |
| Q14 | ≤ 0.001 | ||
| No | 72 (36) | 26 (13) | |
| Yes | 6 (3) | 96 (48) | |
| Q15 | ≤ 0.001 | ||
| No | 71 (35.5) | 57 (28.5) | |
| Yes | 7 (3.5) | 65 (32.5) | |
| Q16 | ≤ 0.001 | ||
| No | 65 (32.5) | 57 (28.5) | |
| Yes | 13 (7.5) | 65 (32.5) | |
| Q17 | ≤ 0.001 | ||
| No | 71 (35.5) | 45 (22.5) | |
| Yes | 7 (3.5) | 77 (38.5) | |
| Q18 | ≤ 0.001 | ||
| No | 73 (36.5) | 70 (35.5) | |
| Yes | 5 (2.5) | 52 (26) | |
| Q19 | ≤ 0.001 | ||
| Good | 66 (33) | 30 (15) | |
| Poor | 12 (6) | 92 (46) | |
| Q20 | ≤ 0.001 | ||
| Never | 21 (10.5) | 0 (0) | |
| Sometimes | 57 (28.5) | 114 (57) | |
| Always | 0 (0) | 8 (4) | |
| Q21 | ≤ 0.001 | ||
| Never | 23 (11.5) | 4 (2) | |
| Sometimes | 53 (26.5) | 90 (45) | |
| Always | 2 (1) | 28 (14) | |
4. Discussion
This study explored the phenomenon of SCD among middle-aged and older adults in Hong Kong. As far as we know, this study marked the inaugural use of the SCD-Q21 for data collection in Hong Kong, aimed at investigating the phenomenon of SCD among middle-aged and older individuals in the region. Regarding the prevalence of SCD, we referenced previous research that used the SCD-Q21 as an indicator to identify individuals who experience it. The study identified a cutoff value of 9.25, indicating that individuals scoring above this threshold are deemed to “potentially have SCD” [28]. A total of 122 individuals in our study had SCD-Q21 scores above the cutoff, indicating a prevalence of 61%. In comparison to neighbouring countries, such as China and Korea, their prevalence rates of 50.8% and 57.3% are lower than our findings [29, 30]. Previous studies have indicated that individuals with ongoing SCD have approximately a 75% probability of developing mild cognitive impairment and dementia within 10 years [31]. A growing number of people in Hong Kong may develop cognitive impairment in the future, highlighting the need for increased attention and intervention.
Previous studies have shown that ageing is a principal factor in cognitive decline, as it leads to alterations in brain structures, including a reduction in the volume of the hippocampus, frontal lobe and temporal lobe [32–35]. In this study, age did not demonstrate a significant correlation with memory decline. However, 176 individuals (88%) reported a perception of declining memory compared with five years ago (Q8), and 173 individuals (86.5%) indicated that they cannot remember things as well as they used to (Q11). Among those considered as “having SCD,” more than 90% agreed that they had experienced cognitive decline. Notably, 34 individuals classified as “non-SCD” reported dissatisfaction with their memory over the past two years (i.e., Q3), indicating a potential cognitive decline.
Furthermore, a greater number of participants in our study indicated increased difficulties in memorising words and numbers. A total of 173 respondents (86.5%) reported difficulties with word recall (i.e., Q21). Concurrently, 140 (70%) and 118 (59%) individuals faced challenges in recalling dates and numerical information, such as phone numbers, while only 84 (42%) indicated that they misplace objects more frequently than usual. Multiple studies pointed out that older individuals exhibited greater difficulty in recalling words and numbers compared with their ability to recall pictures or spatial information since the hippocampus and prefrontal cortex are critical for word recall [35, 36]. Conversely, the memory of images and spatial locations typically engages a broader array of neural networks, including regions that are less influenced by ageing [36].
Mild cognitive decline seems to be a normal and unavoidable process in ageing, but at the same time, some studies have also pointed out that the cognitive abilities of some healthy older adults might not necessarily decline; in fact, they could even improve [37, 38]. Engaging in physical activities has been demonstrated in various studies to enhance cognitive abilities among older adults [39–42]. The research conducted by Kramer et al. [42] showed that in those older adults who completed the aerobic exercise intervention after 6 months, the executive control processes related to the frontal and prefrontal lobe regions of the brain have been improved.
In term of chronic diseases, this study did not establish a between various chronic diseases and memory; however, numerous studies have indicated a correlation between these factors [43, 44]. Different diseases have varied mechanism. For instance, hypertension, the brain is the organ with the highest metabolic activity, and it needs a steady flow of oxygen and nutrients via a massive capillary network in order to function effectively [45]. Hypertension can substantially affect cognitive function by impairing cerebral blood vessels, resulting in diminished cerebral blood flow [46]. Also, it can lead to cognitive deterioration via mechanisms including white matter lesions and microinfarcts [47, 48]. Diabetes mellitus (DM) constitutes a further potential factor for cognitive decline that has been recognised [49, 50]. DM can impact every organ in humans, with particular emphasis on its neural tissues and the cerebrovascular system. Hyperglycemia, a hallmark of diabetes, substantially contributes to cognitive deterioration. Prolonged elevated blood sugar levels can result in oxidative stress, inflammation, and the formation of advanced glycation end products (AGEs) in the brain. These can impair neurons and hinder the functionality of neurotransmitters [51, 52]. Regulating blood glucose levels can influence synaptic plasticity, which is crucial for memory and learning [53]. Moreover, dysregulation of insulin signalling and small vessel disease linked to diabetes may substantially contribute to cognitive impairment [54, 55].
Higher education and continuous learning significantly enhance cognitive abilities through multiple mechanisms. Numerous studies have indicated a correlation between education and cognitive ability. Learning or training enhances cognitive function by improving memory, extending attention span, and developing problem-solving skills among older adults [56–58]. In the research conducted by Stern [59], they pointed out that individuals possessing greater cognitive reserve, frequently enhanced by educational attainment, are better equipped to preserve cognitive function in the face of brain ageing or pathology.
5. Limitations
This study has several limitations that should be considered in further intervention. First, we need increase the sample size. Numerous studies indicate that cognitive decline may arise from several factors, including age, education, and chronic diseases. Research suggests that cognitive functions may start to decline after midlife, with a notable decrease around age 70 [60, 61]. However, the limited sample size and a mean age of 65.05 in our study precluded the establishment of a consistent consensus, as observed in a prior research. Consequently, increasing the sample size may be a suitable method to obtain more significant insights. Second, the sample has an unequal distribution of male and female respondents, which may lead to findings that are inconsistent with previous studies. Some studies pointed out that women have faster cognitive decline than men [62, 63]. In our study, more than 75% of the participants were female. Enlarging the male sample size may facilitate the understanding of cognitive ability disparities between older male and female individuals. Lastly, our sample collection method may not be an exhaustive picture of cognitive decline in all older adults. As our current study employed an online questionnaire for older adults to complete, we did not account for the possibility that there may be some older adults unfamiliar with online platforms and are unable to complete the questionnaire. Therefore, we could look into various approaches for disseminating questionnaires in the future, including collaboration with senior institutions and delivering paper-based questionnaires to older adults in order to obtain a more substantial sample size.
6. Conclusion
Our cross-sectional study revealed that Hong Kong had a much higher prevalence of SCD than neighbouring regions and countries, which highlighted the urgent need to address this phenomenon promptly and with utmost concern. The implications of memory difficulties cannot be overlooked, as they can lead to a profound decrease in one’s quality of life, and if left unattended, they may progress into irreversible brain degenerative conditions such as dementia. It is crucial to recognise that memory loss is not confined to the older population alone. Therefore, early prevention of memory problems becomes paramount in safeguarding the wellbeing of individuals across various age groups. Furthermore, the findings of the study investigated the relationship between cognitive decline and a variety of variables. Unfortunately, the sample size of this study was insufficient to provide an unambiguous proof of consistent results between the Hong Kong situation and previous studies. To elucidate the cognitive abilities of older adults in Hong Kong, future research will endeavour to include a larger sample size. In addition, we aim to develop effective interventions to improve the situation for older individuals who are rated as having greater awareness of cognitive decline. This will help us gain a deeper understanding of the causes of their cognitive impairment.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors did not receive financial support for the research, authorship, and/or publication of this article but was conducted as part of the employment of the authors at the Hong Kong Polytechnic University.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




