Fujian Province β-Thalassemia: A Molecular and Hematological Study in Southeastern China
Abstract
Background: This study aims to investigate the mutation spectrum of β-thalassemia in Fujian Province, China, and to comprehensively analyze the correlation between age, gender, genotype, and hematological parameters in carriers of β-thalassemia.
Methods: Genotypes of 10,350 subjects suspected of having thalassemia were analyzed using reverse dot blotting (RDB) or β-globin gene sequencing. Their hematological indices were analyzed by genotype, gender, and age.
Results: Among the subjects, 1214 (11.73%) were identified as β-thalassemia carriers. The prevalent genotypes included IVS-II-654 (C > T)/N (37.56%), CD 41-42 (-TTCT)/N (30.72%), CD 17 (A > T)/N (9.64%), −28 (A > G)/N (7.00%), CD 27-28 (+C)/N (3.21%), and CD 26 (GAG > AAG)/N (3.05%). Two rare mutations, Cap+22 (G > A) and IVS-II-806 (G > C), were detected, with the latter being part of a double heterozygous condition with hemoglobin (Hb) New York, compound −α4.2/αα, and Hb Q Thailand, marking the first report in Chinese individuals. Hematological analysis revealed that the CD 26 group exhibited higher levels of Hb, mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) compared to the β0 and β+ groups (p < 0.05). Within the β+ group, individuals with −28 (A > G)/N showed significantly higher Hb, MCV, and MCH levels compared to those with IVS-II-654 (C > T)/N. Adult males had higher Hb levels than adult females, and adult patients generally had higher MCV and MCH levels than minors (p < 0.05).
Conclusion: This study represents the first comprehensive molecular epidemiological investigation and hematological analysis of β-thalassemia in Fujian Province, providing support for the optimization of prevention and control strategies for thalassemia.
1. Introduction
Thalassemia is a group of hereditary blood disorders that can seriously threaten human health and result in mortality and disability, which is divided into α-thalassemia (α-thal) and β-thalassemia (β-thal) depending on the globin affected [1]. β-thal mainly arises from mutations in the β-globin gene, which lead to a reduction or complete absence of β-globin synthesis, resulting in an imbalance in the ratio of α to β globin chains and the deposition of excess α-globin responsible for ineffective erythropoiesis and hemolytic anemia [2]. Clinical severities range from asymptomatic carriers with β-thal minor to severe anemia in β-thal intermedia and major, with the latter sometimes requiring lifelong blood transfusions and iron chelation [3].
As reported, β-thal predominantly manifests in the Mediterranean region, the Middle East, India, and Southeast Asia [4, 5]. Recent epidemiological data reveal that carriers of β-thal account for approximately 3% of the global population [6]. Comprehensive studies have shown that the most common mutation type of β-thal in China are mutations within the β-globin gene, including IVS-II-654 (C > T), CD 41-42 (-TTCT), CD 17 (A > T), hemoglobin E (HbE), and a mutation in the promoter region of the β-globin gene −28 (A > G) [6]. Fujian Province, situated in the southern region of China with a population exceeding 40 million permanent residents, exhibits a notably high incidence rate of β-thal. The paucity of large-sample studies on the molecular spectrum and hematological parameters of β-thal in Fujian Province limit our understanding of the disease in this region. A large-scale comprehensive analysis of the correlation between age, gender, genotype, and hematological parameters in Fujian Province was conducted for the first time in this study, potentially providing additional data for genetic counseling and prenatal diagnosis to reduce the incidence of babies born with major thalassemia.
2. Methods
2.1. Ethical Statement
This study was approved by the institutional ethics committee of Fujian Maternity and Child Health Hospital (approval no. 073, 2019). Signed informed consent was obtained from all participants following a detailed description of the purpose of the study. All experiments were performed by relevant guidelines and regulations.
2.2. Study Subjects
From January 2019 to November 2021, a total of 10,350 individuals suspected of being thalassemia carriers were recruited at Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics and Gynecology and Pediatrics. Those meeting one or more of the following inclusion criteria were screened for the thalassemia gene as (1) mean corpuscular volume (MCV) of < 80 fL and/or mean corpuscular hemoglobin (MCH) concentration of < 27 pg; (2) hemoglobin A2 (HbA2) of > 3.5% and/or fetal hemoglobin (HbF) of > 2.0%; and (3) a family history of hereditary thalassemia.
2.3. Genotype of β-Thal
DNA was extracted using the DNA Blood Extraction Kit (Yaneng Biosciences, Shenzhen, China), and 17 common β-thal point mutations in the Chinese population were detected by reverse dot blotting (RDB) using a commercial kit (Yaneng Biosciences, Shenzhen, China). Suspected rare types of β-thal were analyzed by amplifying the full-length β-globin gene using PCR, with purified PCR products sequenced directly on an ABI 3100 DNA sequencer (Applied Biosystems; Foster City, CA, USA). The specific methods for these experiments are described in previous literature [4], and all experiments were performed with three replicates to ensure the accuracy and reproducibility of the data.
β+-thal means that the mutation leads to a reduction in β-globin chain synthesis; if it leads to a complete failure of β-globin chain synthesis, it is called β0-thal. In this study, the β0 group contains CD 41-42 (-TTCT)/N, CD 17 (A > T)/N, CD 27/28 (+C)/N, CD 71 -72 (+A)/N, CD 43 (G > T)/N, initiation codon (ATG > AGG)/N, and IVS-I-1 (G > T)/N; the β+ group includes IVS-II-654 (C > T)/N, −28 (A > G)/N, IVS-I-5 (G > C)/N, and Cap+40-43 (-AAAC)/N.
2.4. Statistical Analysis
The data analysis was conducted using SPSS Version 26 (IBM Inc., Chicago, USA). Normality was assessed with the Kolmogorov–Smirnov test, and variance homogeneity was tested using Levene’s test. Parametric tests (ANOVA or t-test) were utilized for normally distributed and homogeneous data, while the Kruskal–Wallis test was applied for nonhomogeneous data. Continuous variables are expressed as mean ± standard deviation (SD), and categorical variables are presented as frequencies and percentages. Statistical significance was set at p < 0.05 for all tests, with effect sizes calculated to measure the strength of observed differences.
3. Results
3.1. The Spectrum of β-Thal in Fujian Province
Among the 10,350 subjects screened for suspected thalassemia, 1214 cases were diagnosed as β-thal carriers, with a detection rate of 11.73% (1214/10,350). There were 1148 cases (94.56%) with heterozygotes for β-thal, and 66 cases with concurrent α- and β-thal (5.44%). In this study, 17 kinds of β-thal mutations were identified, the most common genotypes were IVS-II-654 (C > T)/N and CD 41-42 (-TTCT)/N, with a remarkable proportion of 37.56% and 30.72%, and the other common genotypes were CD 17 (A > T)/N (9.64%), −28 (A > G)/N (7.00%), CD 27-28 (+C)/N (3.21%), and CD 26 (GAG > AAG)/N (3.05%). The above genotypes accounted for 91.18% of all β-thal genotypes (Table 1).
| Genotype of β-thal | N | Percentage in β-thal (%) | Detected rate (%) | |
|---|---|---|---|---|
| β-thal | βIVS-II-654(C>T)/βN | 456 | 37.56 | 4.41 |
| βCD41-42 (-TTCT)/βN | 373 | 30.72 | 3.60 | |
| βCD17 (A>T)/βN | 117 | 9.64 | 1.13 | |
| β-28(A>G)/βN | 85 | 7.00 | 0.82 | |
| βCD27-28 (+C)/βN | 39 | 3.21 | 0.37 | |
| βCD26 (GAG>AAG)/βN | 37 | 3.05 | 0.35 | |
| βCD71 -72(+A)/βN | 15 | 1.24 | 0.14 | |
| βCD43 (G>T)/βN | 12 | 0.99 | 0.12 | |
| βInitiation codon (ATG>AGG)/βN | 4 | 0.33 | 0.04 | |
| βIVS-II-806(G>C)/βN | 2 | 0.16 | 0.04 | |
| βCap+40-43 (-AAAC)/βN | 2 | 0.16 | 0.02 | |
| βCap+22 (G>A)/βN | 1 | 0.08 | 0.01 | |
| βIVS-I-1(G>T)/βN | 1 | 0.08 | 0.01 | |
| βIVS-I-5(G>C)/βN | 1 | 0.08 | 0.01 | |
| βCD30 (AGG>GGG)/βN | 1 | 0.08 | 0.01 | |
| β-29(A>G)/βN | 1 | 0.08 | 0.01 | |
| βCD14-15 (+G)/βN | 1 | 0.08 | 0.01 | |
| Subtotal | 1148 | 94.56 | 11.24 | |
| Concurrent α- and β-thal | -α3.7/αα, βIVS-II-654(C>T)/βN | 11 | 0.90 | 0.11 |
| -α3.7/αα, βCD41-42 (-TTCT)/βN | 4 | 0.33 | 0.03 | |
| -α3.7/αα, βCD71 -72(+A)/βN | 2 | 0.16 | 0.02 | |
| -α3.7/αα, βCD17 (A >T)/βN | 2 | 0.16 | 0.02 | |
| -α3.7/αα, β-28(A>G)/βN | 1 | 0.08 | 0.01 | |
| -α3.7/αα, βCap+40-43 (-AAAC)/βN | 1 | 0.08 | 0.01 | |
| -α3.7/-- SEA, βCD41-42 (-TTCT)/βN | 1 | 0.08 | 0.01 | |
| -α4.2/αα, βCD41-42 (-TTCT)/βN | 2 | 0.16 | 0.02 | |
| -α4.2/αα, βCD17 (A>T)/βN | 1 | 0.08 | 0.11 | |
| -α4.2/αα/Hb Q Thailand, βIVS-II-806(G>C)/βN/Hb New York | 1 | 0.08 | 0.11 | |
| --SEA/αα, βIVS-II-654(C>T)/βN | 11 | 0.90 | 0.13 | |
| --SEA/αα, βCD41-42 (-TTCT)/βN | 12 | 0.98 | 0.04 | |
| --SEA/αα, β-28(A>G)/βN | 4 | 0.33 | 0.03 | |
| --SEA/αα, βCD27-28 (+C)/βN | 3 | 0.24 | 0.03 | |
| --SEA/αα, βCD17 (A>T)/βN | 3 | 0.24 | 0.03 | |
| --SEA/αα, βCap+40-43 (-AAAC)/βN | 1 | 0.08 | 0.01 | |
| --SEA/αα, βCD26 (GAG>AAG)/βN | 1 | 0.08 | 0.01 | |
| --SEA/-α3.7, βIVS-II-654(C>T)/βN | 1 | 0.08 | 0.01 | |
| --SEA/-α4.2, βCD41-42 (-TTCT)/βN | 1 | 0.08 | 0.01 | |
| ααCS/αα, βCD17 (A>T)/βN | 1 | 0.08 | 0.01 | |
| ααCS/αα, βIVS-II-654(C>T)/βN | 1 | 0.08 | 0.01 | |
| ααWS/αα, βIVS-II-654(C>T)/βN | 1 | 0.08 | 0.01 | |
| Subtotal | 66 | 5.44 | 0.63 | |
| Total | 1214 | 100.00 | 11.87 | |
- Note: N indicates no mutation. SEA, Southeast Asian deletion; THAI, Thailand deletion; WS, Hb Westmead. The bolded sections, “Subtotal” and “Total,” summarize key data on the distribution of β-thalassemia genotypes in the Fujian population. The “Subtotal” sections provide specific insights into the distribution of simple β-thalassemia and concurrent α- and β-thalassemia genotypes within the β-thalassemia population. These figures are crucial for understanding the prevalence of different β-thalassemia types and their detection rates in the studied sample. Meanwhile, the “Total” section offers a comprehensive overview of all β-thalassemia genotypes, giving us the overall incidence and detection rate of β-thalassemia in the entire population. These bolded summaries are essential for grasping the main findings and implications of the study.
Among the 66 cases of concurrent α- and β-thal, 95.45% of the genotypes consisted of common deletions of the α-globin gene (–SEA/αα, −α3.7/αα, −α4.2/αα) combined with a β-globin gene point mutation, and composite –SEA/αα and CD 41/42 (-TTCT)/N was the most common genotype (Table 1).
3.2. Rare Types of β-Thal and Their Hematological Parameters
A case with Hb 142 g/L, MCV 63.9 fL, MCH 22.1 pg, and an elevated HbA2 level of 3.8% was identified, suspecting the individual to be a carrier of β-thal. The routine test results appeared normal. Subsequent sequencing of the full-length β-globin gene revealed a double peak at nt192, G > A, which upon comparison was confirmed as the CAP +22 (G > A) mutation (Figures 1(a) and 1(b)). Three additional cases exhibiting elevated HbA2 or HbF levels underwent sequencing of the full-length β-globin gene, revealing a mutation in the intron 2 region, characterized as G > C. This mutation was subsequently confirmed by comparison as IVS-II-806 (G > C) (Figures 1(c) and 1(d)).
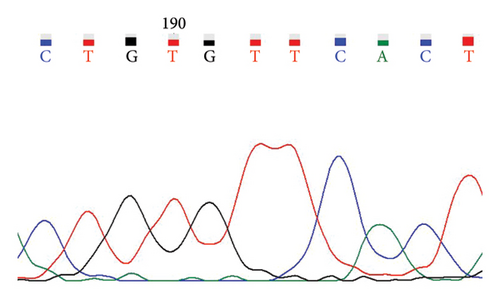
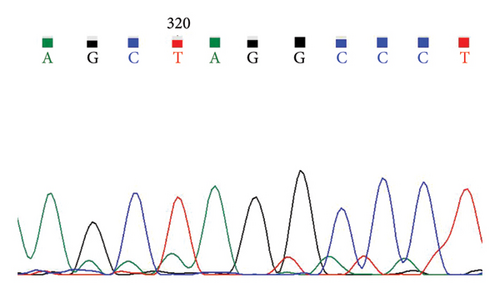
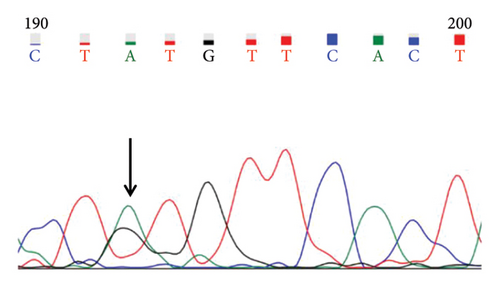
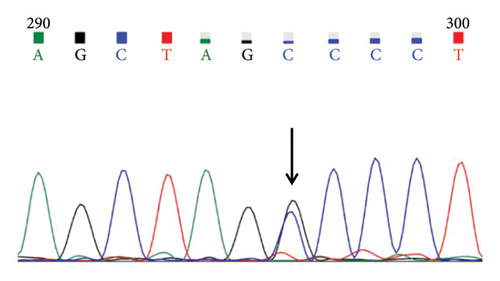
In this study, the hematological indices of cases with mutations in the cap locus of the β-globin gene and IVS-II-806 were further summarized. Subject with Cap +22 (G > A)/N exhibited hypochromia and microcytosis (Hb 142 g/L, MCV 63.9 fL, and MCH 22.1 pg) and elevated levels of HbA2 (3.8%) and subject with Cap +40-43 (-AAAC)/N showed normal or mild microcytosis with normal HbA2 level (Table 2). Two cases with IVS-II-806 (G > C)/N had normal or mild microcytosis with elevated HbA2 level (Hb 126 g/L, MCV 78.9 fL, MCH 27.7 pg, and HbA2 3.6%; and Hb 122 g/L, MCV 80.7 fL, MCH 29.0 pg, and HbA2 3.8%). Another subject was diagnosed with double heterozygous IVS-II-806 (G > C) and Hb New York, compound -α4.2/αα mutation, and Hb Q-Thailand and presented mild microcytic hypochromic anemia with decreased HbA2 level, elevated HbF level, and detectable level of Hb New York (Hb 121 g/L, MCV 73.8 fL, MCH 24.8 pg, HbA2 2.1%, HbF 17.6%, and Hb New York 28.2%) (Table 3).
| Genotype | Age | Gender | Hb (g/L) | MCV (fL) | MCH (pg) | HbA2 (%) | HbF (%) |
|---|---|---|---|---|---|---|---|
| βCap+22 (G>A)/βN | 28 | Male | 142 | 63.9 | 22.1 | 3.8 | 0.5 |
| βCap+40-43 (-AAAC)/βN | 31 | Female | 120 | 79.3 | 24.9 | 2.9 | / |
| βCap+40-43 (-AAAC)/βN | 29 | Male | 138 | 88.7 | 29.9 | 2.8 | / |
| Genotype | Age | Gender | Hb (g/L) | MCV (fL) | MCH (pg) | HbA2 (%) | HbF (%) |
|---|---|---|---|---|---|---|---|
| βIVS-II-806 (G>C)/βN | 27 | Female | 126 | 78.9 | 27.7 | 3.6 | 0.4 |
| βIVS-II-806 (G>C)/βN | 24 | Female | 122 | 80.7 | 29.0 | 3.8 | 0.0 |
| -α4.2/αα/Hb Q, βIVS-II-806(G>C)/βN/Hb New York | 33 | Female | 121 | 73.8 | 24.8 | 2.1 | 17.6 |
3.3. Hematological Parameters in β-Thal Carriers of Common Genotypes
In the cohort with β-thal, the CD 26 group had higher levels of Hb, MCV, and MCH compared to the β0 carrier group and the β+ carrier group (125.84 ± 12.29 g/L vs. 108.99 ± 14.37 g/L and 106.99 ± 17.08 g/L, 75.78 ± 4.18 fL vs. 63.40 ± 6.41 fL and 63.44 ± 7.86 fL, and 25.49 ± 1.60 pg vs. 20.54 ± 3.02 pg and 20.34 ± 3.0 pg) (p < 0.05). As expected, the β+ carrier group had higher levels of Hb (108.99 ± 14.37 g/L) compared to the β0 carrier group (Hb 106.99 ± 17.08 g/L) (p = 0.0029) (Table 4).
| Hb (g/L) | MCV (fL) | MCH (pg) | |
|---|---|---|---|
| βCD26(GAG>AAG)/βN | 125.84 ± 12.29 | 75.78 ± 4.18 | 25.49 ± 1.60 |
| β+/βN | 108.99 ± 14.37 | 63.40 ± 6.41 | 20.54 ± 3.02 |
| β0/βN | 106.99 ± 17.08 | 63.44 ± 7.86 | 20.34 ± 3.08 |
| p valuea | < 0.0001∗∗∗∗ | < 0.0001∗∗∗∗ | 0.0110∗ |
| p valueb | < 0.0001∗∗∗∗ | < 0.0001∗∗∗∗ | 0.0076∗∗ |
| p valuec | 0.0029∗∗ | 0.9976 | 0.9421 |
- aComparison between subjects with βCD26(GAG>AAG)/βN and those with β+/βN.
- bComparison between subjects with βCD26(GAG>AAG)/βN and those with β0/βN.
- cComparison between subjects with β+/βN and those with β0/βN.
- ∗p < 0.05.
- ∗∗p < 0.01.
- ∗∗∗∗p < 0.0001, Kruskal–Wallis test.
Further analysis was conducted on the impact of gender and age on the hematological parameters of patients with β-thal, categorizing individuals below 18 years old as immature and those aged 18 and above as adults. It was observed that adult male patients in the β+ group had statistically higher levels of Hb compared to adult female patients (131.10 ± 10.75 g/L vs. 102.96 ± 10.44 g/L). Similar results were shown in the β0 group (132.08 ± 8.50 g/L vs. 106.46 ± 11.44 g/L). Moreover, both in the β+ group and the β0 group, adult males had statistically higher levels of Hb, MCV, and MCH compared to immature males (131.10 ± 10.75 g/L vs. 104.09 ± 10.42 g/L, 63.64 ± 2.38 fL vs. 58.83 ± 4.62 fL, and 19.91 ± 0.85 pg vs. 19.27 ± 1.91 pg and 132.08 ± 8.50 g/L vs. 102.66 ± 16.00 g/L, 64.45 ± 4.27 fL vs. 58.22 ± 7.79 fL, and 20.29 ± 1.65 pg vs. 18.73 ± 3.11 pg) (p < 0.05). Moreover, both in the β+ group and the β0 group, adult females had higher MCV and MCH than the immature females (64.34 ± 3.88 fL vs. 60.72 ± 6.71 fL and 20.55 ± 1.70 pg vs. 19.67 ± 2.84 pg and 65.66 ± 5.25 fL vs. 60.04 ± 6.26 fL and 21.08 ± 1.63 pg vs. 19.26 ± 1.96 pg) (p < 0.05). However, there was no obvious difference between adult female patients and immature female patients in Hb levels both in the β+ group and the β0 group (102.96 ± 10.44 g/L vs. 103.27 ± 11.13 g/L and 106.46 ± 11.44 g/L vs. 104.11 ± 10.05 g/L) (p > 0.05) (Supporting Table 1).
The hematological parameters of different genotypes within the β+ and β0 groups were analyzed, focusing on those with case numbers exceeding three. Further comparisons were made between the data of subjects with the −28 (A > G)/N mutation and those with IVS-II-654 (C > T)/N in the β+ group, as well as between subjects with CD 17 (A > T)/N, CD 71-72 (+A)/N, CD 41-42 (-TTCT)/N, CD 27-28 (+C)/N, and CD 43 (G > T)/N in the β0 group. In the β+ carrier group, subjects with −28 (A > G)/N had statistically higher levels of Hb, MCV, and MCH compared with IVS-II-654 (C > T)/N (110.62 ± 13.13 g/L vs. 107.82 ± 14.31 g/L, 67.08 ± 3.90 fL vs. 62.13 ± 5.95 fL, and 21.61 ± 1.44 pg vs. 20.12 ± 3.05 pg) (p < 0.05), and in the β0 carrier group, subjects with CD 17 (A > T)/N, CD 71-72 (+A)/N, CD 41-42 (-TTCT)/N, CD 27-28 (+C)/N, CD 43 (G > T)/N, and initiation codon (ATG > AGG)/N had no statistically significant difference (p > 0.05) (Supporting Table 2).
4. Discussion
Thalassemia is a globally prevalent hereditary hemolytic disease and is highly prevalent in Southern China, such as Guangxi, Guangdong, Hainan, and Fujian [1]. According to the molecular epidemiological survey of thalassemia in Fujian Province in 2019, the prevalence of β-thal in Fujian was 1.87% [7]. However, there are few studies on the molecular spectrum of β-thal in Fujian Province based on a large sample, and this is the first large-scale comprehensive analysis of the correlation between age, gender, genotype, and hematological parameters in Fujian Province.
In this study, β-thal genes were detected in 1214 out of 10,350 individuals (11.73%) suspected of having thalassemia. Of these, 17 kinds of β-thal mutations were identified, of which the most common mutation types were IVS-II-654 (C > T) (37.56%), CD 41-42 (-TTCT) (30.72%), CD 17 (A > T) (9.64%), −28 (A > G) (7.00%), CD 27-28 (+C) (3.21%), and CD 26 (GAG > AAG) (3.05%) accounting for 91.18% of all mutation types, indicating a high genetic heterogeneity of β-thal in Fujian Province, which was similar to our previous studies [7]. The most common gene mutation types of β-thal in the Fujian Province were IVS-II-654 (C > T) and CD 41-42 (-TTCT), which were different from the reports from Guangxi [8], Guangdong [9], and Hainan [10], where the most common gene mutation was CD 41-42 (-TCTT). In Chongqing [11], the most common gene mutation was CD 17 (A > T), indicating that there was an obvious geographical variation in the gene mutation of β-thal.
Two rare mutations were also detected in this study: Cap +22 (G > A) and IVS-II-806 (G > C). The Cap +22 (G > A) mutation was first identified in Turkey [12] and reported for the first time in China in 2013 by Huang et al. [13]. The 5′UTR of the β-globin gene is about 50 bp from the Cap site to the translation initiation site, and Cap +40-43 (-AAAC), Cap +1 (A > C), +10 (-T), +20 (C > T), +22 (G > A), +33 (C > G), +45 (G > C), and other mutations have been reported [14]. These point mutations generally do not affect the coding sequence of β-globin but affect the transcription level of the β-globin gene [15]. Upon analyzing the hematological parameters of mutations Cap +22 (G > A) and Cap +40-43 (-AAAC), it was observed that heterozygous cases with Cap locus mutations exhibited mild or no significant symptoms of anemia. However, in contrast to cases with the Cap +40-43 (-AAAC) mutation, those with the Cap +22 (G > A) mutation displayed characteristics of hypochromia and microcytosis (MCV 63.9 fL and MCH 22.1 pg), along with an elevated level of HbA2 (3.8%). This suggests that the Cap +22 (G > A) mutation may have a certain inhibitory effect on the transcriptional activity of the β-globin gene, leading to a decrease in the synthesis of β-globin chains and thus affecting the morphology and function of red blood cells. Furthermore, while some studies consider mutations at the Cap site to be merely polymorphisms without significant genetic effects, our research results indicate an association between the Cap +22 (G > A) mutation and anemia phenotype. This finding emphasizes the importance of in-depth research on rare mutations to better understand their role in the pathology of β-thalassemia [16].
The IVS-II-806 (G > C) mutation was first reported in China by Chen et al. in 2020 [17]. The mutation has not been included in the HbVar database and its clinical significance is unknown. In this study, three cases of IVS-II-806 (G > C) mutation were identified, of which two were the only heterozygous mutations of IVS-II-806 (G > C), with hematological parameters such as Hb, MCV, and MCH within normal ranges and slightly elevated HbA2. This is not completely consistent with the three reported blood parameters in the literature, indicating that the phenotype expression of IVS-II-806 (G > C) mutation varies under different genetic backgrounds or environmental factors. This emphasizes the necessity of further studying the molecular mechanism and clinical significance of this mutation under different conditions [18]. The genotype of a double heterozygous individual carrying the IVS-II-806 (G > C) mutation along with Hb New York and compound -α4.2/αα mutation, as well as Hb Q-Thailand, was reported for the first time. This case presented with Hb 121 g/L, MCV 73.8 fL, MCH 24.8 pg, HbA2 2.1%, HbF 17.6%, and Hb New York 28.2%, indicative of mild microcytic hypochromic anemia. Moreover, this case had high levels of HbF, which may be due to the presence of the Hb Q-Thailand mutation, which co-migrates with HbF in capillary electrophoresis [19]. And as this case also carries the Hb-New York mutation, it had an abnormal Hb in zone 11 of capillary electrophoresis [20]. Therefore, compared with IVS-II-806 (G > C)/N cases, cases with IVS-II-806 (G > C) mutations combined with abnormal Hb mutations have lower HbA2 levels and higher HbF levels. These differences may have significant implications for the diagnosis and clinical management of β-thal, highlighting the necessity to account for genetic heterogeneity among individuals in clinical practice.
The clinical symptoms of patients with β-thal vary in severity, but most of them are characterized by chronic progressive hemolytic anemia with altered hematological phenotypes such as Hb, MCV, MCH, MCHC, and HbA2 concentrations [21]. Different mutation types of the β-globin gene have different effects on the function of the β-globin gene, β+-thal means that the mutation type leads to a reduction in β-globin chain synthesis, and if it leads to a complete failure of β-globin chain synthesis, it is called β0-thal. In the present study, the hematological phenotypes of β0-thal, β+-thal, and CD 26 were analyzed, showing that subjects with β0-thal had statistically lower levels of Hb and MCV than subjects with β+-thal (p < 0.05), while the subjects with CD 26 (GAG > AAG) mutation had higher levels of Hb, MCV, and MCH compared to the β0 carriers and the β+ carriers, which is consistent with previous studies [22]. CD 26 (GAG > AAG) refers to the mutation of glutamate to lysine at position 26 of the β-globin peptide chain, which reduces the amount of β-globin gene synthesis but synthesizes HbE that is still functional with abnormal structure and reduces the degree of anemia in patients [23]. Therefore, patients with mutation type 26 (GAG > AAG)/N, who have a clinical phenotype of β+-thal, have little change in their hematological phenotype and very mild or even no significant symptoms of anemia. However, patients who co-inherited severe β+ or β0-thal alleles might be more severely affected [24].
This study evaluated the effect of age, gender, and genotype on phenotype in β-thal and found that in the β+ carrier group, subjects with −28 (A > G)/N had statistically higher levels of Hb, MCV, and MCH compared with IVS-II-654 (C > T)/N, and in the β0 carrier group, subjects with CD 17 (A > T)/N, CD 71-72 (+A)/N, CD 41-42 (-TTCT)/N, CD 27-28 (+C)/N, and CD 43 (G > T)/N had no statistically significant difference (p > 0.05). Interestingly, our study revealed that adult male patients with β-thal had significantly higher Hb levels than adult female patients, a phenomenon observed in both the β+ and β0 groups. This discrepancy might be attributed to gender-related physiological differences, such as the significant increase in testosterone levels during male puberty (p < 0.05), which promoted the development of muscles and bones, as well as the enhancement of cardiopulmonary function, leading to generally higher Hb levels in males compared to females [25].
In this study, MCV and MCH levels in adult β-thal individuals were found to be significantly higher than those in immature individuals (p < 0.05), indicating a maturation effect on hematological parameters with age, consistent with trends seen in healthy populations [26, 27]. However, the difference in Hb levels between adult and immature females was not statistically significant (p > 0.05), which was inconsistent with results from healthy populations [28]. This might be due to the significant participation of pregnant individuals in this study and during pregnancy, the increase in blood volume led to the dilution of Hb, resulting in lower Hb levels [29, 30]. These findings suggest that clinicians should consider gender and age factors when assessing hematological indicators in β-thal patients to diagnose and monitor the disease state more accurately. The results of the study underscore the importance of establishing customized reference ranges for β-thal patients, especially for children and immature patients, to prevent underdiagnosis and misdiagnosis.
While this study presents a comprehensive molecular epidemiological investigation and hematological analysis within a substantial sample population from Fujian Province, several limitations must be acknowledged. Firstly, the generalizability of our findings to the broader Chinese population or other regions may be constrained by the geographic specificity of the sample cohort. Secondly, our research, which concentrated on molecular and hematological parameters, did not account for potential influencing factors such as environmental aspects and lifestyle habits. Additionally, although we considered variables like gender, age, and genotype, there may be unmeasured confounding factors, including the patients’ overall health status and lifestyle, that could influence hematological parameters. Nonetheless, our investigation offers novel insights by elucidating the complex interplay among age, gender, genotype, and hematological parameters within the β-thal phenotype in Fujian Province. The innovative aspect of our study is its detailed analysis of a large dataset, yielding region-specific understanding of β-thal’s molecular epidemiology. We are confident that our findings will enhance diagnostic and therapeutic strategies for β-thal in Fujian and may provide valuable insights for other regions.
5. Conclusion
5.1. Comprehensive Molecular Epidemiological Investigation and Hematological Analysis
This study represents the first comprehensive molecular epidemiological investigation and hematological analysis of β-thal in Fujian Province, a significant contribution toward optimizing thalassemia prevention and control strategies.
5.2. Novel Findings in Hematological Parameters and Genotype
Notably, this study reports for the first time the hematological parameters and genotype in a patient with compound heterozygosity of Hb New York and IVS-II-806 (G > C) mutation with −α4.2/αα and Hb Q-Thailand. This finding offers valuable insights for prenatal genetic counseling.
This study also reveals that gender and age are the key factors affecting the hematological parameters of β-thal patients and emphasizes that the individualized differences of gender and age shall be considered in the clinical diagnosis and treatment of β-thal.
Ethics Statement
This study was approved by the Ethics Review Committee of Fujian Maternity and Child Health Hospital (approval no. 073, 2019). Written informed consent to participate was obtained from all of the parents of the participants in the study, and the study was conducted in accordance with the Declaration of Helsinki.
Consent
Please see the Ethics Statement.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Junhao Zheng, Meihuan Chen, and Siwen Zhang conceived and designed the study, coordinated the research activities, and contributed to drafting the manuscript. Aixiang Lv and Min Zhang were responsible for collecting samples, conducting experiments, and analyzing the results. Na Li and Lingji Chen interpreted the data and were primarily involved in writing the manuscript. Liangpu Xu and Hailong Huang contributed to the conception and design of the research and played a significant role in revising and finalizing the draft. All authors have read and approved the final manuscript for publication. Junhao Zheng, Meihuan Chen, and Siwen Zhang contributed equally to this work.
Funding
This work was funded by the Major Scientific Research Program for Young and Middle-Aged Health Professionals of Fujian Province, China (no. 2023ZQNZD009), National Natural Science Foundation of China (no. 81970170), Joint Funds for the Innovation of Science and Technology, Fujian Province (nos. 2021Y9173, 2021Y9174, and 2023Y9364), Fujian Provincial Natural Science Foundation of China (no. 2023J011217), Innovation Platform Project of Science and Technology, Fujian Province (no. 2021Y2012), and National Key Clinical Specialty Construction Program of China (Obstetric).
Acknowledgments
We also acknowledge the use of Kimi for language editing and manuscript improvement. Kimi significantly enhanced the clarity and readability of the manuscript through its language polishing capabilities. Kimi is an AI assistant developed by Moonshot AI, which provides language editing and text improvement services (available at: https://kimi.moonshot.cn (accessed Nov 10, 2023)).
Supporting Information
Supporting Table 1: the table shows hematological parameters of β-thal in different genders. Supporting Table 2: the table shows hematological parameters in β-thal carrier of common genotype.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.




