The Slow Pandemic: Emergence of Antimicrobial Resistance in the Postadvent of SARS-CoV-2 Pandemic
Abstract
Background: The unprecedented outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has dramatically changed the global approach to public health, emphasizing the importance of measures to control and prevent infections. In response to the COVID-19 crisis, stringent hygiene practices and surface disinfection have become the norm, with an unprecedented surge in the use of disinfectants and antiseptics (DAs).
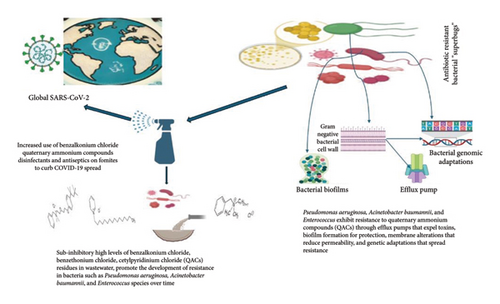
Main Text: While these measures have been crucial in curbing the spread of the virus, an emerging concern has taken center stage: the potential impact of the prolonged and widespread use of antimicrobial compounds in these products on the development of antibiotic resistance. Antimicrobial resistance (AMR) has long been recognized as one of the most pressing global health threats. Quaternary ammonium compounds (QAC) such as benzalkonium chloride, benzethonium chloride, and cetylpyridinium chloride, which are extensively used in DAs formulations, have gained less attention in the context of AMR.
Conclusion: A high abundance of QACs was detected in wastewater, and certain bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterococcus species developed resistance to these compounds over time. We analyzed the available evidence from the scientific literature, examining the presence and concentrations of QACs in different water sources, and their resistance mechanisms. This review aimed to shed light on the multifaceted challenges that arise from the dual battle against the COVID-19 pandemic and the ongoing global fight against AMR.
1. Introduction
In an era characterized by remarkable medical advancements and improved life expectancy, the shadow of antimicrobial resistance (AMR) looms over the progress made by the introduction of antibiotics and synthetic antimicrobials. AMR is an escalating global challenge that jeopardizes the progress made in modern healthcare, making routine infections difficult to treat and turning once-treatable illnesses into potentially fatal threats [1]. This phenomenon occurs when microorganisms, including bacteria, viruses, fungi, and parasites, acquire resistance to antibiotics intended to eradicate them, thereby diminishing the efficacy of therapeutic interventions [2]. The emergence of AMR can be attributed to the excessive and inappropriate use of antimicrobial agents, including antibiotics, across the human, animal, and agricultural domains [3]. Extended exposure to these agents provides opportunities for microbes to adapt and evolve, thereby leading to the development of mechanisms that counteract the effects of medications. Consequently, the difficulty in managing infections escalates, resulting in extended periods of illness, elevated healthcare expenditure, and an increased likelihood of mortality [4]. The World Health Organization (WHO) has recognized AMR as a significant peril to global public health, necessitating immediate attention and collaborative efforts from states worldwide [5].
Incorrect utilization of antibiotics is a significant contributing factor to the dissemination of AMR. Patients commonly exhibit nonadherence to recommended antibiotic regimens and prescribing antibiotics for viral infections despite their lack of efficacy in such cases. The use of antibiotics in agricultural practices to enhance the growth of animals exacerbates this issue [6]. These practices facilitate the development and spread of drug resistance in microorganisms. The consequences of AMR are far-reaching, affecting not only individual health, but also global economies and healthcare systems [7]. Frequently performed medical operations, such as surgery, chemotherapy, and organ transplants, might pose significant risks among immune-deficient individuals because of their heightened vulnerability to infections [4]. The financial implications of managing resistant infections in healthcare are significantly elevated, placing extra pressure on already overwhelmed health systems and perhaps exacerbating the economic vulnerability of marginalized groups.
The outbreak of the COVID-19 pandemic elicited an unprecedented global response, characterized by a significant emphasis on preventing infection and implementing control measures to curb the spread of the virus [8]. One of the most widely adopted strategies is to increase the use of DAs in public spaces, healthcare facilities, and homes. These measures are crucial in reducing the transmission of the virus; however, there is growing concern that excessive and inappropriate use of these agents could inadvertently contribute to the evolution of AMR [9]. DAs are chemical substances specifically formulated to eradicate or impede the proliferation of bacteria on various surfaces and on the skin. They play a vital role in reducing the risk of infections in healthcare settings and their significance has become even more apparent during pandemics [9]. However, the overuse or misuse of these agents can result in unintended consequences. When DAs are used, or when pathogens are exposed to sublethal concentrations of these chemicals, not all microorganisms can be eliminated or completely killed, which allows them to develop new ways to survive. This could be achieved by increasing the activity of efflux pumps, biofilm formation, or other defense mechanisms that make bacteria less susceptible to both antibiotics and antiseptics. Some microorganisms may survive because of their natural or acquired resistance to genetic mutations. Furthermore, repeated exposure to these chemicals can lead to the selection for and proliferation of resistant strains of microorganisms, which is a significant concern in healthcare settings and beyond [10]. This phenomenon underscores the importance of judicious use of such agents to help combat the growing problem of AMR. For instance, benzethonium chloride, benzalkonium chloride, and cetylpyridinium chloride are quaternary ammonium compounds (QAC) often found in DAs mixtures [9]. In addition to antibiotics and biocides, bacteria can become resistant to other harmful environmental compounds found in the soil, water, food, and feed [11]. The public is continually bombarded with messages in various media, encouraging them to purchase and use antimicrobials during all phases of their lives. Although these chemicals have been shown to kill or slow the growth of bacteria, their unnecessary or long-term use might facilitate the development of AMR in the future. Therefore, this review intends to uncover the multifaceted challenges that arise from the dual battle against the COVID-19 pandemic and the ongoing global fight against AMR, and to elucidate the mechanisms underlying the development of co-resistance.
2. Role of QAC in the Efficacy of Antiseptics and Disinfectants
DAs play crucial roles in maintaining hygiene standards, mitigating the spread of infections, and controlling microbial growth in various settings such as healthcare facilities, households, food industries, and paper industries [12, 13]. QACs are a broad class of positively charged nitrogen-containing chemicals employed as hydrophobic integrating dyes, industrial surfactants, and biocides [14]. The antibacterial activities of the R groups can also be affected by their length [15]. Typically, the strongest antibacterial action occurs in the methyl group lengths of C12–C16. QACs disrupt bacterial cytoplasmic membrane integrity and ionic stability. In addition to DNA binding, QACs also interact with intracellular targets.
At concentrations ranging from 0.5 to 5 mg/L, QACs exhibit algistatic, bacteriostatic, tuberculostatic, sporostatic, and fungistatic properties [14]. At concentrations ranging from 10 to 50 mg/L, the microbicidal properties of these substances were observed across the categories, with the efficacy varying based on the specific organism and formulation. A sequence of events describing the mechanism of action of QACs against microorganisms has been proposed to include: (i) adsorption and penetration of QACs into the cell wall; (ii) subsequent interaction of QACs with the cytoplasmic membrane, either through lipid or protein binding, leading to membrane disorganization; (iii) release of lower-weight intracellular components due to membrane permeability; (iv) degradation of proteins and nucleic acids within the cell; and (v) eventual lysis of the cell wall, facilitated by autolytic enzymes [16]. Different formulations of QACs have demonstrated efficacy against diverse microbial species [14]. A range of biocides have been prohibited for application to items intended for disposal through drainage systems. Therefore, it is imperative to prioritize chemicals that remain permissible and continue to be utilized in substantial quantities for research [17]. According to the United States Food and Drug Administration (FDA), benzalkonium chloride is classified as a Category III antiseptic active component. Ingredients were classified as Category III because there was insufficient information to determine their safety and effectiveness, necessitating further study. Two examples of antimicrobial agents commonly used in soaps are benzalkonium chloride, which is often found in bar soaps, and benzethonium chloride, which is commonly used in liquid-hand soaps. Benzethonium chloride, benzalkonium chloride, and cetylpyridinium chloride are chemical compounds with unique functions in the composition of DAs [7].
2.1. Benzalkonium Chloride
Benzalkonium chloride (BAC; CAS 8001-54-5) (Figure 1) is a quaternary ammonium chemical that has several functions, including application as a biocide, cationic surfactant, and phase transition agent [18]. Furthermore, it serves as an antiseptic agent, employed for the preservation of pharmaceuticals, or as a disinfectant for cleaning agents. BAC consists of a blend of alkyl benzyl dimethyl ammonium chloride, wherein the alkyl chain exhibits varying numbers of carbon atoms, typically n-C12H25 (dodecyl), n-C14H29 (tetradecyl), and n-C16H33 (hexadecyl). The antimicrobial potency is directly proportional to the length of the alkyl chain. The C12-homolog demonstrates efficacy against fungal and mold species, whereas the C14 variant exhibits activity against Gram-positive bacteria, and the C16 variant targets Gram-negative bacteria. Owing to the ability of BAC to rupture the cell membranes of microbes, it is frequently included in antiseptic compositions and demonstrates efficacy against a diverse array of bacteria, fungi, and certain types of viruses at low concentrations. The introduction of novel coatings and disinfection formulations based on QACs has led to a rise in their overall usage. Consequently, there has been an accompanying spike in reservations regarding the potential environmental and health risks associated with QAC exposure.

2.2. Benzethonium Chloride
Benzethonium (CAS 121-54-0) (Figure 2) is a synthetic quaternary ammonium salt that exhibits surfactant characteristics, possesses antiseptic properties, and demonstrates a wide range of antibacterial actions. Benzethonium chloride is primarily applied in its salt form as a skin disinfectant. It is also present in cosmetic products and personal care items, including mouthwashes and ointments, and is designed to alleviate itching. The efficacy of this substance in facilitating antibacterial activity against bacteria, fungi, molds, and viruses has been previously demonstrated. The concentrations often employed for this purpose range from 0.1% to 0.2%. These concentrations have been determined to be appropriate for use, as stated by the U.S. FDA [22]. Benzethonium is a member of the cationic detergent group that disrupts lipid bilayers. It is widely used because of its broad-spectrum antimicrobial activity against bacteria, viruses, and fungi [23]. Benzethonium chloride in antiseptic solutions kills bacteria by interfering with the integrity of microbial cell membranes. This leads to the leakage of cell contents and eventual cell death [24]. Benzethonium chloride is an active component of certain hard-surface disinfection solutions listed by the Environmental Protection Agency (EPA).
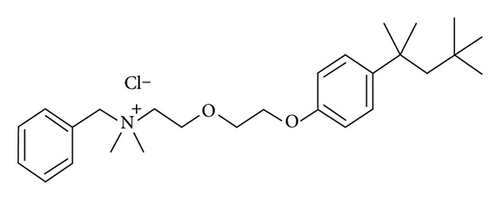
2.3. Cetylpyridinium Chloride
Cetylpyridinium chloride (CAS 123-03-5) (Figure 3) is another QAC used in various oral hygiene products and disinfectants [26]. It acts as an antiseptic by disrupting the cell membranes of microorganisms, resulting in inactivation. Cetylpyridinium chloride is utilized in oral hygiene solutions to effectively manage bacterial proliferation, mitigate plaque production, and deter the occurrence of halitosis [27]. The function of this entity is vital for the preservation of oral health and prevention of oral infections. Like other QACs, the excessive and prolonged use of goods containing cetylpyridinium chloride might result in the emergence of bacterial resistance. Strains that exhibit resistance have the potential to have diminished susceptibility to other antimicrobial agents, such as antibiotics, because of the common mechanisms of resistance.
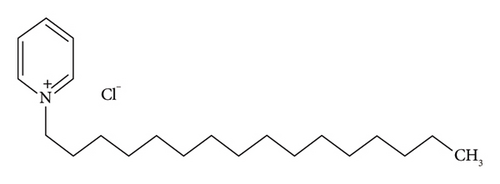
Benzethonium chloride, benzalkonium chloride, and cetylpyridinium chloride are valuable components for the formulation of antiseptics and disinfectants. Their distinct mechanisms of action, ranging from cell membrane disruption to inhibition of bacterial growth, contribute to their effectiveness in preventing infections, controlling microbial growth, and maintaining public health in various settings.
3. Fate and Impact of QACs on the Environment
The distribution and incidence of QACs in the environment are influenced by their inherent properties. The presence of smaller QACs in wastewater and water can be attributed to their low solubility and hydrophobicity [28]. The permanent positive charges of QACs facilitate sorption to negatively charged solids, particularly phyllosilicate clay minerals, which are commonly found in soils and sediments. The vapor pressures of QACs restrict volatilization following indoor applications, thereby contributing to their persistence on indoor surfaces. The persistence of QACs is determined by their architecture and prevailing environmental conditions. Despite the potential for aerobic degradation and passive photolysis of QACs, their relevance is constrained by their pronounced affinity for the adsorption of particles and chemicals. [28, 29]. The detailed occurrences of the selected QACs in the environment are highlighted in Table 1.
| QACs | Continent | Country(s) | Sources | Concentration μg/L | References |
|---|---|---|---|---|---|
| C12-benzalkonium chloride | Europe | Austria | Wastewater treatment plants (WWTPs) | 170 μg/L | [30, 31] |
| Europe | Germany | Wastewater | 7.7 μg/L | [32] | |
| North America | USA | Surface water downstream from WWTPs | 2.7–5.8 μg/L | [33] | |
| Africa | South Africa | WWTPs | 7.805 μg/L | [34] | |
| BAC C-14 | Europe | Austria | WWTPs | 110 μg/L | [31] |
| North America | USA | Surface water downstream from WWTPs | 6.3–36.6 μg/L | [33] | |
| Benzalkonium chlorides | Southeast Europe and West Asia | Turkey | Surface water and wastewater | 40 and 500 μg/L | [35] |
| BAC C12, BAC C14 and DADMAC C10 | Europe | Austria | Surface water | 1.9 μg/L | [29] |
QACs are ubiquitous in the environment globally, and their presence is not limited to household wastewater and sludge but also extends to treated effluent, surface water, and sediment [14]. Despite undergoing conventional wastewater treatment, QACs are not eliminated from the treated effluents. QACs are partially removed through processes such as sorption of biosolids and biological biodegradation [24]. In addition, QACs can still be found in the surface waters that receive them, especially at higher concentrations further downstream from the points where municipal wastewater treatment plant (WWTP) effluents and effluents from hospitals and industries such as laundry and food processing are released. Furthermore, the presence of nonlinear isotherms during adsorption suggests that QACs have a greater affinity for smaller amounts of organic particulates.
The notable levels of QAC present in the environment can be linked to the manufacturing of chemicals. As a result, with the rising global demand for QACs, these compounds are progressively being introduced into the environment via point source pollution, the land application of biosolids, or the discharge of treated municipal and industrial effluents [36]. Consequently, the occurrence of BACs in WWTPs, surface waters, and sediments could negatively impact various forms of life due to their toxic characteristics. Due to their widespread application, QACs are commonly found in influent, effluent, and receiving water bodies at levels that can be toxic. Approximately 25% of QACs ultimately find their way into the surrounding environment. Ventullo and Larson [37] found that QAC levels below 1 mg/L might induce ecologically important reactions.
The presence of QACs in ambient environments can be attributed to their extensive production and widespread usage. Hence, as the worldwide demand for QACs continues to increase, these substances will progressively infiltrate the environment via point-source pollution, application of biosolids to land, or discharge of treated municipal and industrial effluents. Consideration of chemical persistence is important when determining the prioritization of substances that are of interest. Existing regulations commonly establish criteria for determining the long-term stability of chemicals by considering their half-lives in various environmental nexuses. These criteria generally establish boundaries in the range of months, assuming situations that are favorable for biodegradation, such as high oxygen levels and the presence of acclimated bacteria. However, it is important to note that these standards can differ significantly and may not accurately represent the ambient conditions relevant to QACs [38]. Although they can degrade in the environment, they are classified as pseudo-persistent compounds. Furthermore, the sorption affinity of QACs in nonaqueous phases coupled with their persistent charges has the potential to hinder the biodegradation rate. Consequently, this can result in an extended half-life (spanning years) when QACs are sorbed into soil and sediment, thereby satisfying the persistence criterion [39].
In aquatic environments, three main processes facilitate the attenuation of QAC: photolysis, biodegradation, and the sorption of suspended particles, which ultimately results in settling. The quality assurance of QACs chemicals is generally recognized for its stability and relatively moderate degradation rates, influenced by processes such as hydrolysis, photolysis, and microbial activity. Research on the biodegradation of (QACs) has primarily utilized activated sludge or enrichment cultures. Notable data indicate that aquatic microbes can degrade and mineralize alkyltrimethyl ammonium compounds (ATMACs) and BACs to carbon dioxide within a period ranging from three days to two weeks [40], as described by [40, 41]. The biotransformation pathways of various QACs require complex bacterial consortia, rather than single species, to govern the fate of BAC in both natural and artificial systems. QAC concentrations were detected in both surface water and wastewater effluent [44]. These concentrations were measured to be as low as 0.002 μg/L and as high as 60 μg/L, respectively [45, 46]. Additionally, QACs in influent wastewater were significantly more concentrated, with concentrations up to 170 μg/L, representing a multiple-fold increase in concentration.
The utilization of nonpharmaceutical methods during and after the SARS-CoV-2 (COVID-19) pandemic has led to a significant increase in advertising and use of disinfectants containing QACs in healthcare and community environments. Disinfectants containing QACs account for more than 50% of items designated by the United States EPA and Health Canada as effective against SARS-CoV-2 [47, 48]. The recent COVID-19 pandemic has resulted in a significant increase in the use and environmental release of QACs [49]. The concentrations of QACs in aqueous environments range from 10−3 to 10−1 mg/L, with levels in wastewater being 10 times higher [28, 29, 50]. Consequently, it is imperative to recognize QACs as an emerging chemical class of concern.
However, there is a growing apprehension that extensive utilization of QACs might facilitate the development of bacterial resistance to QACs or trigger the development of resistance to antibiotics. Improper disinfectant use, a significant increase in QACs in untreated wastewater in specific regions, significantly elevated QAC levels in household dust compared to prepandemic levels, and the discovery of QACs in blood and breast milk samples from individuals during the pandemic have raised concerns about their safety [51]. The toxicity of QACs increases with prolonged exposure, as well as with physiological characteristics that provide resilience to QACs [52]. A study conducted by Alaa et al. [53] in Iran aimed to investigate the correlation between antibiotic resistance in bacteria and resistance to QACs in maternity wards. Enterobacter cloacae, Escherichia coli, certain serotypes of Staphylococcus hominins, and Aeromonas hydrophila were isolated from maternity wards and demonstrated resistance to disinfectants containing QACs.
Baseline activation of chromosomally encoded nonspecific efflux pumps confers innate resistance to QAC. The efflux determinants typically provide resistance against various agents, including antibiotics from the last resort class, and play physiological roles, such as conferring resistance against naturally occurring substances produced by the host, including bile hormones and host defense molecules [53]. Tolerance to QACs can be altered by sublethal exposure, which leads to relative changes. This is accomplished by temporarily modifying the density and nature of porins in the outermost cell membrane [53, 55]. Additionally, hyperexpression of efflux pumps is regulated in response to oxygen deprivation. Tolerance can also be affected by self-induced mutations. The acquisition of QAC-specific efflux pumps is facilitated by mobile recombinant genetic materials, such as plasmids and integrons [56]. Numerous aerobic and facultative microorganisms develop resistance to QACs by modifying their outer cell layer, specifically by altering the mix of fatty acids, phospholipids, and polysaccharides found within the cell membrane [57]. After these changes, the cellular composition becomes more negatively charged and hydrophobic, which makes it more difficult for the QAC to pass through the cell membranes. These effects could contribute to the colonization and long-term survival of bacteria within the host.
Co-resistance refers to the simultaneous selection of multiple genetic elements that confer resistance, including plasmids, transposons, and integrons, which are transferred concurrently [58]. Genes were individually expressed to determine their resistance. Cross-resistance occurs when a microorganism exhibits resistance to multiple unrelated compounds via a common mechanism such as employing the same efflux pump to counteract different antibiotics. Cross-resistance to QAC, for example, benzalkonium chloride, has also been reported. Merchel Piovesan Pereira et al. [23] subjected 40 E. coli strains to subinhibitory concentrations of 10 commonly utilized DAs. Seventeen strains showed cross-resistance to ampicillin, chloramphenicol, and norfloxacin. These results indicated that BAC induced cross-resistance. Membrane-related mutations are prevalent, and most strains exhibit enhanced biofilm-forming abilities [59].
In a separate investigation, E. coli strains were obtained from pigs, pig carcasses, and pork and were then exposed to BAC and other disinfectants. The strains were subsequently tested for the presence of eight antibiotics. BAC induced cross-resistance to at least three of the eight antibiotics. The efflux pump inhibitor (EPI) phenylalaninearginine-naphthylamide (PAbN) showed resistance to chloramphenicol and trimethoprim, while maintaining resistance to other antibiotics [60].
Recent findings during the COVID-19 pandemic highlighted the ability of Listeria monocytogenes to adapt to biocides, particularly QACs, and suggested a potential link between this adaptation and the selection of resistance to critical antibiotics like ciprofloxacin. The data indicate a potential association between the widespread application of QACs from production to consumption and the emergence of biocide and antibiotic resistance in pathogenic bacteria, including Listeria monocytogenes [61].
Jia et al. [48] investigated the molecular mechanisms of antibiotic resistance induced by mono- and twin-chained QACs, revealing a range of mutations associated with QACs. These mutations involve negative regulators (acrR, marR, soxR, and crp), outer membrane proteins and transporters (mipA and sbmA), and RNA polymerase (rpoB and rpoC), which may contribute to elevated multidrug resistance (MDR). Upon the removal of QACs stress, the phenotypic resistance caused by subinhibitory concentrations of QACs was found to be reversible, in contrast to the irreversible nature of resistance induced by inhibitory concentrations of QACs. The evolution and potential spread of antibiotic resistance due to rising environmental levels of (QACs), particularly dodecyl dimethyl ammonium chloride (DDAC), deserves critical attention.
3.1. Adaptation, Tolerance, and Resistance Mechanisms of Bacterial Pathogens to QAC (Pseudomonas aeruginosa, Enterococcus Species, and Acinetobacter baumannii)
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. (ESKAPE) pathogens frequently exhibit MDR in both developed and developing countries, indicating their ability to withstand more than three classes of antibiotics, which significantly contributes to the increasing prevalence of antibiotic resistance [62]. ESKAPE pathogens pose a significant global health challenge, complicating treatment options for severe infections, escalating the burden of disease, raising mortality rates due to treatment failures, impacting the achievement of sustainable development goals, and necessitating a unified global approach for surveillance of AMR [63].
Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterococcus spp. have become prominent nosocomial pathogens, primarily owing to their remarkable ability to rapidly acquire resistance to a wide range of antimicrobial agents, and their ability to persist in the environment, humans, and animals [64–67].
3.2. Pseudomonas aeruginosa
Pseudomonas is a diverse genus of bacteria that is widely distributed in environmental niches, including water, wastewater, and plant surfaces. Furthermore, Pseudomonas aeruginosa is a significant nosocomial bacterium found in tap, recreational, and surface water and has been implicated in several infectious outbreaks in immunocompromised individuals [68, 69]. It is a widely distributed bacterium that thrives in many ecological environments because of its exceptional capacity to adapt, which is supported by its genome that is flexible and highly adaptable to different diets [70]. The remarkable ability of the bacterium to evade antimicrobial drugs is attributed to a wide array of innate and acquired resistance mechanisms within its genetic diversity.
These populations have been shown to adapt to subinhibitory concentrations by changing the structure of cell membranes, increasing the expression of efflux pumps, and accelerating biofilm growth and the capacity for persistent infections in both animal and human hosts [28, 71]. These attributes render this species especially appropriate for studying the impact of consistent subinhibitory levels of QACs. Lower concentrations of QACs can selectively affect the types of microorganisms that live in an area, possibly leading to the emergence of QAC-resistant populations. The primary cause of aerobic biodegradation of QACs has been identified as a bacterial species belonging to the Pseudomonas genus. The aerobic degradation of QACs is contingent on the presence of monooxygenases, dioxygenases, amine dehydrogenases, and/or amine oxidases [36]. The enzyme amine oxidase can significantly decrease BAC toxicity to bacteria by a factor of multiple hundred-fold. Approximately a decade ago, a gene cluster that encodes transporters, namely integrase and dioxygenase, was shown to be crucial for the biotransformation of BAC in aerobic environments [35]. The presence of multidrug efflux pump genes, including sugE, PmpM, mexAB-oprM, and mexEF-oprN, in the BAC-degrading community suggests that these efflux pumps and oxidase enzymes contribute to the enhancement of microbial resistance to QACs [72, 73].
3.3. Enterococcus Species
Among the more than 60 recognized Enterococcus species, Enterococcus faecalis and Enterococcus faecium are mostly associated with opportunistic human infections and are among the most common in the human gut microbiota [74]. It is important to examine how DAs affect the evolution of the Enterococcus microbiome, because this organism is one of the most common MDR healthcare-associated infections worldwide [75]. Inadequate cleaning during disinfection, improper biocide application, or the use of adulterated DAs in veterinary, food chain (e.g., food industry and farms), or human domestic contexts may lead to the use of QACs at subinhibitory concentrations in these specific contexts. Diverse microbiomes frequently encounter varying concentrations of QACs, potentially leading to alterations in the cellular composition of pathogens and fostering the selection of populations with reduced susceptibility to QACs and other antimicrobial agents (biocides or antibiotics) via co- or cross-selection mechanisms. The diminished sensitivity of Enterococcus species to QAC after adaptation may be attributed to several factors, including alterations in membrane fatty acid content, differential expression or mutations in efflux pumps, and stress responses. These findings reveal the complex bacterial adaptation mechanisms of QACs, potentially affecting disinfection strategies in healthcare. Understanding these processes may improve antimicrobial formulation and infection control protocols, thereby enhancing food and patient safety.
3.4. Acinetobacter baumannii
Acinetobacter is ubiquitous in nature and has been isolated from both clinical and environmental samples. MDR Acinetobacter baumannii has substantial clinical importance due to its role in hospital-associated nosocomial epidemics worldwide [76]. The potential impact of QAC and its derivatives on the spread of antimicrobial-resistant genes/bacteria via co-selection processes in manure and WWTPs, or in environments where Acinetobacter baumannii and QAC come into close contact [77]. High concentrations of BZK mostly cause membrane damage, although low concentrations have been demonstrated to disturb the balance of proteins in cells, ultimately resulting in the death of A. baumannii cells. The resistance and tolerance of Acinetobacter baumannii to QACs are frequently linked to mobile genetic elements (MGEs), which facilitate the extensive spread of AMR genes and bacteria. A. baumannii’s array of multidrug efflux pumps contribute to its high disinfectant (chlorhexidine and benzalkonium chloride) tolerance, which gives it a unique capacity to persist for extended periods of time in hospital conditions [78, 79]. Currently, resistance to disinfectants is an adaptive advantage. However, many A. baumannii efflux pump genes have been found in all species or genera, suggesting that they have a common function in the past [80]. The arrays of bacterial pathogens exhibiting QAC resistance patterns are shown in Table 2.
| QAC resistant pathogens | Continent | Country | Sources | References |
|---|---|---|---|---|
| Acinetobacter bohemicus strain | Europe | Germany | Pig manure | [76] |
| ESBL-producing Escherichia coli | Europe | Central Slovenia | Human (lower respiratory infection) | [81] |
| Escherichia coli | North America | United States of America | Retailed meat | [12] |
| Enterobacter cloacae | Europe | Norway | Fish farm | [82] |
| Escherichia coli | Africa | Egypt | Chicken | [83] |
| Escherichia coli | Africa | Egypt | Diseased broilers and environmental sources | [84] |
| Acinetobacter baumannii | North America | United States of America | Clinical isolates | [85] |
| Acinetobacter baumannii | Asia | Malaysia | Admitted patients | [86] |
| Klebsiella pneumoniae | Asia | Saudi Arabia | Clinical specimen | [87] |
| Pseudomonas aeruginosa | Asia | Saudi Arabia | Clinical specimen | [87] |
| Acinetobacter baumannii | Asia | Saudi Arabia | Clinical specimen | [87] |
| Acinetobacter spp. | South America | Brazil | Food sample | [88] |
| Acinetobacter baumannii | South America | Venezuela | Patients with nosocomial infections | [89] |
| Pseudomonas aeruginosa | South America | Brazil | Clinical isolates | [90] |
| Pseudomonas aeruginosa | Australia | Australia | Ocular isolates | [91] |
| Staphylococcus spp. | Europe | Norway | Food industry | [90] |
| Listeria monocytogenes | Europe | Norway | Food industry | [92] |
| staphylococci | Europe | Norway | Clinical strains | [93] |
| Listeria monocytogenes | North America | United States of America | Fresh produce environment | [94] |
| Lysinibacillus sp. | Asia | China | Soil | [95] |
| Bacillus aryabhattai | Asia | China | Soil | [95] |
| Bacillus cereus | Asia | China | Soil | [95] |
| Exiguobacterium acetylicum | Asia | China | Soil | [95] |
| Bacillus thuringiensis | Asia | China | Soil | [95] |
| Klebsiella pneumoniae | Asia | China | Soil | [95] |
| Enterobacter ludwigii | Asia | China | Soil | [95] |
| Salmonella enterica | Asia | China | Retail meat, retail layer and human faeces | [96] |
4. Mechanism of QAC Resistance
When disinfectants are used incorrectly or bacteria are exposed to doses below what is needed to kill them, some types of bacteria may be able to survive, become less sensitive, and develop resistance. QAC-resistant microorganisms are frequently obtained from sewage or activated sludge due to the discharge of QACs into the environment via runoff [36, 97, 98]. This phenomenon gives rise to concentration gradients in QACs, facilitating microbial adaptability to subinhibitory concentrations of disinfectants [100]. Antibiotic resistance has received increased attention since the 1960s. However, it is important to note that the escalation of resistance to disinfectants and antibiotics poses a substantial risk to biosafety and human health. In addition to responses to exposure to antibiotics, bacteria could adapt to changes in their ambient environment, including exposure to antimicrobial chemicals, and thereby exhibit resistance to disinfectants. Because of this, changes have been seen in the membranes of cells, the appearance of mutant enzymes with new functions and metabolic pathways, and the activation of more resistance genes, such as those linked to horizontal gene transfer (HGT), biofilm formation, different efflux pumps, and biotransformation.
4.1. HGT of QAC and Antibiotic Genes
Drug-susceptible bacteria can acquire antibiotic resistance, primarily through genetic mutations or gene transfer, with HGT being the predominant mechanism. Mutations occur randomly and are typically spread through vertical inheritance; however, HGT is more concerning because it allows for the phylogenetic jumps of genes [7]. This suggests that the majority of the resistance genes found in microbiomes are part of the intrinsic resistome. The primary concern regarding HGT mechanisms in disinfectants and the spread of antiseptic resistance is conjugation. QACs facilitate the transfer of genetic material between bacteria via direct cell-to-cell contact [7]. Hu et al. [50] systematically investigated the effects of various QAC with differing structures and carbon chain homology on the conjugative transfer of antibiotic resistance genes (ARGs). Their findings indicated that most QACs at environmental concentrations could facilitate the horizontal transfer of ARGs from E. coli DH5α to E. coli MG1655 and S. sonnei through various mechanisms. The reported concentrations of QACs in aqueous environments range from 10−3 to 10−1 mg/L. Without accounting for the synergistic or antagonistic effects of other environmental factors, it is important to highlight the potential risks of QACs facilitating the horizontal transfer of ARGs in the environment. S. sonnei is a pathogen that affects the human intestine, with its infection rate rising annually. This study demonstrated that S. sonnei can obtain ARGs from environmental bacteria through HGT, with exposure to QACs that enhance the transfer rate.
4.2. Efflux Pumps as a QAC Resistance Mechanism
Efflux pumps are the basic mechanisms of resistance to DAs. The concentrations of antimicrobial agents within cells are reduced by actively transporting agents out of the cells [101], as described by [99]. Efflux pumps can exhibit either particularity towards a substrate or possess a broad spectrum of substrates, as seen by MDR pumps. Efflux-mediated intrinsic resistance is a mechanism that enables tolerance to QACs in amounts that are considered environmentally harmful, as shown in Figure 4. Efflux-mediated QAC resistance has received attention because of its genetic basis, ability to create simultaneous resistance to antibiotics, and potential for HGT across different microbial species [103]. Proteins facilitate active expulsion of antimicrobials within bacterial cells. Efflux systems can actively transport a diverse array of chemically and structurally dissimilar substances, such as QACs, using energy- or proton-dependent mechanisms, while maintaining the integrity of transported chemicals [104, 105]. There are various categories of multidrug efflux systems, including (i) resistance-nodulation-division (RND), (ii) major facilitator superfamily (MFS), (iii) adenosine triphosphate (ATP)-binding cassette (ABC) family, (iv) small multidrug resistance (SMR) family, (v) multidrug and toxic compound extrusion (MATE) family, and (vi) proteobacterial antimicrobial compound efflux (PACE) [104, 106] MDR efflux systems, including the MFS, ATP-ABC, and SMR systems, are frequently observed in Gram-positive bacteria. Conversely, the RND, NorA, and QacA/A families of efflux pumps are more commonly associated with Gram-negative bacteria. The MATE family pump, known as PmPM, is present in several species of bacterial, including P. aeruginosa, Acinetobacter baumannii, Haemophilus influenzae, Clostridium difficile, Vibrio parahaemolyticus, Bacteroides thetaiotaomicron, and Staphylococcus aureus [107]. The upregulation of efflux pumps results in a 2- to 8-fold increase in resistance to QAC and other associated substrates transported by the pump. ABC transporters belong to the basic family class, whereas the remaining families are regarded as the advanced family class.
RND efflux pumps are mostly found in Gram-negative bacteria. They have three parts: a cytoplasmic membrane pump, a periplasmic protein, and an outer membrane protein channel. The AcrAB-TolC system, which belongs to the RND family, is prevalent in Gram-negative bacteria, including Escherichia coli. This system has a broad substrate specificity, including dyes, fluoroquinolones, and BAC. Proportion of subpopulations persisting significantly decreased by 1000-fold when the tolC gene was knocked out. This observation suggests that TolC facilitation of the efflux mechanism is crucial for the persistence of BAC tolerance in subpopulations. These results are in line with those of other studies that have found links between differences in the location of the AcrAB-TolC efflux pump, differences in tolC expression, and antibiotic resistance. The results of a recent study suggested that persistent cells exhibit greater tolerance to bactericidal antibiotics because of enhanced efflux mechanisms [100]. Further studies are required to validate this mechanism. This hypothesis posits that the random expression of the marRAB system, which serves as a regulator of acrAB and tolC51, can potentially contribute to the survival of artificial chromosomes in bacteria by inducing heterogeneous gene expression.
Resistance against ATMACs through efflux pumps might be attributed to two distinct processes: First, efflux pumps are overexpressed in response to exposure to high concentrations of QACs, which then results in resistance against QACs; Second, internally generated QAC stress can trigger overexpression of efflux pumps, resulting in resistance against QACs. Exposure to such stressors can activate a regulatory mechanism responsible for modulating the expression of efflux determinants. Alternatively, stress can cause one or more mutations in the genetic elements, which results in increased production of the efflux driver or makes its outflow more effective [108, 109]. In this context, genes encoding proteins associated with efflux pumps are often encoded within chromosomes or acquired through MGEs and exhibit activities against a diverse range of antimicrobial drugs. QAC resistance genes, such as class-1 integrons are frequently located in the same genetic elements as the antibiotic resistance genes. This colocalization potentially facilitates the emergence of co-resistance between QACs and antibiotics because they might possess comparable modes of action [52, 110]. Exposure to comparable sub-inhibitory concentrations of QACs or DAs can lead to the survival of potentially pathogenic microbes. Survival could result in the emergence of cross-resistance against medications that share structural or functional similarities, including antibiotics that are often used in medical interventions [111]. This phenomenon presents a plausible mechanism for producing antibiotic-resistant bacteria in the surroundings contaminated with QACs. It is possible for QAC efflux pump genes to be moved from one cell to another through conjugation, which can be accomplished by integrons, plasmids, transposons, and conjugative elements [112]. The qac gene provides an initial selection advantage by conferring resistance to DAs. Hence, the increasing use of QACs facilitates the fixation of additional genetic elements that are new and distinct. Integrons serve as reservoirs for resistance cassettes and can efficiently acquire supplementary resistance determinants through plasmids. The present vehicle technology demonstrates high efficiency in facilitating interspecies mobility. It is common for Gram-negative bacteria to become resistant to QACs by having qac genes, and for Gram-positive bacteria to become resistant by having class 1 integrons [113]. It is important to acknowledge that reduced sensitivity and sometimes resistance against QACs is facilitated by efflux pumps, among other possible mechanisms. Numerous studies have indicated that efflux pumps might facilitate biofilm formation either through direct or indirect mechanisms [114, 115]. Empirical and genetic evidence supports the idea that QAC environmental residues might potentially have a significant impact on the evolution of AMR, and the transfer of genes linked to AMR [98]. Because of its extensive array of efflux pumps, which can expel many antimicrobial agents, including QACs and antibiotics, the genus Pseudomonas has significant relevance in the context of AMR, owing to its extensive distribution in many environments. Its inclusion as one of the most notorious drug-resistant bacteria poses significant challenges in the treatment of bacterial infections [28, 116]. QACs facilitated the transfer of RP4-resistant plasmids into E. coli at low doses. The quantities of QACs that maximized the enhancement of trans-conjugate efficiency were comparable to those observed in the environment. This indicates that the presence of residual QACs in the environment might worsen environmentally induced resistance of bacteria to antimicrobials and/or antibiotics [117].
4.3. Biofilm Formation as a QAC-Resistant Mechanism
Compounds such as benzalkonium chloride and chlorhexidine can potentially induce biofilm development. Biofilms, which are intricate assemblies of microorganisms surrounded by a protective extracellular matrix, serve as physical barriers that can confer protection against antimicrobial agents, such as antibiotics. It has been suggested that EPS matrices enhance recalcitrance by impeding the diffusion of disinfection solutes into biofilms. This hindrance can occur through mechanisms such as size exclusion, interactions, and reactions with solutes [118]. Biofilm formation contributes to a decrease in the vulnerability of both Gram-positive and Gram-negative bacteria to disinfectants. Gram-negative bacteria have a greater propensity to acquire resistance against QACs because of the composition of their cell walls [119]. Furthermore, the extracellular matrix contains other constituents, including enzymes, that can contribute to the detoxification of harmful substances. For example, P. aeruginosa can withstand exposure to disinfectants through the formation of persistent cells, effectively hindering the manufacture of antibiotic targets [120, 121]. According to Qin et al. [122], the presence of molecules derived from persistent cells can sustain their viability and replenish biofilms. In the absence of disinfectants, bacteria regain growth and viability, resulting in the development of chronic infections. The effectiveness of disinfectants against biofilms is significantly lower than that of planktonic organisms [123].
The resistance of biofilms to disinfectants is contingent on factors such as the efficacy of disinfectants, temperature, and surface characteristics [125]. In addition, bacterial biofilms formed within wastewater treatment systems can function as reservoirs of AMR. Biofilms provide a shield against antimicrobial agents, allowing bacteria to survive and potentially exchange resistance genes within the biofilm community [10]. The coexistence of various microorganisms within the surrounding ecosystem may potentially contribute to increased survival when exposed to disinfectant-induced stress as shown in Figure 4. For example, Listeria monocytogenes monocytes have been shown to protect against Pseudomonas putida when exposed to low levels of unclassified BAC [126]. The treatment selectively targeted and eradicated L. monocytogenes. Another discovery was that the addition of Pseudomonas fluorescens to dual-species biofilms made Salmonella enterica more resistant to a group of unknown QACs [121, 127].
4.4. Biotransformation as a QAC-Resistance Mechanism
Most types of antibiotics can be broken down by photo- or hydrolytic activity in the environment because of the way their molecules are structured and the physical and chemical properties they have [124]. The initial steps of QAC biodegradation are a multistep process conducted by microorganisms, which is similar to the degradation of antibiotics. Metabolism requires the recognition of the molecule and efficient binding between enzymes and substrates [129]. This phenomenon is contingent on the arrangement of the molecule and its physicochemical characteristics. The metabolic processes of QACs are influenced by their structural compositions. Consequently, the presence of hydrophobic alkyl chains has been shown to significantly affect the biodegradation and toxicity of QACs. Specifically, owing to their lower solubility, the elongation of alkyl chains has been shown to reduce biodegradability [130, 131]. Hence, reduced solubility restricts the availability of microbial species. DAs might have less of an effect on the ecosystem if there is a diverse community of microbes that include species with moderate to high tolerance and the ability to break down antimicrobial compounds. Because biotransformation reduces the concentrations of antimicrobials, exposure-susceptible species may reach subinhibitory concentrations. This could facilitate the development of resistance. Previous studies have demonstrated that certain bacteria possess the ability to metabolize disinfectants, such as QACs. These microbes respond to antimicrobial agents and may potentially acquire resistance through several metabolic pathways [132, 133].
Biotransformation is likely to be the primary method for removing disinfectants such as QACs from wastewater treatment facilities. When a community of microbes is exposed to QACs, the genera that are naturally resistant or that can biotransform QACs are given more attention [134]. For instance, a previous study reported that after exposure to BAC, microbial communities exhibited a decrease in activity and were composed of Pseudomonas sp., as described by [99, 131]. P. nitroreducens, P. aeruginosa, and P. putida were the Pseudomonas species that were most often seen after being exposed to BAC in the special enrichment medium. Additional taxa, including Citrobacter sp., Klebsiella sp., Salmonella sp., Enterobacter sp., and Achromobacter, have been identified [36]. Relative to enhanced microbial communities, bacterial artificial chromosomes (BACs) undergo degradation via dealkylation, resulting in the formation of BDMA. This is followed by successive demethylation, resulting in the production of dimethylamine and methylamine [99]. With the development of resistance, microbes may acquire the capacity to use antimicrobials as a viable source of nutrition or energy. These microbes, which are related to P. nitroreducens and P. putida, have been linked to the process of assimilation and degradation of BAC [135]. This phenomenon not only provides advantages to the destruction of microorganisms but might also offer protection to other members of the community because of the influence effect.
The continuous emergence of QAC resistance in the environment necessitates urgent development of next-generation disinfectants for human health. It is essential to balance antibiotic efficacy with environmental factors in order to successfully combat the emergence of bacterial resistance [135]. Recent research has shown that biscationic quaternary phosphonium compounds (bis-cationic QPCs) are very effective against both Gram-positive and Gram-negative pathogenic bacterial strains that can acquire QAC resistance mechanisms [135].
5. Conclusions
QACs are becoming more popular as preferred agents for chemical disinfection of microorganisms in healthcare, household, and industrial settings. Improper utilization of these substances has the potential to exert selection pressure, contributing to the development of resistance, including AMR, which is currently a widespread phenomenon worldwide. It is very important to make sure that policies are put in place to encourage responsible and smart use, as this will help lower the risks that come with the spread of antimicrobial-resistant strains in the years after COVID. Policymakers should mandate the disclosure of QACs and their possible effects to encourage responsible usage by consumers, while fostering stewardship among producers. In addition, the use of environmentally sustainable QACs or alternative chemicals, together with improved practices for the treatment and control of wastewater containing QACs, presents encouraging avenues for mitigating or eradicating the discharge of QACs into progressively strained ecosystems. Moreover, it is important to enhance monitoring systems to effectively monitor the establishment and dissemination of AMR, which is crucial for the development of successful intervention approaches. Moreover, DAs and antibiotics play a crucial role in controlling infections caused by MDR bacteria. Therefore, the research community must continue to support studies that generate practical data to guide the development of policies. This can be achieved by offering incentives for research and development, which in turn can facilitate the development of novel antimicrobial drugs that specifically combat resistant infections. Nevertheless, despite some findings from experiments conducted in controlled laboratory environments, there is a dearth of definitive data indicating that QACs are directly responsible for the extensive proliferation of AMR in real-life scenarios. Further research is required to investigate the evolutionary patterns of pathogens that exhibit tolerance and resistance to QACs and antibiotics in real-world environments. Therefore, a more comprehensive understanding of the potential impact of QAC use on the promotion of clinically significant resistance is required.
Nomenclature
-
- COVID-19
-
- Coronavirus disease 2019
-
- SARS-CoV-2
-
- Severe acute respiratory syndrome coronavirus 2
-
- QAC
-
- Quaternary ammonium compounds
-
- AMR
-
- Antimicrobial resistance
-
- FDA
-
- Food and drug administration
-
- BAC
-
- Benzalkonium chloride
-
- DNA
-
- Deoxyribonucleic acid
-
- EPA
-
- Environmental protection agency
-
- ADBAC
-
- Alkyl dimethyl benzyl ammonium chloride
-
- WWTP
-
- Wastewater treatment plant
-
- ATMAC
-
- Alkyl trimethyl ammonium compounds
-
- RND
-
- Resistance-nodulation-division
-
- MFS
-
- Major facilitator superfamily
-
- ATP
-
- Adenosine triphosphate
-
- ABC
-
- ATP-binding cassette
-
- SMR
-
- Small multidrug resistance
-
- MATE
-
- Multidrug and toxic compound extrusion family
-
- PACE
-
- Proteobacterial antimicrobial compound efflux
-
- MDR
-
- Multiple-drug resistance
-
- MFS
-
- Major facilitator superfamily
-
- AcrB
-
- Acriflavine resistance B
-
- DA
-
- Disinfectant and antiseptic
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ayodeji Osunla: conceptualization of the review topic and writing the original draft. John Giesy: conceptualization of the review topic and review draft. Femi Oloye: conceptualization of the review topic and review draft. Adeoye Kayode: review draft. Oluwabunmi Femi-Oloye: review draft. Ayomide Okiti: review draft. Mark Servos: review draft.
Funding
This work received funding from the Global Water Futures.
Acknowledgments
We are grateful to the Global Water Futures for sponsoring our projects.
Open Research
Data Availability Statement
No data are generated in this manuscript.




