Emerging Material Design Trends in Photothermal Water Vapor Generation
Abstract
Photothermal steam generators hold significant potential as sustainable and green technologies for applications such as water desalination, wastewater treatment, sterilization, and photothermal electric generation by efficiently harnessing solar energy to produce steam. However, their performance heavily depends on the precise design and optimization of advanced materials, which present notable challenges. Essential material properties include high solar light absorption, facilitated by enhanced crystallinity and nanostructured designs, low thermal conductivity achieved through defect engineering, and vacancy creation. Additionally, optimal wettability, supported by surface roughness and hydrophilicity, ensures efficient water transport to the evaporation interface. Salt resistivity, crucial for preventing performance degradation, is managed by controlling thermal gradients and employing strategies such as vertically aligned pores and a leaning structure for salt management. Floatability, achieved through reduced density and increased porosity, is also essential for maintaining high system efficiency. Integrating these diverse properties into a single material system requires a delicate balance of optical, thermal, and mechanical characteristics, along with long–term durability. This review outlines the key material attributes driving the performance of photothermal steam generators, explores recent advances in material design, and proposes strategies to overcome existing challenges. Furthermore, it assesses the technology readiness levels (TRLs) of these systems, identifying areas for future development to improve their practical feasibility.
1. Introduction
Photothermal steam generation has emerged as a promising method for harnessing solar energy by converting incident photons captured by solar absorbers into heat [1–3]. This sustainable approach involves the design and development of photothermal materials that efficiently convert sunlight into thermal energy, which drives the evaporation process in applications such as seawater desalination, water purification, photothermal electricity generation, wastewater treatment, and sterilization (Figure 1) [4, 5]. The core principle of this technology is that photothermal materials absorb solar radiation, convert it into thermal energy, and transfer this heat to water, facilitating its evaporation. This localization of heat raises the temperature at the liquid–air interface, leading to rapid evaporation [6, 7]. During the solar steam generation process, bulk water is transported to the evaporation surface through capillary action. The resulting steam can then be condensed to produce freshwater, while salts and contaminants are left behind [8, 9].
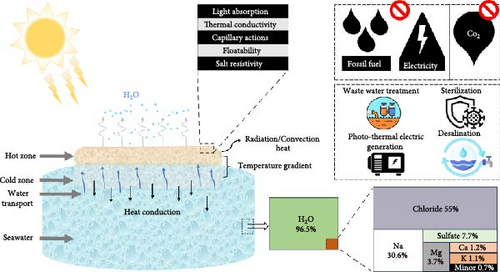
This method offers several advantages over traditional desalination technologies, such as RO and multistage flash distillation [10–12]. Solar-driven photothermal systems operate without the need for external electricity, making them suitable for remote areas. Additionally, they have the potential to reduce operational costs and have positive environmental impacts like the purification of contaminated water without chemical additives, reducing reliance on fossil fuels, biodegradability, and minimal carbon footprint [13–15]. However, conventional solar evaporation methods often suffer from inadequate solar light absorption and significant heat losses, resulting in photothermal efficiencies, frequently referred to as photothermal conversion efficiencies, which is the ratio of the energy utilized for water evaporation to the total solar energy incident on the material, ranging from 30% to 45% [16]. This limitation affects their ability to produce substantial amounts of steam (presently: 10–215.8 L m–2 day–1) [17, 18].
The development of novel materials is crucial for enhancing the efficiency of photothermal steam generators. These materials must be designed to maximize light absorption, achieved through techniques such as crystal engineering or band gap modulation, and to minimize heat loss by reducing thermal conductivity via defect engineering or the use of thermally insulating materials (Figure 1). Additionally, salt accumulation must be mitigated through strategies like the incorporation of the Donnan effect or the use of vertically aligned pore channels. Other critical properties include floatability, which can be achieved through highly porous nanostructures, and optimal wettability, which can be enhanced by surface roughness and the incorporation of hydrophilic functional groups such as hydroxyl, carboxyl, and amino groups [19–23]. Materials must exhibit strong light absorption of almost 100%, particularly across the solar spectrum (250–2500 nm) [24]. Numerous studies have aimed to address these factors by focusing on intense solar absorption by incorporating multi–scattering inside the porous structure and energy conversion efficiency near theoretical limits (100%) with high stability [25, 26]. Key considerations for achieving optimal system efficiency include localizing heat at the surface, minimizing heat loss to bulk water, ensuring a smooth supply of water via capillary action, and maintaining performance integrity to resist salt accumulation [13]. Salt accumulation on photothermal materials during steam generation can obstruct nanopores, hindering efficient water transport, degrading both the material and its performance, and reducing light absorption by blocking nanostructured pathways. This, in turn, lowers evaporation efficiency and necessitates frequent maintenance for cleaning [27, 28]. To maintain consistent steam generation, photothermal materials must exhibit excellent water–wicking properties to prevent the drying out of the active surface. Failure to do so can significantly reduce the overall efficiency of the evaporation process, leading to lower water production [29, 30]. In addition to high performance, photothermal materials must be resistant to corrosion and fouling to ensure long–term durability and minimize the need for frequent maintenance or replacement. The economic viability of photothermal steam generation systems is closely tied to the scalability of these materials, an area still under development with ongoing challenges and notable progress [31]. These materials should be cost–effective, abundant, and easy to fabricate on a large scale, particularly to enable widespread deployment in resource–constrained regions. Recent advances include the development of a 1 m2 steam generator prototype, produced at a cost of approximately $27.30 using 3D printing technology, achieving an evaporation rate of 2.67 kg m–2 h–1 [32]. In contrast, the CBCP–F photothermal conversion material, reported by the Liang group, was fabricated at a cost of $0.91, with an evaporation rate of 1.25 kg m–2 h–1 [33]. Current research efforts are focused on improving the durability, cost–effectiveness, and overall efficiency of these systems to enable large–scale deployment [34].
Recent advancements in photothermal material design have introduced semiconductors with low bandgaps, such as TiO2, Fe2O4, and black titania, alongside metallic nanoparticles like Ag, Au, and Ru, which exhibit excellent light absorption properties [9, 35–39]. Commonly employed photothermal conversion materials include plasmonic metals (e.g., Au, Ag, Al, and Pd), plasmonic ceramics (e.g., TiN, Ti3C2), semiconductors (e.g., Cu2–xS, Cu2–xSe, Cu2–xTe, TiO2, Ti2O3, MoO3, Fe3O4, WO3–x, Fe1–xS2, ZnO, and CdO), carbon–based materials (e.g., exfoliated graphite, CNT, GO, rGO, carbon dots, carbon aerogels, porous carbon, fullerene), organic polymer nanomaterials (e.g., polythiophene, polypyrrole, polyaniline, and polydopamine), and MXenes [40–44]. Combining these photothermal materials with supporting structures such as paper, wood, foam, membranes, gels, or cellulose enhances solar absorption, improves light scattering, and boosts floatability while ensuring adequate water supply [41]. However, photothermal materials are prone to degradation when exposed to prolonged solar radiation, ultraviolet (UV) light, and harsh environmental conditions. UV radiation can lead to the breakdown of chemical bonds, particularly in organic or polymer–based materials, causing photo–oxidation [45]. Thermal fatigue, caused by daily cycles of sunlight and nighttime cooling, may induce microcrack formation within nanostructures, while chemical degradation, exacerbated by salt, further diminishes material performance [45–47]. Additionally, the design of steam generation systems must include efficient vapor collection and condensation mechanisms. Minimizing heat loss during evaporation and condensation, sloping the condensation surface towards a water reservoir for optimal collection, and ensuring continuous vapor flow to the condensing zone is critical for maximizing freshwater output.
This review explores the fundamental design principles of photothermal steam generation to achieve optimal performance, with a focus on material design requirements, recent advancements, and key modifications. It identifies existing research gaps and outlines future directions for advancing fundamental studies in photothermal steam generation systems. Essential factors influencing stable and efficient steam generation—such as crystal engineering, band gap modulation, vacancy creation, and optimized structural design—are examined in detail. The review also provides insights into strategies for reducing water vaporization enthalpy within the porous structures of photothermal materials. Photothermal steam generators harness interdisciplinary principles from material science, physics, and chemical engineering to enhance solar–driven water evaporation. In material science, the development of advanced photothermal materials—including semiconductors, plasmonic nanostructures, carbon–based absorbers, organic materials, and metal–organic frameworks (MOFs)—is crucial for optimizing light absorption and heat localization. Physics governs light–matter interactions, thermal transport, and fluid dynamics, which collectively influence energy conversion efficiency and evaporation rates. Chemical engineering further refines the process by enhancing water transport, controlling surface wettability, reducing evaporation enthalpy, and facilitating salt resistivity. Key physicochemical phenomena, such as the Wenzel model, the Marangoni effect, and the Donnan effect, play a pivotal role in ensuring system stability and long–term operational efficiency.
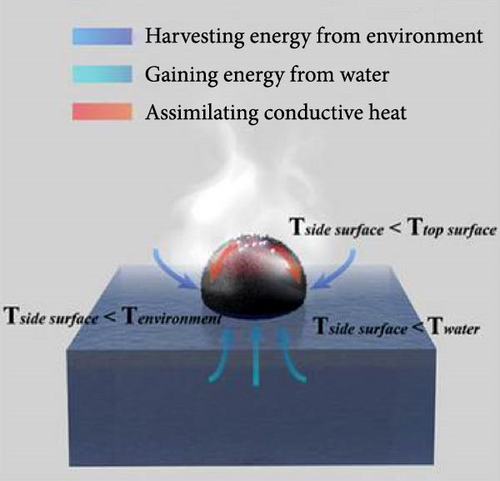
2. Fundamentals of the Photothermal Mechanism
Photothermal materials harness solar light to convert it into heat. This solar thermal energy is then used to drive the evaporation of water (the latent heat of vaporization ~2.25 MJ kg–1 at 0.1 MPa), which can be leveraged for energy savings and various industrial applications [48–52]. A photothermal absorber captures incident photons to generate heat, which in turn drives the evaporation of water. The heat generated is partly transferred with the evaporating water (termed evaporative cooling) and partly dissipated to the surroundings (Figure 2). The continuous heat transport in this process can be fundamentally categorized into three types of transpiration. A localized high–temperature surface dissipates heat to the surrounding environment through convection and radiation, while in bulk water, heat is transported primarily by conduction. It is also crucial to consider heat loss from the photothermal material through the surface and side walls, as these losses can significantly impact the overall evaporation rate [53, 54].

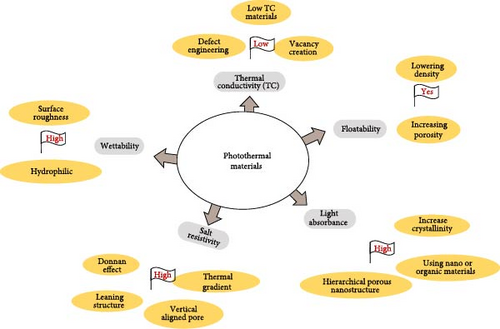
The design of efficient photothermal materials requires careful consideration of several critical factors. Key properties such as light absorbance, floatability, thermal conductivity, wettability, and salt resistivity must be optimized to develop materials with a comprehensive set of features that enhance efficiency, performance, stability, ease of fabrication, and cost–effectiveness (Figure 3) (Table 1). The subsequent sections will discuss the detailed requirements for these properties, their improvements, and related factors.
| Category | Semiconductor | Organic | Plasmonic | Carbon–based |
|---|---|---|---|---|
| Mechanism | Absorb photons and convert to heat via nonradiative recombination | Molecular vibrations generate heat upon light absorption | LSPR enhances light absorption and heat generation | Broad spectrum absorption with excellent light–to–heat conversion via multi–scattering |
| Solar–thermal conversion efficiency | ~96.00% | ~98.00% | ~98.00% (tunable to higher with appropriate substrate) | ~178.60% |
| Light absorption range | Selective (bandgap dependent, UV–visible range) | Narrow (specific absorption wavelengths) | Tunable (adjustable plasmon resonance in visible and NIR) | Broadband (absorbs UV, visible, and NIR) |
| Heat localization | Moderate (depends on material composition) | Poor (heat dissipation to surroundings) | High (due to strong light–matter interactions) | High (localized heating due to high surface area) |
| Cost (USD per kg) | 10–50 (e.g., TiO2, MoS2) | 5–30 (organic dyes, polymers) | 500 or more (Au, Ag, Noble metals) | 5–20 (graphene, CNTs, porous carbon) |
| Evaporation rate | ~4.50 Kg m–2 h–1 | ~18.88 Kg m–2 h–1 | ~29.10 Kg m–2 h–1 | ~7.60 Kg m–2 h–1 |
| Durability (time before degradation) | Over 2000 h | ~50 h | ~120 h | ~ 96 h |
| Fabrication complexity | Medium (synthesis and doping required) | Low (easy to synthesize and process) | High (precise synthesis of nanostructures needed) | Low to medium (scalable carbon processing) |
| Scalability | Moderate (depends on material synthesis and processing) | High (organic materials are easy to synthesize in bulk) | Low to moderate (noble metals are expensive and challenging for large–scale synthesis) | High (carbon materials can be produced in large quantities) |
| Environmental impact | Moderate (some toxic precursors used) | Low (biodegradable options available) | Moderate to high (noble metals can have toxic effects but recycling is possible) | Low (carbon materials are eco–friendly) |
| Fabricated weight ∗ | 3–5 g cm–3 | 1–2 g cm–3 | 8–19 g cm–3 | 0.5–2 g cm–3 |
| Stability in harsh environments | Moderate (can degrade under prolonged UV exposure) | Low to moderate (prone to degradation in extreme conditions) | High (resistant to extreme conditions but may oxidize) | High (chemically and thermally stable) |
| Hydrophilicity | Moderate (can be engineered for hydrophilic surfaces) | High (organic molecules can enhance hydrophilicity) | Low (metals are usually hydrophobic) | High (carbon–based materials like graphene oxide have high wettability |
| Biocompatibility | Moderate (some semiconductors are biocompatible) | High (commonly used in medical applications) | Low (noble metals may be cytotoxic in high doses) | High (graphene oxide and CNTs have biomedical applications) |
| Commercial availability | Moderate (MoS2, TiO2 widely available) | High (organic dyes and polymers are mass–produced) | Low to moderate (platinum–group metals are scarce and expensive) | High (carbon materials are abundant) |
- ∗Based on their study and expertise, the author estimates the weight of photothermal materials per cubic centimeter.
2.1. Solar Light Absorption and Conversion Mechanism to Heat
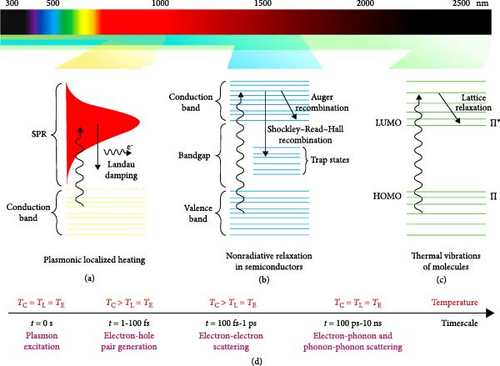
Photothermal materials harvest solar energy and convert it into heat through several key mechanisms, including photon absorption, thermal vibrations, electron–hole generation and relaxation, and plasmonic heating [76]. When photothermal materials absorb photons from sunlight, the excitation of electrons occurs, causing them to elevate to higher energy states. This absorbed energy induces aggressive molecular vibrations, generating vibrational heat. Additionally, the excited electrons return to their original energy states, releasing heat in a process known as electron–hole recombination [40, 77]. Metal–based nanoparticles absorb light, leading to a collective oscillation of electrons, which generates significant heat, known as the plasmonic effect. Both organic materials (e.g., polymers, dyes, macromolecules) and inorganic materials (e.g., semiconductors, carbon, metals) utilize different mechanisms to convert solar energy into thermal energy (Figure 4) [78–81]. The following sections provide a detailed discussion of these mechanisms.
2.1.1. Semiconductors: Non–Radiative Relaxation
When a semiconductor material is excited by photon energy, electron–hole pairs are generated if the photon energy is sufficient enough to separate as required by the bandgap [82]. Upon excitation, two main phenomena can occur: (i) the excited electron releases energy in the form of an emitted photon or (ii) the excited electron transfers its energy to the material’s lattice through nonradiative relaxation [83]. Nonradiative relaxation can occur via processes such as the Shockley–Read–Hall (SRH) mechanism or Auger recombination [84].
In semiconductors, the presence of defects or impurities can create mid–gap electronic energy states, known as trap levels or defect states. An excited electron from the conduction band may transfer to these defect states and subsequently relax to the valence band, where it recombines with a hole. This SRH process results in the exchange of thermal energy with the material. If the defect states are near the surface, the process is termed trap–assisted surface recombination and depends on the defect density [85].
In Auger recombination, three carriers are involved: an electron and a hole recombine, releasing energy that is transferred to a third carrier—either an electron in a higher conduction band state or a hole deeper in the valence band [86]. This third carrier then thermalizes back to the band edge through lattice vibrations. The likelihood of Auger recombination increases as the bandgap decreases [87].
2.1.2. Organic Molecules Thermal Vibration
Organic or polymer–based materials exhibit photothermal effects through light absorption and subsequent heat generation, primarily involving lattice vibrations [88]. In organic polymers, bonds such as C─C, C─O, and C─H have high excitation energy and are hard to excite due to the significant energy difference between the σ and σ ∗ orbitals. However, electrons in loosely bonded π orbitals can be excited under low–energy irradiation, transitioning from π (highest–occupied molecular orbital, HOMO) to π ∗ (lowest–unoccupied molecular orbital, LUMO) orbitals. The relaxation of these excited electrons occurs through vibrational–electron coupling back to the ground state, releasing excess energy as heat [89, 90]. As the number of π bonds increases, the energy gap between the HOMO and LUMO decreases. So, conjugation, or hyperconjugation can modify electron transitions between the HOMO and LUMO, affecting the photothermal efficiency.
2.1.3. Light–to–Heat Conversion in Plasmonic Materials
Noble metals such as Ag, Au, Pd, and Pt, along with base metals like Al and Ni, exhibit localized surface plasmon resonance (LSPR) properties when exposed to light irradiation. Plasmonic nanostructures with metallic subwavelength–sized dimensions can confine incident light to the nanoscale, significantly enhancing light–matter interactions [91, 92]. LSPR arises from the collective oscillation of conduction band electrons within the nanostructure, with the quanta of these oscillations termed plasmons, analogous to photons for light waves and phonons for sound waves. These excited plasmons generate strong electric fields at the surface and, critically, provide large absorption and scattering cross–sections at the resonance frequency. The combined effects of these plasmonic nanostructures make them highly effective for light harvesting and energy concentration, leading to efficient light–to–heat conversion. The efficiency of plasmonic effects depends on several factors, including particle size, shape, dielectric environment, and material composition [93]. Electrons excited to higher energy states, known as hot electrons, have short lifespans and release their gained energy through electron–electron, and electron–phonon interactions. This energy is subsequently dissipated into the surrounding environment as thermal energy through phonon–phonon relaxation.
2.1.4. Micro/Nano–Structured Materials Multiple Interactions
Micro– and nanostructured photothermal materials demonstrate exceptional light absorption capabilities due to their unique size–dependent optical properties and enhanced multi–scattering phenomena [93]. The intricate arrangement of micro/nano structures within these materials creates a complex light propagation environment, where light undergoes multiple scattering events, including reflection, refraction, and diffusion by hierarchical porous features, effectively functioning as a black body [94]. This repeated scattering significantly extends the optical path length, thereby increasing the interaction of light with the material and enhancing the absorption of incident photons, which are subsequently converted into heat. Micro/nanostructured carbon–based materials, black silicon, black GaAs, MXenes, and metals exhibit superior light–absorbing performance compared to their regular structured counterparts [95]. By optimizing the geometry and spacing of these structures, researchers can fine–tune the multi–scattering effects to maximize the performance of photothermal materials, thereby enhancing their efficiency for specific technological applications.
2.2. Strategic Approaches to Heat Localization
Efficient heat management in photothermal systems requires localized heat at the liquid–air interface to maximize evaporation efficiency. The solar–to–heat conversion efficiency in photothermal heat generation is determined by both solar absorption and interfacial heat localization, which are critical factors. Heat localization on the evaporative surface can be achieved using materials with low thermal conductivity, such as carbon foam, aerogels, graphene oxides (GOs), cellulose, and wood. The thermal conductivity of photothermal materials should be lower than that of water (0.599 W m–1 K–1) in both dry and wet conditions. Various strategies have been employed to achieve efficient heat localization on photothermal surfaces for effective light–to–heat conversion.
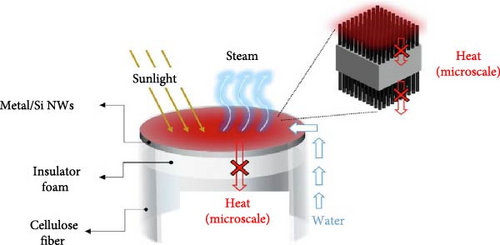
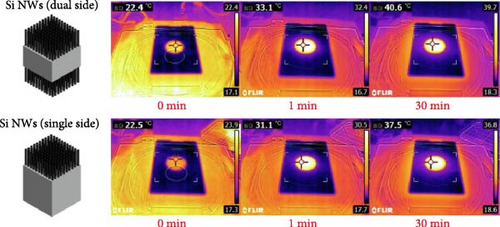
Kang group designed a solar steam generator using plasmonic Si nanowires, which act as a broadband light absorber (300–2500 nm), along with a water transport layer (microporous cellulose fiber) utilizing capillary action, and a macroscale thermal insulator (expanded polystyrene foam) (Figure 5a). The combination of low thermal conductivity Si nanowires and expanded polystyrene foam between the absorber and bulk water reduces heat conduction losses to the surroundings, promoting heat localization (40.6°C at 30 min) at the liquid–air interface (Figure 5b) [96].
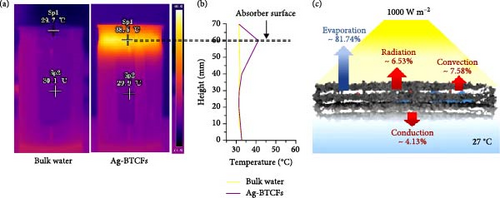
Chen et al. developed a material structure to localize heat at the evaporation surface, minimizing heat losses from solar energy. The design consists of a double–layer structure (DLS) with a 10 mm thick carbon foam layer that is thermally insulating, hydrophilic, and porous, paired with a 5 mm thick graphite layer that is also hydrophilic, porous, and solar light absorbing. The hydrophilic nature of both layers supports water transport to the surface through capillary action. The DLS exhibits thermal conductivities of 0.117 W m–1 K–1 in dry conditions and 0.426 W m–1 K–1 in wet conditions, both lower than that of water [97]. The Fu group developed self–floating plasmonic Ag/black TiO2/carbon porous layered foams (Ag–BTCFs) for photothermal evaporation systems. The design utilizes a 3D cross-linked porous carbon foam as an efficient sunlight–harvesting layer and an insulating layer that minimizes heat transfer or loss to the sublayer (Figure 6) [98]. A robust heat–blocking layer is integrated to concentrate heat at the evaporation interface. Temperature measurements indicate that the evaporation interface of Ag–BTCFs reaches 42.1°C, while the temperature below the evaporation interface is 32.3°C. Further details on thermal conductivity are discussed in Section 3.1.
2.3. Water Flow Dynamics in the Hot Zone
The created hot zone should be continuously supplied with water, as the evaporation rate depends on the appropriate water flow. Water is drawn from the bulk zone to the hot zone through porous channels, where it subsequently evaporates at the liquid–air interface. To maintain a consistent and uninterrupted water flow, it is crucial to have uniform and suitably structured porous channels that are free from blockages to ensure continuous water supply. Li et al. [99] used computational fluid dynamics (CFD) to model water dynamics over their photothermal materials, comparing scenarios with and without interconnected pore channels (Figure 7). Their simulations revealed that vortex regions form due to the collision of water molecules with the walls of interconnected pore channels. In the absence of such channels, water flow is laminar, exhibiting distinct gradient shifts due to variations in cross–sectional area. Singh et al. [100] produced aluminum (Al) surface layers through femtosecond–laser treatment, which demonstrated excellent water–wicking properties due to Al’s intrinsic hydrophilicity and the super water–absorbing (SWSA) properties of the treated surface. This process and the resulting surface modifications maintain limited contact between the solar absorber and water, minimizing water loss from the bulk and maximizing heat localization at the SWSA surface. In another study, Cao’s group demonstrated an evaporation rate of 14.09 kg m–2 h–1 in brine water under 1–sun irradiation using a 1D multichannel photothermal rod (MCPR) with a height of 10 cm [101]. A higher evaporation rate was observed with increased height and adjusted incident angles. The research utilized hydrogenated TiO2 (H–TiO2) modified carbonized rattan plants as photothermal materials, taking advantage of the natural vertical and horizontal microchannels for efficient water transport, rather than commonly used photothermal materials. The experimental results showed faster water transport by MCPR compared to widely used solar–driven evaporation materials like polyurethane sponge, carbonized balsa wood, and carbonized wicker, presenting promising findings consistent with CFD analysis regarding the water–wicking properties of highly curved channels, lowly curved channels, and vertical channels. The study highlighted the excellent water transport capacity of the MCPR, as the vertical channels decrease water resistance.
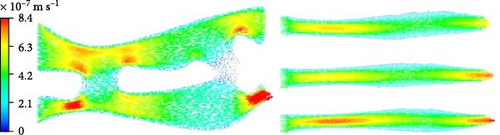
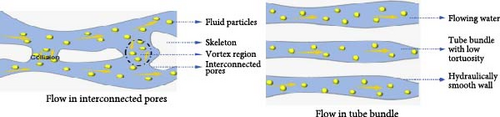
The findings emphasize the importance of structured porous channels in optimizing water transport for efficient solar-driven evaporation. Computational and experimental analyses confirm that vertical and without interconnected microchannels enhance water–wicking properties, minimize losses, and improve heat localization.
2.4. Water Evaporation From the Hot Zone
Here η, , hLV, and Qin represents photothermal efficiency, evaporation rate (kg m–2 h–1), total enthalpy of latent heat (J kg–1), and energy of incoming sunlight (W m–2). The evaporation rate can be maximized by controlling the efficiency by augmenting the total enthalpy of latent heat. Recent studies emphasize that decreasing the latent heat of vaporization can greatly improve performance by utilizing the unique behaviors of free, intermediate, and bound water, where intermediate water vaporizes with lowered latent heat within the porous structure of photothermal materials [103].
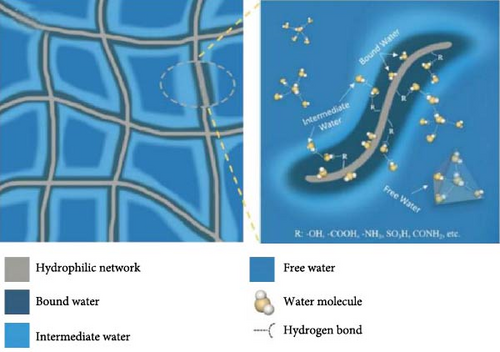
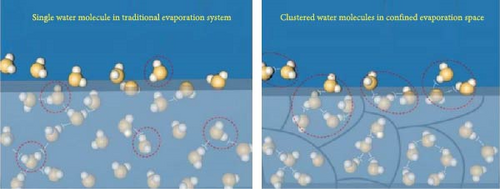
The techniques utilized for water vaporization in the hot zone of photothermal materials differ markedly from traditional vaporization methods. Within hierarchical photothermal materials, such as hydrogels, water exists in three distinct states: free water, intermediate water, and bound water [104]. Free water closely resembles bulk water, characterized by minimal interactions among functional groups. In contrast, bound water exhibits strong interactions with various functional groups, while intermediate water occupies a transitional position, displaying weak interactions, such as hydrogen bonds, among polymeric chains (see Figure 8a, b). By manipulating the levels of intermediate and bound water, it is possible to reduce the enthalpy of water vaporization [104, 106]. The temperature within the hot zone of photothermal materials typically ranges from 40 to 65°C. In conventional scenarios, the vaporization enthalpy of pure water at 40°C is ~2406.17 kJ kg–1 [107]. However, the presence of a polymeric water network within the hierarchical porous structure of photothermal materials leads to a decreased latent heat of vaporization as water vaporizes in cluster form due to intermediate and bound water.
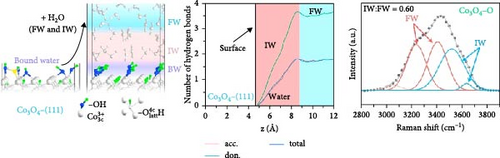
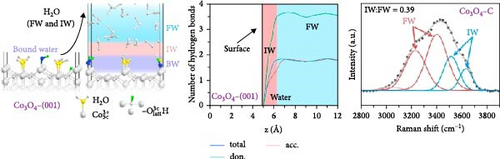
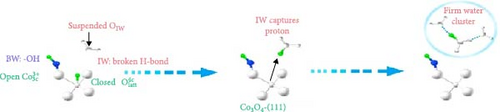
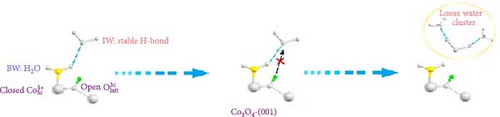
Based on this advancement, Wang et al. [108] fabricated bilayer–structured solar evaporator (p–SDWE) with porous polydopamine (p–PDA) as the top layer and thermo–responsive sporopollenin–engineered (PN10–g–SEC) as the bottom layer on Ni foam (NiF). The study demonstrated an evaporation rate of 3.58 kg m–2 h–1 with 93.9% efficiency under 1–sun irradiation due to the steady supply of thin water layer and reduced evaporation enthalpy to 1080 Jg–1. The research achieved this evaporation enthalpy by forming increased intermediate water above the lower critical solution temperature, breaking bound water as sporopollenin provided a thermo–responsive behavior. Moreover, this action transformed wettability characteristics, switching from 2° to 20°C to 153° to 35°C, demonstrating the potential for effective thin–layer water management, thermal management, and salt resistance. Linhui and co–workers employed crystal plane engineering in their synthesized material Co3O4 and demonstrated three different structures: nanocube Co3O4–C, truncated octahedra Co3O4–T, and regular octahedra Co3O4–O with exposed crystal planes of (001), (001) + (111), and (111) respectively [105]. The steam generator’s performance was evaluated by loading this different crystal–structured Co3O4 onto hydrophilic polytetrafluoroethylene (PTFE). Here, Co3O4 also provided a good wettability, exhibiting a contact angle of 50° to 0° within 10 s. The effect of crystal engineering increased the intermediate water. It promoted the transfer of protons that formed firm water clusters bonding with broken hydrogen bonds in the intermediate water layer (see Figure 8c–f). Though the light absorption was almost identical for every sample, the effect of different water states in Co3O4–O exhibited higher evaporation of 2.23 kg m–2 h–1 compared to Co3O4–C (1.71 kg m–2 h–1), and Co3O4–T (1.92 kg m–2 h–1) respectively with a reduction in the evaporation enthalpy of Co3O4–O (1806.9 J g–1) remarkably lower than Co3O4–C (2194.2 J g–1) and Co3O4–T (2047.9 J g–1).
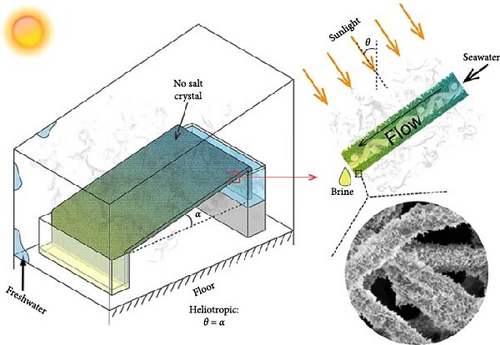
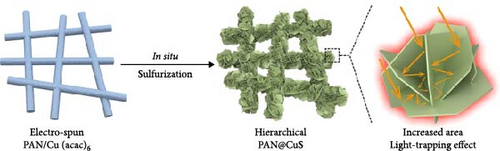
In another study, the Chen group developed a PAN@CuS photothermal fabric aimed at heliotropic steam generation, achieving an evaporation enthalpy of 1956.32 kJ kg–1 (Figure 9) [109]. Their findings indicated that increasing the CuS content further reduced the latent heat of evaporation, while their heliotropic model effectively mitigated salt accumulation. Following the sulfurization of PAN@CuS fabrics, an increase in surface area was observed due to the formation of CuS nanosheets. This enhanced surface area facilitated greater light absorption within the structure, resulting in the formation of a higher number of H2O–S polymeric chains, which contributed to the reduction of latent heat of vaporization. The study of Yang’s group of hydrogel–based solar steam generators was synthesized by optimizing Ti3C2Tx MXene and 18 mg rGO (MRH-3). Here, they observed a rapid drop in water vaporization enthalpy, which is 905 kJ kg–1 [110]. This reduction of vaporization enthalpy is attributed to the weakening interactions of hydrogen bonds between water molecules as the high presence of hydrophilic groups in the hybrid hydrogel. By augmenting the supply of bound and intermediate water to the photothermal hot zone while minimizing the latent heat of vaporization, the efficiency of the photothermal steam generation process can be significantly improved.

The discussion provides the idea of promoting the rapid evaporation of intermediate water, which requires less energy than free water due to its disrupted hydrogen bonding network. By minimizing bound water’s energy–intensive phase change, photothermal systems can achieve higher photothermal conversion efficiencies with minimal energy loss.
3. Design Strategies for Photothermal Materials Based on Key Factors
Many researchers characterize the key factors for photothermal materials differently; however, the fundamental properties can be summarized as follows: (a) low thermal conductivity, which minimizes heat loss; (b) high light absorption to maximize energy capture; (c) efficient conversion of light to heat; (d) floatability, which facilitates the positioning of the materials at the liquid–air interface; (e) wettability, which influences water transport and evaporation dynamics; (f) optimized material geometry for enhanced surface area and heat localization; and (g) salt resistivity to ensure performance stability in saline environments [52, 81].
3.1. Strategies for Achieving Low Thermal Conductivity in Materials
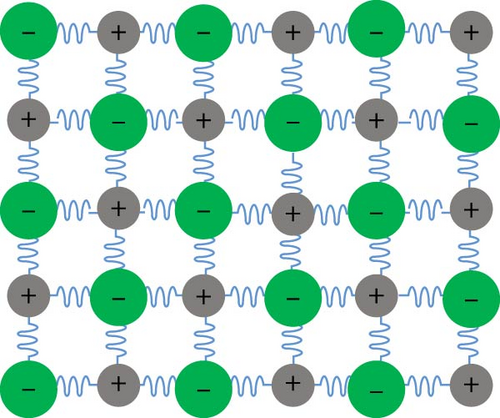
Strategic engineering of materials can modulate thermal conductivity, which is ideally minimized for effective photothermal processes. As discussed earlier, non–radiative relaxation involves heat transfer through phonon scattering and relaxation. The thermal conductivity of photothermal materials can be managed by controlling the mean free path (MFP) of phonons, which is the distance a phonon travels before transferring its energy to another carrier. Reducing the MFP of scattered phonons by introducing lattice defects can effectively decrease thermal conductivity [112]. The thermal conductivity (κp) of defective materials is inversely proportional to the square root of the phonon scattering coefficient (Γ). This relationship indicates that shortening the MFP increases phonon scattering at the sites of defects. Defects can be introduced into materials by creating vacancies or substituting atoms in the molecular structure (Figure 10). Increasing the concentration of defects in photothermal materials enhances phonon scattering and thus reduces thermal conductivity. Vacancies or substituted atoms, which represent either an absence of mass or a mismatch in volume at crystalline sites, contribute to lower thermal conductivity [113]. Vacancies can be categorized into two types: oxygen (anion) vacancies and cation vacancies. Atom substitution can involve larger, smaller, or smaller–sized atoms relative to the host lattice. However, the impact of vacancies on lowering thermal conductivity is generally more effective and efficient compared to substitutions, due to greater deviations in molecular mass and size caused by vacancies compared to substitutions [113].
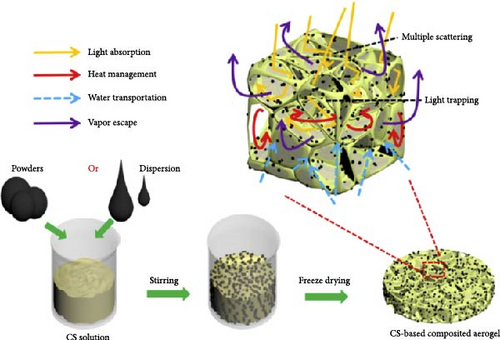
To achieve efficient water evaporation at the liquid–air interface, it is crucial to develop materials with thermal conductivities lower than that of pure water (0.599 W m–1 K–1) and seawater (0.6 W m–1 K–1) to enable effective heat localization. Gu et al. [114] designed a solar steam generator using chitosan (CS) aerogel composite with various concentrations of carbonized pomelo peel (PP), graphite, TiN, MnO2, GO, multiwall carbon nanotubes (CNTs), and commercial carbon black ink powder dispersed into the aerogel (Figure 11a). The CS aerogel enhances light absorption through light trapping and multiple scattering within its highly porous structure, which has a porosity of 96%. Under dry conditions, the CS aerogel exhibited a low thermal conductivity of 0.033 W m–1 K–1, significantly lower than that of pure water. This low thermal conductivity is attributed to the aerogel’s properties, which provide insulation similar to air, and the aerogel’s freezing process also plays an important role. Among the freezing methods used—refrigeration (R), lyophilization (L), and liquid nitrogen (N)—lyophilized CS showed the lowest thermal conductivity of 0.033 W m–1 K–1, compared to 0.038 W m–1 K–1 for liquid nitrogen freezing and 0.036 W m–1 K–1 for refrigeration. The temperature at the hot zone reached 42.3°C within 1 h of light irradiation (1 kW m–2), demonstrating the effectiveness of the lyophilized aerogel in concentrating heat at the liquid–air interface. However, the study did not thoroughly evaluate the primary factors contributing to the lower thermal conductivity of the lyophilized CS aerogels compared to those prepared by refrigeration or liquid nitrogen freeze drying. Based on the evidence provided, this difference could be due to variations in pore size and structure: lyophilized aerogels exhibit a 3D honeycomb pore structure with a size range of 80 μm, whereas aerogels prepared by refrigeration and liquid nitrogen freeze drying display irregular square shapes (150 μm) and vertical tube shapes (10 μm), respectively.
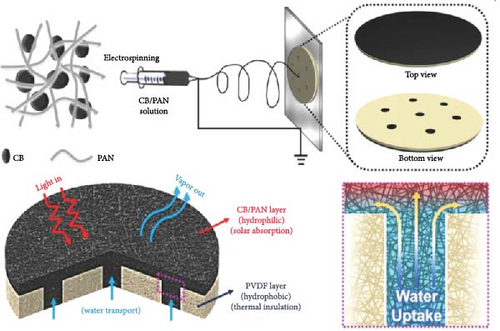
The Wang group developed a solar steam generation system using polypyrrole (PPy) coated on bamboo as a photothermal material [117]. PPy is effective for full–spectrum light absorption, demonstrating high sunlight absorption and efficient photothermal conversion performance. The bamboo substrate, a low–cost material, exhibits a low thermal conductivity of 0.2533 W m–1 K–1 [35]. Their study reported an evaporation rate of 1.125 kg m–2 h–1 under 1–sun illumination, with the liquid–air interface temperature reaching 34.8°C within 1 h. Gao et al. [115] successfully synthesized a bilayer solar evaporator consisting of carbon black/polyacrylonitrile (CB/PAN) on the top layer and polyvinylidene fluoride (PVDF) on the bottom layer, chosen for its low thermal conductivity properties. Typically, PVDF exhibits a thermal conductivity range from 0.11 W m–1 K–1 to 0.19 W m–1 K–1. However, in their study, the PVDF layer demonstrated thermal conductivities of 0.046 W m–1 K–1 in the dry state and 0.075 W m–1 K–1 in the wet state. This reduction in thermal conductivity is likely due to the electrospinning synthesis process, which creates open pores within the PVDF and embeds CB in the PAN electrospun layer atop the PVDF nanofibers (Figure 11b). The PVDF nanofiber layer reduces direct contact between bulk water and the CB/PAN absorption layer, resulting in a temperature increase of 34°C within 10 min and an evaporation rate of 1.2 kg m–2 h–1.
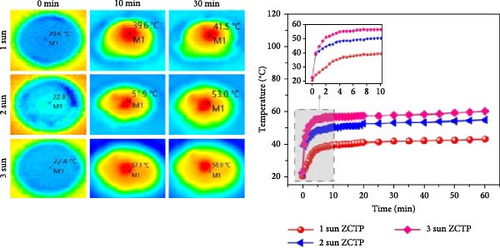
Zhao et al. [116] developed a solar steam generator using a zeolite/chitosan/TiO2@PPy aerogel (ZCTP), where PPy serves as the light–absorbing material and the ZCT aerogel provides support, water transport, and insulation. The ZCTP aerogel exhibits low thermal conductivities of 0.062 W m–1 K–1 in the dry state and 0.1969 W m–1 K–1 in the wet state, due to the synergistic effects of zeolite, chitosan, and TiO2. As a result, their system achieved a high evaporation rate of 1.66 kg m–2 h–1 with a temperature increase of 39.6°C within 10 min (Figure 11c). Further reductions in thermal conductivity could be explored through material engineering of TiO2, such as creating defects or vacancies.
3.2. Strategies for Enhancing Light Absorption
Selecting or designing a suitable material that behaves as an ideal black body and exhibits significant broadband light absorption is crucial for effective photothermal materials (Figure 12a). For light absorption in a solar steam generator, two key properties must be considered: the absorption range across the solar spectrum and the intensity of absorption at individual wavelengths. An ideal solar absorber should exhibit high absorbance across a broad spectrum, ranging from 250 to 2500 nm (ASTM G–173, AM 1.5), capturing as much solar energy as possible. The solar absorptance of photothermal materials can be defined by Equation (3) [78, 120].
Photothermal materials with highly hierarchical porous nanostructures are advantageous for the continuous scattering of incident light. When light strikes a flat surface, it may be reflected depending on the angle of incidence. However, in porous nanostructures, incident light is trapped within the pores, leading to repeated scattering and re–absorption. This repeated interaction enhances light absorption, significantly improving the photothermal efficiency of the material (Figure 12b) [121]. Fu et al. [122] developed a hierarchical porous steam generator using a metallic glass (Pt57.5Ni14.7Cu5.3P22.5) that exhibits broad wavelength absorption ranging from 380 to 2500 nm with an absorbance efficiency of 90%. The material demonstrated very low reflectance, less than 5% in the visible region and less than 10% in the near–infrared region. The extensive light absorption capabilities are attributed to the multiple reflections of incident rays within the hierarchical porosity and the inherent black properties of the metallic glass.
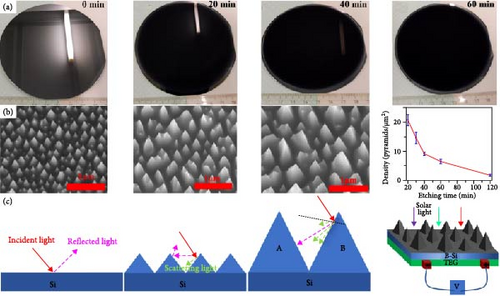
Studies have found that the surface texture, particularly the height and tilt of nano–roughness, plays a crucial role in trapping light within nanostructures through multiple scattering [123, 124]. Addressing this phenomenon, Cheng et al. [125] synthesized black silicon (b–Si) photothermal materials using a dry etching method. They successfully created nanopyramids with varying sizes, averaging 407 nm from top to bottom, on the pristine Si surface, which significantly reduced the reflectance of light and rendered the Si black (Figure 13a) [125]. The blackening of Si stabilized after 60 min of etching when the density of surface nanopyramids relative to the area reached a steady state, with a tilt angle of 20°. The incident light on the b–Si surface undergoes multiple scattering within the nanopyramids, converting photon energy into phonon energy (Figure 13b, c). This heat generation mechanism makes b–Si suitable for applications in photothermal steam generation, as well as in photovoltaic (PV) and thermoelectric generator (TEG) devices.
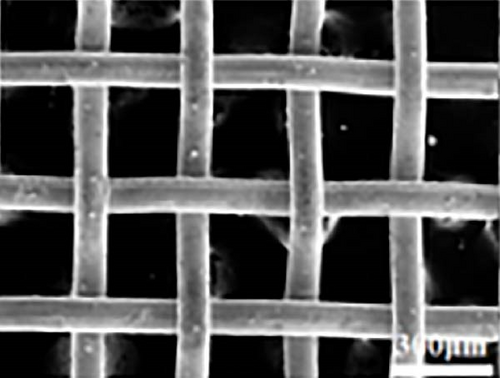
Carbon–based materials such as carbon black, CNTs, graphene, GO, and reduced graphene oxide (RGO) have conjugated π–electrons, allowing them to absorb light across the solar spectrum, including the infrared (IR) region. Extensive research has been conducted on incorporating these carbon–based materials into various supporting matrices. For instance, Chen’s group developed a solar steam generator by embedding CNTs into polyacrylonitrile nonwoven fabric, achieving a light absorption efficiency of 90.8% in the wet state across a broad solar spectrum (350–2500 nm) [126]. This high absorbance is primarily attributed to the superior light–absorbing properties of CNTs. The thickness of the nonwoven fabric and the electrospinning preparation method showed minimal impact on light absorption capacity. However, the concentration of CNTs significantly influenced the light absorption efficiency (Figure 14). Overall, the concentration of different carbon–based photothermal materials plays a critical role in enhancing light absorption capacity.

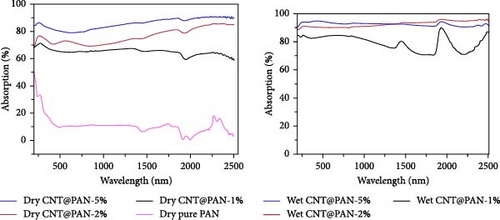
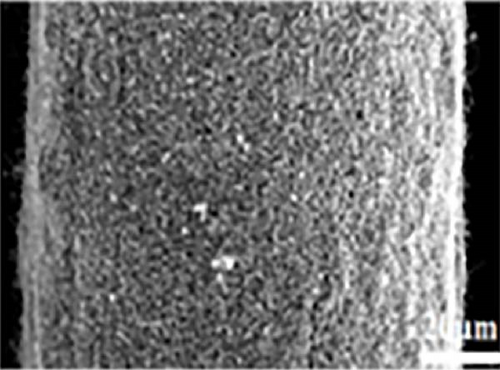
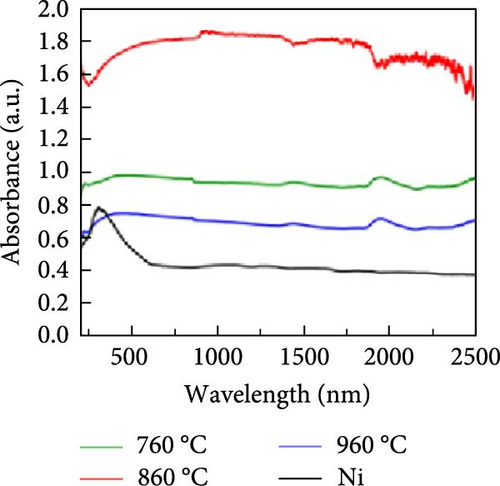
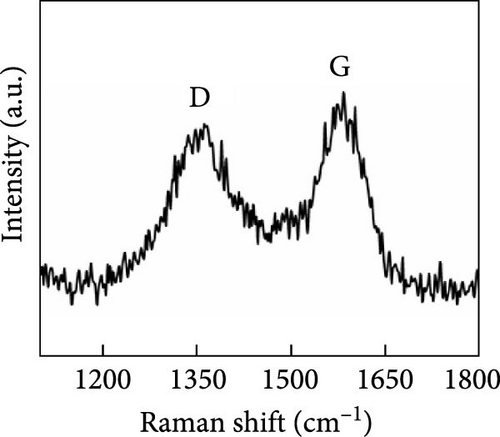
Another study involving CNTs embedded into a nickel (Ni) mesh reported a high light absorption capacity of 94.3% across a solar spectrum ranging from 200 to 2500 nm [127]. The study also explored the effects of varying preparation temperatures (760, 860, 960°C) on the light absorption properties of the Ni/CNT photothermal material, revealing a novel approach to enhancing light absorption capacity. As shown in Figure 15, pure Ni exhibits very limited light absorption, whereas CNTs prepared at an optimal temperature of 860°C demonstrated significantly higher and broader light absorption. At 860°C, CNTs with a uniform diameter of 45 nm were found to grow uniformly on the Ni mesh surface in a crystallized form. Additionally, a prominent peak at the D–band was observed, indicating defect–induced disorder in sp2 bond vibrations. In contrast, CNTs synthesized at 760°C had smaller diameters, and at 960°C, the Ni mesh showed only a few irregularly formed CNTs. This study underscores that preparation methods, synthesis temperatures, and defect engineering are critical factors that can enhance the light absorption capabilities of solar steam generators.

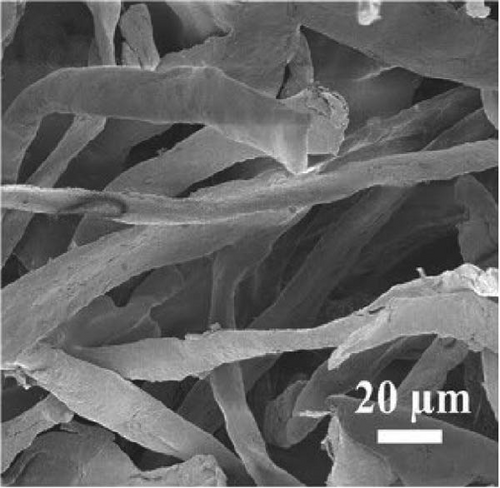
Carbonized wood and other naturally derived carbon–based materials are highly accessible and can serve as effective photothermal materials with broad light absorption properties [128]. Zhou et al. [129] developed an economical steam generator using carbonized tofu (CT) derived from soybeans, which demonstrated light absorbance across the 300–2500 nm wavelength range, achieving 90% absorption efficiency post–carbonization. This high efficiency is attributed to the formation of abundant pores (50–400 μm) during the carbonization process at 600°C, which enhances light absorption and reduces thermal conductivity due to the well–packed porous structure and graphene–like high–crystallinity features. In contrast, tofu processed via freeze–drying becomes soft and brittle in water, making it unsuitable for long–term applications. The carbonization process thus provides superior structural integrity and performance in photothermal applications compared to freeze–drying.
Metal nanoparticles, such as gold (Au), silver (Ag), and aluminum (Al), are known for their wide solar absorbance and are widely utilized as effective materials in solar steam generators. The size, shape, and crystalline structure of these nanoparticles significantly influence their light absorption capacity. Liu et al. [130] developed a photothermal steam generator by combining abundant paper with a gold nanoparticle film (PGF), resulting in high surface microstructure roughness due to the paper substrate. While the gold nanoparticle film alone exhibited a light absorption of 64%, the PGF achieved an enhanced absorption of 87% due to the uniform distribution of nanoparticles and the multi–scattering of incident light. In another study, Cao et al. [131] synthesized aerogel–based photothermal materials composed of bacterial cellulose (BC), GO, and silver nanoparticles (Ag NPs). The absorbance of these materials was reported as 15.7% for BC, 77.6% for BC/GO, and 84.1% for BC/GO/Ag in the 300–3500 nm range of the solar spectrum [131]. The increased absorbance efficiency of the BC/GO/Ag composite is attributed to the localized surface plasmon resonance (LSPR) effect of the silver nanoparticles.
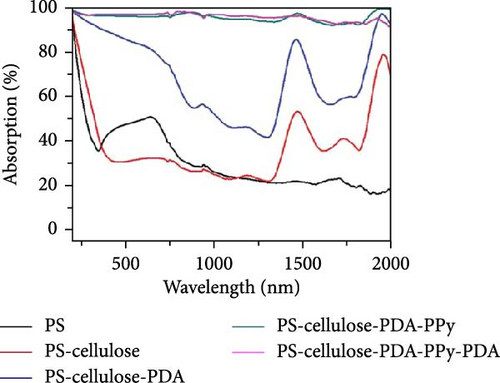
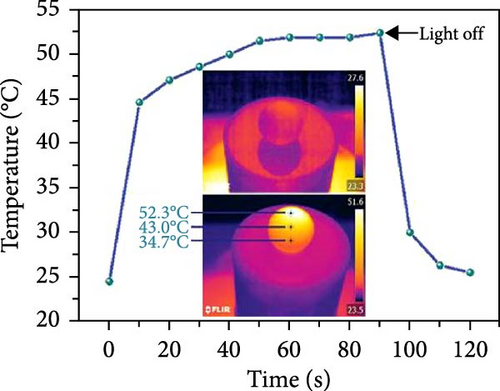
Depositing organic materials such as polypyrrole (PPy) and polydopamine (PDA) have been shown to significantly enhance solar light absorption across a wide range of wavelengths. Sun et al. [132] developed a 3D mirror–assisted, pyramid–shaped evaporator by depositing PPy on nonwoven fabric (PCNF). This evaporator demonstrated broad light absorbance from 220 to 2500 nm with an efficiency of over 95%, achieving an evaporation rate of 1.55 kg m–2 h–1 [132]. In a comparative study, the performance of PCNF was evaluated against GO–coated nonwoven fabric (GONF), which showed a lower evaporation rate of 1.04 kg m–2 h–1, highlighting the superior light–absorbing properties of PPy. Additionally, Tariq et al. [133] developed a PPy–coated sulfur steam generator that effectively absorbs light across the spectrum from 300 to 2400 nm, attributed to the material’s optimal surface roughness, high porosity, and broad light absorption capability. The concentration of oxidant groups also plays a crucial role in enhancing light absorption capacity. Another group designed a dual–zone steam generator by coating polystyrene spheres sequentially with α–cellulose, PDA, PPy, and PDA, resulting in a PS–cellulose–PDA–PPy–PDA structure [134]. This configuration demonstrated remarkable light absorption enhancement across the entire spectrum, with an efficiency exceeding 92% (Figure 16a–f).
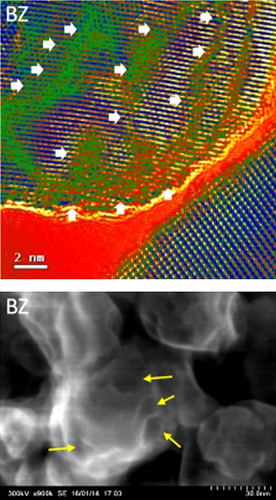
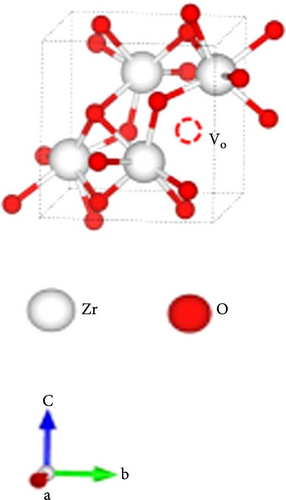
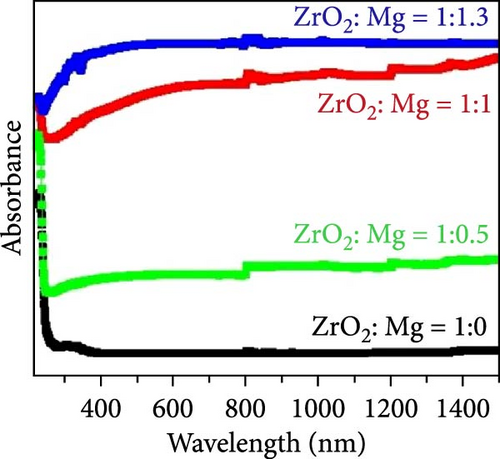
Semiconductor materials, particularly transition metal oxides such as TiO2 and MoO3, are widely utilized in the development of solar steam generators due to their tunable light absorption properties. These materials are ideal candidates for steam generation as their light absorption can be tailored by narrowing the bandgap. In the case of TiO2, doping with non–metal anions such as C, N, S, and P at oxygen sites, or with transition metal cations like Fe, Co, Cu, Cr, and Ni at titanium sites, can adjust its bandgap. Additionally, bandgap engineering can be achieved by introducing surface disorder or creating oxygen vacancies. The surface disorder can alter lattice periodicity and the edges of conduction and valence bands, while oxygen vacancies act as traps that reduce the recombination of photogenerated charge carriers. Sinhamahapatra et al. [135] synthesized black ZrO2 (BZ) from white ZrO2 (WZ) as a precursor for solar light absorption, using varying ratios of Mg particles. Their study found that a higher molar ratio of Mg resulted in enhanced light absorbance (Figure 16g–i) [135]. This increased absorption was attributed to surface disorder and oxygen deficiency, as Mg reacted with oxygen to form MgO.
3.3. Strategic Engineering of Floatability in Solar Evaporators
Designing photothermal materials with floating capabilities is crucial for enhancing their effectiveness and efficiency as evaporators, particularly in desalination applications. The formation of the hot zone at the liquid–air interface is vital for maximizing solar absorption and evaporation efficiency. Floating materials ensure direct exposure to sunlight, allowing for optimal light absorption while minimizing heat loss to bulk water through improved thermal management. The desired floating conditions are illustrated in Figure 17a–c, where partially immersed photothermal materials generate more steam due to the effective management of thermal gradients between the hot and cold zones.
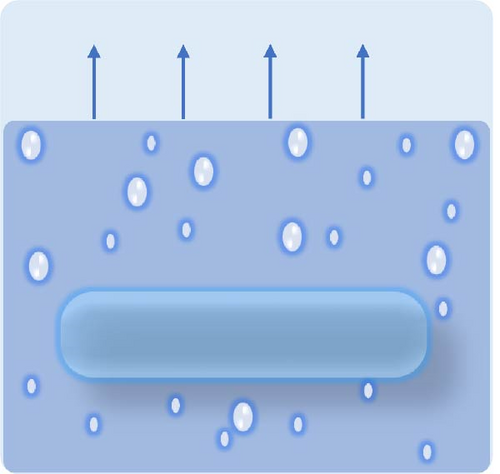
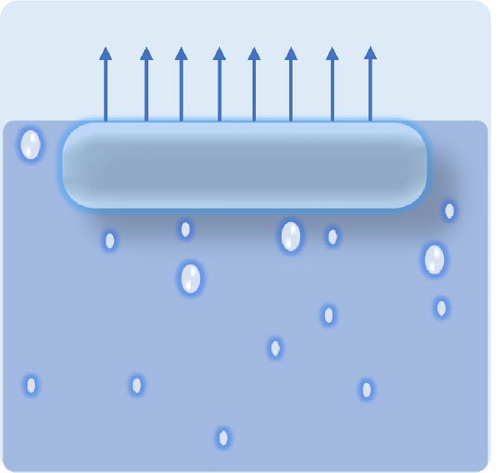
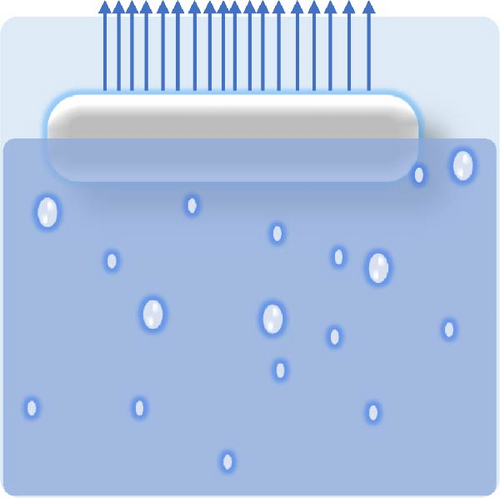
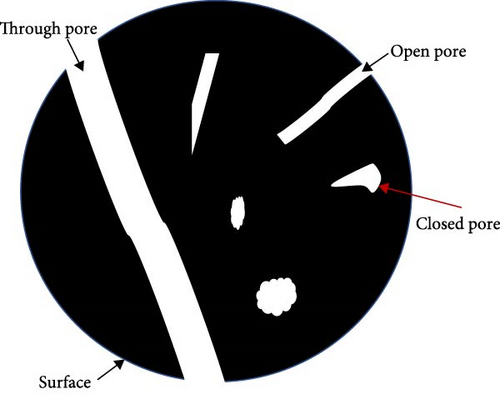
Floatability can be achieved by designing highly porous materials that reduce the overall density to below that of water. Hierarchical porosity is also crucial, as it enables water to wick from the bulk to the hot zone, facilitating efficient steam generation. Pores are empty spaces within a material that affect not only its buoyancy but also water transport and thermal efficiency. They are classified as closed, open, and through pores, each serving a distinct role in solar steam generators (see Figure 17d). Closed pores are enclosed air pockets that enhance buoyancy by lowering material density and preventing water infiltration. However, it is detrimental to effective water transport. Open pores are interconnected voids connected to the steam generator’s surface, enabling controlled water absorption and achieving a balance between buoyancy and capillary–driven water transport to the evaporation surface. Through pores are continuous channels that run through the entire thickness of the material, allowing efficient water replenishment and steam escape, but they can increase water saturation if not optimized. A well-balanced combination of these pores ensures stable buoyancy, adequate water supply, and improved solar steam generation performance.
The type of pores plays a significant role in floatability: closed pores contribute to buoyancy, not water wicking, whereas open pores alone cannot provide buoyancy but efficient water wicking as per both of their structure defines. Therefore, a combination of both open and closed pores is ideal for photothermal structures, allowing them to float on the water’s surface while maintaining effective water transport to the evaporation interface [97]. Implementing various types of floating porous materials, such as paper, anodized aluminum oxide (AAO), polymer foam, and wood, can enhance the self–floating capabilities of photothermal materials [137–140]. Bai et al. [141] developed a self–floating, highly porous photothermal material by dual doping of nitrogen and oxygen into carbon foam, achieving a solar light absorbance of 99% and an evaporation rate of 2.4 kg m−2 h−1. The interconnected porous structure of the carbon foam was instrumental in providing self–floating ability. Wood is particularly advantageous due to its low density, capillary–induced hydrophilic nature, and low thermal conductivity [136]. Its self–floating ability is attributed to open microchannels, which reduce its overall density below that of water [136, 142]. These open microchannels can be compared with through pore. Through pores are random with interconnected voids, whereas open microchannel is organized with directional pathways (see Figure 17e). Hi et al. [143] synthesized wood–based photothermal materials using tannic acid (TA) and Fe3+, where the wood acted as both a heat barrier and a floating support. Carbonization has become a popular strategy for converting wood into effective photothermal materials, enhancing its light absorption and structural properties for solar steam generation applications.
Wang et al. [139] demonstrated self–floating photothermal materials by incorporating melamine foam (MF), polyvinyl alcohol (PVA), and polyaniline, comparing the floatability of their photothermal composite (PCPF) with that of pure MF. They reported that the addition of PVA enhanced the buoyancy of the PCPF [139]. Another study proposed a self–floating aerogel steam generator prepared by dispersing polyaniline (PANI) and diatomite (DE) into a PVA/MF composite foam. The self–floating property was attributed to the inner membrane of the PANI/DE@PVA/MF composite, which provided additional buoyancy [144]. Lei et al. developed highly self–floating photothermal materials inspired by seaweed, using zwitterionic sulfobetaine (ZSB), carbon black (CB), and hollow glass microbeads. Their materials exhibited densities of 0.72 g cm−3 in the wet state and 0.42 g cm−3 in the dry state, both significantly lower than the density of water (0.997 g cm−3). Overall, these efforts effectively enable the photothermal evaporators to float efficiently, reducing heat loss to bulk water through improved thermal management.
3.4. Importance of Wettability on the Capillary Zone and Heat Zone Surface
Enhancing the efficiency of photothermal steam generation relies significantly on proper water supply, which is influenced by the wettability of the steam generator. Wettability describes a liquid’s ability to maintain contact with a solid surface and provide a capillary rise, driven by the interactions between the liquid and the surface molecules. A hydrophilic surface (<90°) promotes even spreading of water, ensuring maximum exposure to the photothermal material and facilitating efficient heat transfer from the material to the water, which is critical for steam generation. Additionally, wettability plays a crucial role in the thermal management of the system. Hydrophilic surfaces help reduce heat loss by maintaining a continuous water layer on the photothermal surface, which acts as a heat sink and minimizes convective heat loss. Therefore, the steam generation rate of photothermal steam generators is directly linked to the wettability of the material; hydrophilic surfaces typically result in more efficient steam generation due to improved water management and heat transfer properties. However, excessive hydrophilicity can lead to the formation of a thick water film over the hot zone, potentially impairing thermal management in photothermal steam generation [145]. Thus, achieving optimal hydrophilicity is essential for the effective performance of photothermal steam generators.
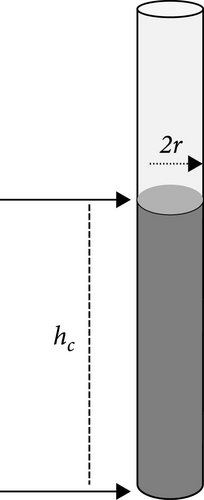
The micro– and nanoscale textures, material composition, and surface coatings of photothermal materials significantly impact their wettability. Surface roughness can enhance the hydrophilicity of a material, thereby improving its water absorption capacity. A rough surface of the inner pore increases the number of micro–and nanoscale textures, which enhance capillary action by creating microchannels and nanoscale voids. According to the Lucas–Washburn equation, the capillary rise is inversely proportional to the pore radius (see Equation (4)) [146]. This implies that smaller pore sizes, resulting from the roughness of the pore’s inner surface, increase the material’s hydrophilicity and promote water transport to the photothermal surface (see Figure 18a).
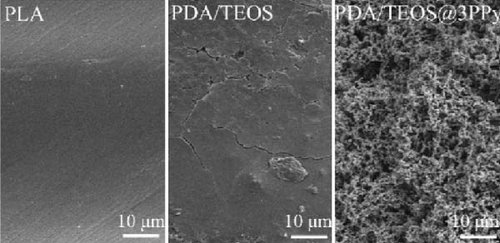
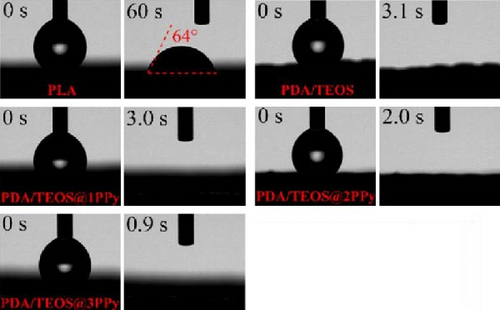
Hydrophilic coatings, such as polydopamine (PDA) or titanium dioxide (TiO2), can also be applied to modify and enhance the wettability of photothermal materials. For instance, Li et al. [147] fabricated photothermal materials using 3D printing technology with polylactic acid (PLA) Due to the inherently poor hydrophilicity of PLA, the group employed a PDA/TEOS@PPy coating, which increased the evaporator’s hydrophilicity. Further enhancement was achieved by depositing multiple layers of polypyrrole (PPy) on the surface of the evaporator material. This improved hydrophilicity is attributed to the increased surface roughness resulting from the coated layers (Figure 18d, e).
The inherent properties of a material, including its chemical composition and structure, significantly influence its wettability. Materials such as graphene and GO, which possess hydrophilic properties, are often preferred in photothermal applications due to their favorable interaction with water [149]. The fabrication methods of photothermal materials also play a crucial role in modulating their surface properties, including the transition from hydrophobic to hydrophilic behavior. Ghasemi et al. [97] synthesized a double–layer structure (DLS) composed of carbon foam at the bottom and exfoliated graphite at the top. Initially, the graphite flakes exhibited a hydrophobic nature with a contact angle of 100°, but after exfoliation, the contact angle reduced to 40°, indicating a transition to hydrophilicity. Additionally, the carbon foam was treated with 4% nitric acid to further enhance its surface hydrophilicity.
Strategies to optimize wettability often involve surface engineering techniques that utilize external stimuli, such as UV irradiation, to enhance the presence of oxygen–containing groups. Babak’s group investigated the wettability properties of a GO/ZnO nanocomposite on an aluminum (Al) surface [150]. Heat treatment of the Al–rGO/Zn sample resulted in the reduction of functional hydroxyl groups, increasing the contact angle from 5° to 137°, indicating a shift towards hydrophobicity. However, when the heat–treated sample was exposed to UV irradiation, it became hydrophilic, as demonstrated by a significant decrease in the water contact angle to 20°. This change was attributed to the formation of oxygen vacancies on the ZnO lattice, facilitated by the generation of electron–hole pairs within the ZnO lattice during the UV treatment for 10 h.
3.5. Influences of Materials Geometry
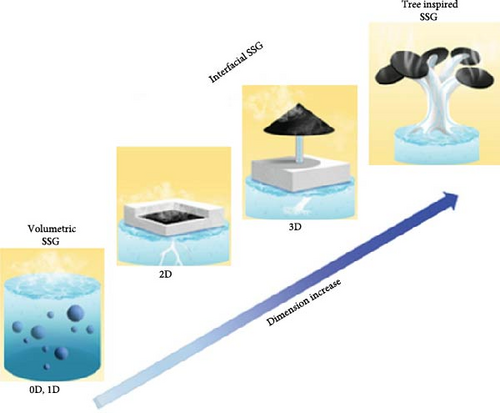
Material geometry is a critical factor in the design and optimization of photothermal steam generators, as it directly affects light absorption, heat management, water transport, and overall efficiency. The geometry can significantly influence the extent of sunlight absorption, with structures that possess a high surface area enhancing light trapping by increasing the interactions between light and the material. Additionally, material structure affects the distribution and dissipation of heat, directing it toward the water surface and minimizing heat loss to the surroundings. Furthermore, geometry can enhance the durability of photothermal steam generator materials by improving their mechanical strength. Zhou et al. [151] reviewed the evaluation of photothermal materials ranging from zero–dimensional to three–dimensional geometries (see Figure 19a). Their study reported that three–dimensional materials exhibit superior light absorption, reduced heat loss, and enhanced water supply compared to other geometries. However, two–dimensional steam generators have been more extensively researched, demonstrating a wide variety of applications. There is no exact specification for the thickness of photothermal material to classify it as a 2D material. The thickness of thin photothermal films varies across different ranges. After reviewing several articles, it can be concluded that a thickness of a few 100 micrometers (less than 500 µm) may be considered 2D photothermal materials.
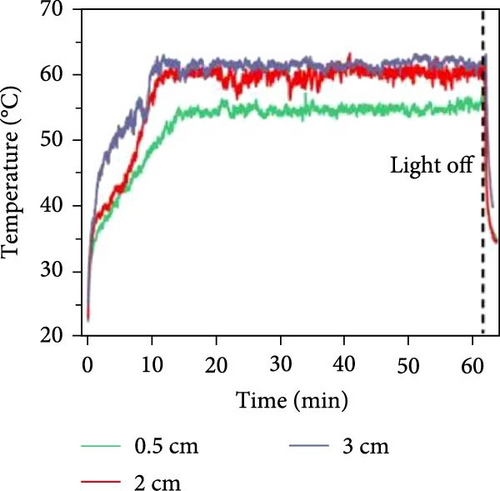
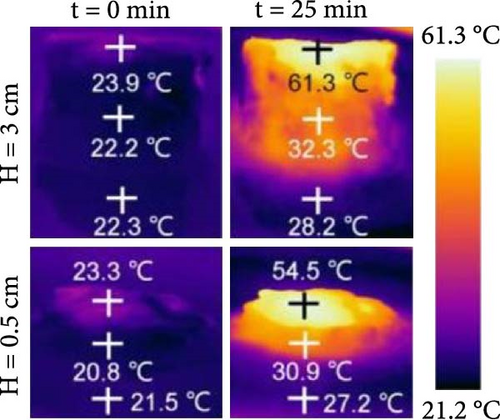
Zhang’s group prepared a black TiO2 film with a thickness of 20 µm and reported a photothermal efficiency of 77.14% alongside an evaporation rate of 1.16 kg m–2 h–1 without any thermal insulation management [153]. Kyubock and colleagues synthesized a photothermal generator that was 160 µm thick, made with polyethyleneimine (PEI) treated activated carbon (AC), and reported an evaporation rate of 1.27 kg m–2 h–1 under one sun irradiation with a photothermal conversion efficiency of 85.66% [154]. Wei et al. [155] fabricated a photothermal steam generator by applying a thin composite film of CNF@RGO–n (where n represents RGO content) with a controllable thickness of several micrometers on the top surface of polystyrene foam (PSF), demonstrating an evaporation rate of 1.47 kg m–2 h–1 under 1–sun irradiation with an efficiency of 83%. In a recent study, Wang et al. [156] synthesized 2D photothermal materials CNT/B–TiO2/PDA in thicknesses of 3.1, 4.8, 6.4, and 8 µm. This study demonstrated the highest evaporation rate of 2.33 kg m–2 h–1 under 1–sun irradiation of brine solution due to adequate water supply [156]. The research indicated that thinner photothermal materials exhibited a thick water film, obstructing the water supply to the evaporation surface. Moreover, relatively thicker photothermal materials could not provide sufficient water supply due to increased mass transfer resistance. This water supply issue can be solved by a 3D solar steam generator along with heat loss.
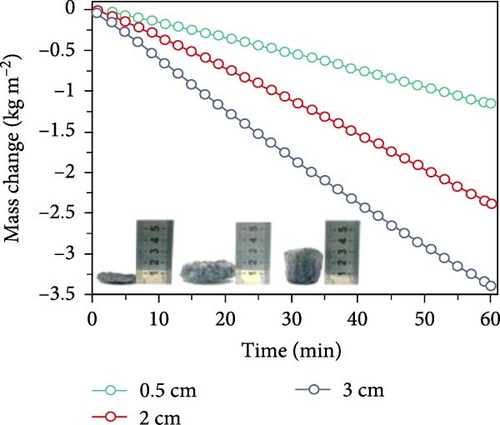
Chen et al. [152] fabricated 3D xerogel photothermal materials composed of PVA, carbon black, and CaCl2 (PXF–x, where x represents the mass percentage of CaCl2) with varying heights. The study demonstrated that increasing the height of PXF–90 led to a rise in temperature at the hot zone and enhanced the evaporation performance. As shown in Figure 19b–e, the results depict changes in evaporation mass and temperature over time, highlighting the differences between 2D and 3D evaporator configurations. The findings indicate that increasing the height of the 3D structure improves the temperature gradient and evaporation rate, achieving a rate of 3.39 kg m–2 h–1 under 1 sun irradiation.
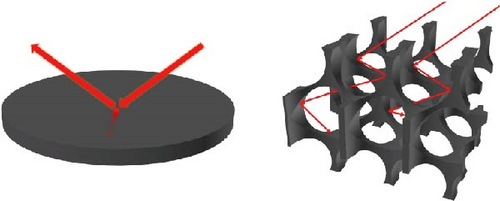
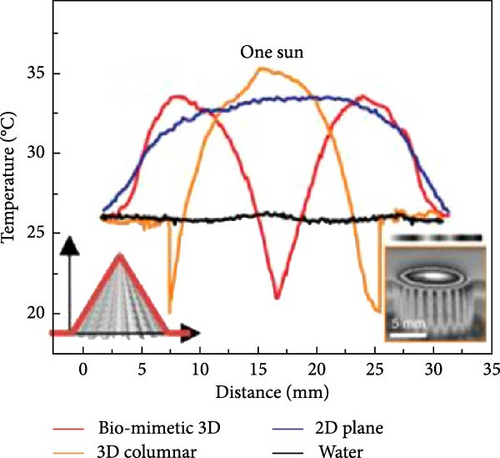
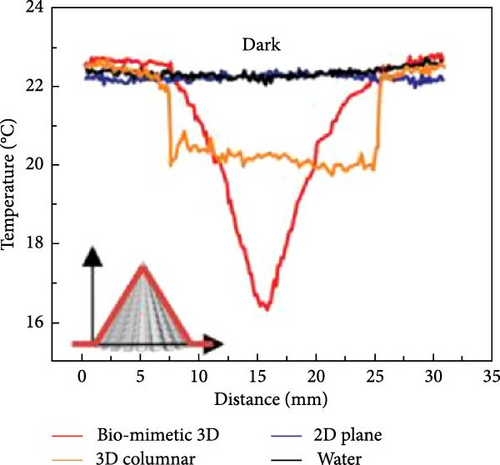
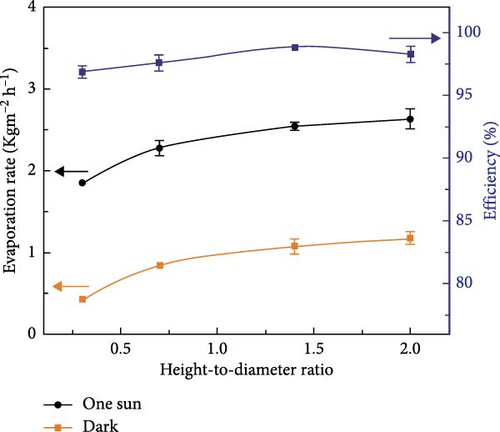
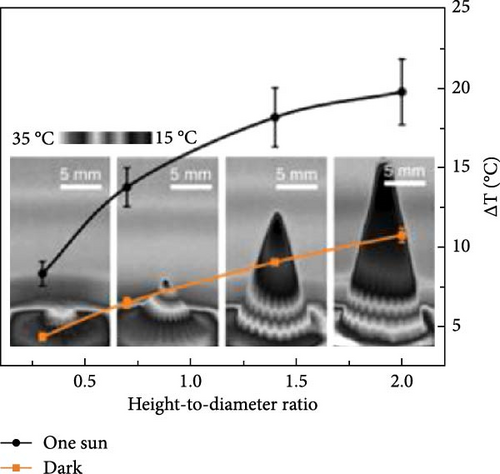
3D materials exhibit strong solar light absorbance with lower heat loss due to reflection and radiation compared to 2D materials [23]. The height or thickness of these materials is a critical factor in optimizing evaporator performance, as it influences the negative and positive temperature gradients within the structure. Between these temperature zones, evaporative cooling zones are formed, which facilitate water evaporation [99]. Wu et al. [157] proposed a 3D photothermal bird beak–shaped evaporator composed of CNTs with hierarchical nanostructures and a microporous surface, which induces the Marangoni effect. The Marangoni effect refers to liquid flow caused by a surface tension gradient between steam and water, driven by a temperature gradient. This flow also aids in reducing salt accumulation on the photothermal materials. The study reported evaporation rates of 1.07 kg m–2 h–1 for pure 2D materials and 2.28 kg m−2 h−1 for 3D biomimetic materials under one sun irradiation, with the 3D structure generating Marangoni flow. Figure 20 illustrates the temperature variation with distance, showing a high–temperature gradient at the apex of the 3D biomimetic structure, whereas the 3D columnar model exhibits a lesser gradient. This suggests that steam dissipates energy from the apex, allowing the structure to continuously produce steam (Figure 20a–d). The evaporation rate and efficiency of 3D biomimetic photothermal materials can be tuned by adjusting their height and diameter, and accumulated salt can dissipate through the leaning of the structure (Figure 20e).

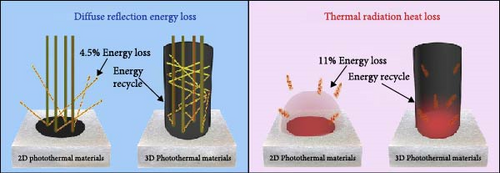
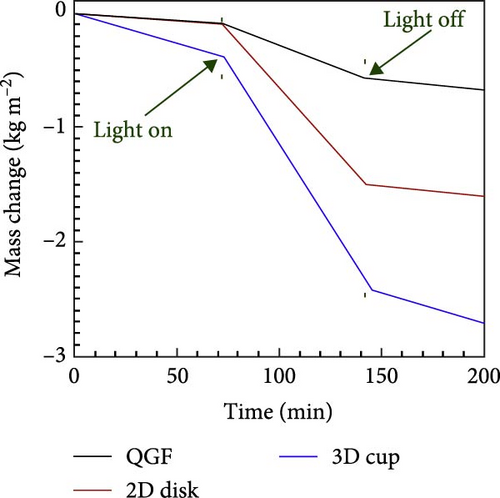
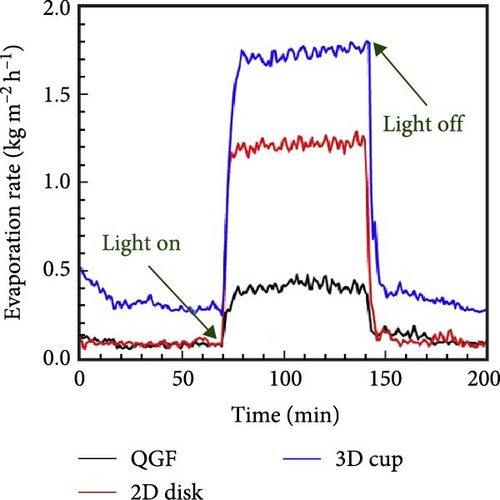
Shi et al. [23] developed a 3D photothermal evaporator that exhibited a higher evaporation rate of 2.04 kg m−2 h−1 compared to the 1.22 kg m−2 h−1 observed for a 2D evaporator. The study focused on reducing energy loss and enhancing solar light absorption in the 3D model relative to the 2D model. The cup–shaped 3D photothermal steam generator demonstrated reduced energy loss due to multiple scattering of solar light (Figure 21a). As a result, the 3D material showed superior evaporation performance (Figure 21b, c). In a recent study, Erçarıkcı et al. [158] synthesized a PDI/GGrS photothermal steam generator with a thickness of 12 mm. They demonstrated an evaporation rate of 3.5 kg m−2 h−1 due to effective water management and low heat loss. By meticulously designing material geometry, the performance of photothermal steam generators can be significantly improved, enhancing their efficiency, durability, and cost–effectiveness. Such advancements are particularly beneficial for applications, including desalination, water purification, and energy generation. For that reason, 3D solar steam generators are favored over 2D models. They provide greater evaporation efficiency by increasing the surface area for water transport and photothermal absorption, which minimizes heat loss. The 3D structure enhances capillary–driven water wicking, ensuring a steady water supply to the evaporation surface while preventing drying and salt accumulation. Moreover, 3D designs capture more solar energy, boosting thermal retention and achieving higher solar–to–vapor conversion efficiency compared to 2D flat designs.
3.6. Salt Resistance Capability Enhancement Strategy
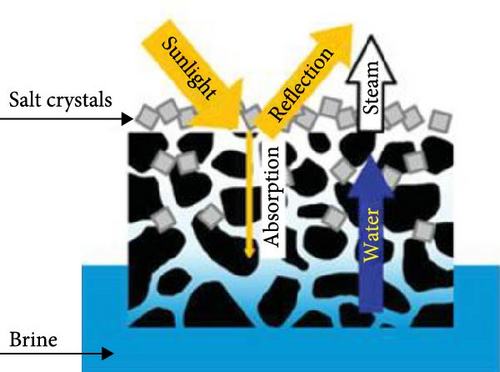
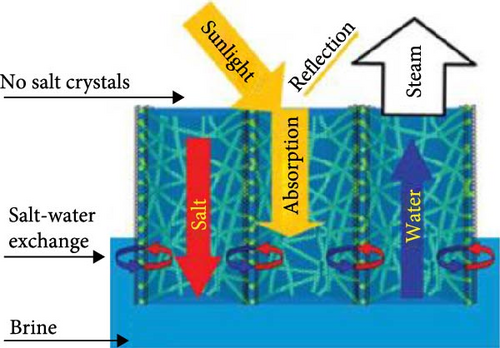
The solar desalination process faces significant challenges due to salt crystallization on the surface of solar evaporators, which drastically reduces the evaporation rate. Fundamentally, the salinity on the evaporator surface increases continuously as evaporation progresses. The absence of highly interconnected pores with vertical channels in the evaporator materials hinders the resistance to salt crystallization, as there is insufficient opportunity for the salt to return to the bulk water. When accumulated salt cannot be transported back to the bulk water, it forms crystals on the evaporator surface, reducing light absorption efficiency, obstructing smooth water transport, and ultimately decreasing the water evaporation rate [159]. This can even lead to the mechanical failure of the evaporator materials. To address this issue, precise design and engineering strategies are required to prevent salt crystallization on the evaporator surface, taking into account various influencing factors. One approach involves hydrophilizing the evaporator materials to prevent salt accumulation and crystallization [159]. However, this can cause salt to crystallize within the internal structure of the evaporators, potentially destroying the internal structure and blocking water transport, as well as resulting in heat loss to the bulk water. Another strategy employs millimeter–scale perforated material designs to prevent salt accumulation and facilitate the diffusion of salt back into the bulk water. While this method efficiently reduces salt crystallization, it also allows light to pass through the evaporators, thereby reducing the light absorption efficiency of the materials.
Recent studies have focused on the synthesis of nanofiber–based evaporators, which hold significant potential for preventing salt crystallization. The preparation of such nano–fibers typically utilizes electrospinning methods, resulting in relatively densely packed membranes that lack efficient connectivity and sufficient porosity. Consequently, these membranes fail to provide effective transportation of salt from the evaporation surface to the bulk water. Dong et al. [160] have addressed this challenge by designing CNTs on SiO2, forming CNT–nanofibrous aerogels (CNAFs). These CNAFs demonstrate efficient salt transportation from high to low salinity regions through the pores in their vessel–like walls (Figure 22). This efficient salt transport reduces salinity at the evaporation interface, thereby preventing salt crystallization. The vessel–like structure and the excellent light absorption properties of CNTs synergistically provide outstanding photothermal conversion and efficient water evaporation performance. The flexible SiO2 nanofibers confer robust mechanical integrity to the CNAFs. Under testing, CNAFs showed no signs of salt crystallization on the aerogel surface even in 20% brine under 6–sun irradiation. Additionally, CNAFs demonstrated an excellent light absorption capacity of 98% and a moderate evaporation performance of 1.50 kg m–2 h–1 under 1–sun irradiation compared to the salt–resistant effect. Their research lacks of higher evaporation rate due to effective water management. When the water evaporation started on the liquid–vapor interface, salt accumulation grew on the evaporator surface as well as the pore channel. Then the salt concentration reached a saturation level that caused salt crystallization over the surface and blocked the pores. At the same time, the salt concentration of seawater remains at initial conditions which is lower than the saturation level of salt at the evaporator surface or pores. That creates a salt concentration gradient for which salt is dissipated into the bulk seawater by convection and diffusion via the vertical–aligned pore channels. Fick’s law can represent the convection of crystal salt. Gravitational force derives the salt diffusion to the balk water. Convection and diffusion are considered as two main mechanisms for salt transportation within bulk water.


A study on photothermal materials involving reduced GO (rGO)/cotton fabric/vertically oriented porous polyacrylonitrile foam (VOPPF) demonstrated the synthesis of a self–desalting system. This system operates by converting salt into bulk water driven by concentration and temperature gradients. The temperature gradient significantly enhances salt solubility in bulk water [161]. In this study, rGO/cotton fiber served as the photothermal layer, while VOPPF functioned as the thermal management and water supply layers. The system achieved an evaporation rate of 1.47 kg m–2 h−1. Notably, the research found no accumulation of salt crystals on the materials, attributable to the unique design of vertically aligned channels.
A recent study demonstrated that a nanofibrous aerogel composed of SA/MXene/CNTs (SMC) exhibits notable salt resistivity due to the synergistic effects of vertically aligned channels and the presence of Ca2+ ions, which induce the Donnan effect [162]. The SMC aerogel achieved a photothermal evaporation rate of 2.41 kg m–2 h−1, surpassing the works of Deng group (CNT with SiO2, evaporation rate 1.50 kg m–2 h–1) and Wang group (RGO/cotton fabric, evaporation rate 1.47 kg m–2 h–1) [160, 161]. Specifically, the Donnan effect is an emerging concept in salt diffusion within bulk water. It involves the separation of permeable ions (e.g., Na+, Cl−) in bulk water from impermeable ions (e.g., Ca2+, Fe3+) incorporated into photothermal materials by a semi–permeable membrane. Due to concentration and electric gradients, only permeable ions can traverse the semi–permeable membrane, resulting in unequal ion distribution. This continuous ion movement prevents accumulation on the surface of the photothermal materials, thereby resisting salt deposition [145]. However, as surface salt concentration gradually increases, saturation occurs, weakening the Donnan effect [163].
Building on this concept of Donan effects, Zhang et al. [145] developed an advanced photothermal steam generator incorporating Fe3+ ions within a GO membrane as the top layer, with phytic acid (PA) and PVA as the bottom layer. The inclusion of Fe3+ ions effectively inhibited the accumulation of salt ions (Na+, Cl–) on the evaporator surfaces and within the pores, thereby facilitating the selective transfer of water molecules (Figure 23a, b). The inclusion of Fe3+ ions effectively inhibited the accumulation of salt ions (Na+, Cl−) on the evaporator surfaces and within the pores, thereby facilitating the selective transfer of water molecules. The Fe3+@GOM exhibited a salt rejection efficiency of 92.16% for 0.50 wt.% NaCl, compared to 81.49% for pure GO without Fe3+ ions. Additionally, Fe3+@GOM achieved an evaporation rate of 3.54 kg m–2 h−1 in saltwater with concentrations ranging from 10 to 25 wt.%.
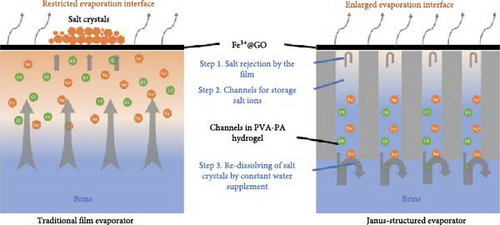




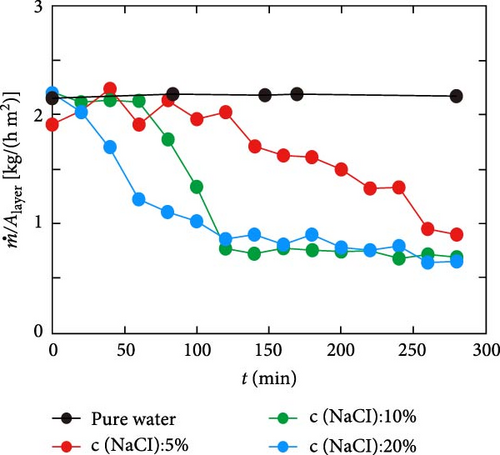
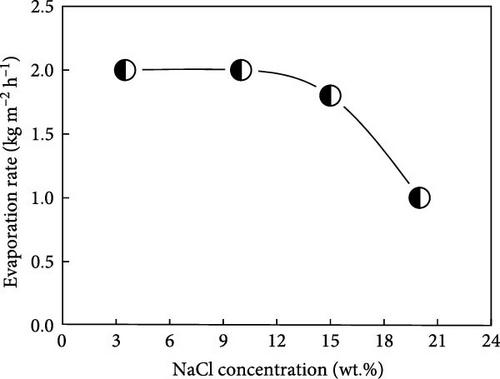

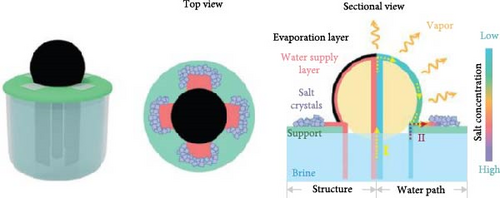
These findings suggest that incorporating charged particles into photothermal semi–permeable membranes, or designing materials with vertical pore channels or secondary water pathways, can effectively enhance salt resistance. This approach highlights the potential for developing advanced photothermal materials through the combination of charged particles within semi–permeable membranes and nanofibrous vertically aligned pore channels along with secondary water pathways as per geometry.
4. Costs Comparison and Technology Readiness Levels (TRLs)
The analysis of key factors influencing the performance of photothermal steam generators provides valuable insights into the design of photothermal materials. Among their various applications, desalination has emerged as a key area of focus. Photothermal desalination (PD) leverages abundant solar energy, producing zero carbon emissions and minimizing reliance on fossil fuels for steam generation. In contrast, conventional desalination techniques—including thermal processes such as multistage flash (MSF), multieffect distillation (MED), and vapor compression (VC), as well as membrane methods like reverse osmosis (RO) and electrodialysis (ED)—require external energy inputs and are associated with higher carbon emissions [169, 170]. Consequently, the costs related to materials and operational expenses in PD can differ markedly from those of traditional methods. When factoring in environmental impact, the overall cost of PD may be significant. Table 2 outlines the costs and estimated energy consumption for various conventional desalination technologies.
| Process | Scalability: water production (m3 day–1) | Water treatment | Production cost (USD/m3) | Electricity consumption (kWh/m3) |
|---|---|---|---|---|
| MSF | 23,000–528,000 | Desalination | — | 2.50–5.00 |
| MED | 91,000–320,000 | 0.56–1.75 | 2.00–5.00 | |
| 12,000–55,000 | 0.52–1.01 | |||
| <100 | 2.00–8.00 | |||
| VC | 30,000 | 0.87–0.95 | 1.60–12.00 | |
| 1000 | 2.00–2.60 | |||
| RO | 100,000–320,000 | 0.45–0.66 | 4.00–6.00 | |
| 15,000–60,000 | 0.48–1.62 | |||
| 1000–4800 | 0.70–1.72 | |||
| RO | 40,000 (large capacity) | Waste | 0.26–0.54 | 1.50–2.50 |
| 20–1200 (medium) | 0.78–1.33 | |||
| Very small capacity | 0.56–12.99 | |||
| ED | Large capacity | 0.60 | 2.60–5.50 | |
| Small capacity | 1.05 | |||
| Photothermal evaporation ∗ | 0.01–0.22 | Desalination/waste | — | — |
- ∗For photothermal evaporation (water production per square meter), the technology readiness levels (TRLs) are not yet mature enough to tentatively evaluate certain parameters.
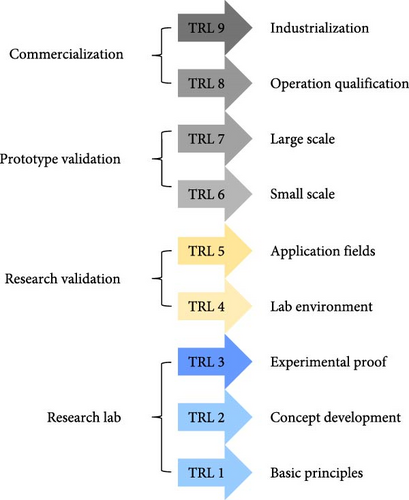
Few studies have examined the preliminary costs of steam generation through photothermal conversion. It has been observed that the cost of freshwater production is lower for conventional methods compared to photothermal techniques, primarily due to the higher maturity and readiness of conventional technologies. The technology readiness levels (TRLs) model is widely used across industries and research domains to standardize the assessment of technological development [172–174]. This model enables researchers, engineers, and policymakers to evaluate the feasibility of innovations, mitigate developmental risks, and allocate resources more efficiently. A comprehensive understanding of TRLs is essential for bridging the gap between theoretical research and commercially viable solutions. The TRL framework is a standardized metric used to assess the maturity of specific technologies or processes, ranging from levels 1–9 [175]. TRL 1 represents the observation of basic scientific principles, while TRL 9 signifies a fully operational system validated in real–world conditions (see Figure 25a).
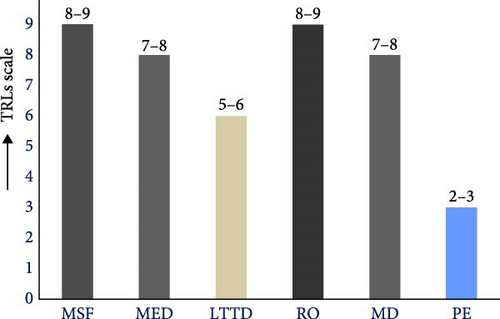
Currently, the TRLs for PD are estimated to be between levels 2 and 3, reflecting its early stage of research and development (see Figure 25b). In contrast, conventional desalination technologies have achieved higher TRLs due to decades of optimization. As such, the maturity of PD technology is not yet sufficient to provide an accurate estimate of operational costs for freshwater production. A study by Ibrahim et al. [176] reported that the cost of freshwater production using photothermal materials ranges from 0.05 to 0.09 USD/L. This study involved the preparation of photothermal materials made from PVA, cellulose acetate (CA), glutaraldehyde (GA), SA, and GO, with an average thickness of 5.0 × 10–3 m. The samples were irradiated at an intensity of 1 sun for 30 days, with 12 h of daily exposure. The production costs were found to be influenced by the choice of chemicals and the sample preparation methods.
5. Summary and Future Research Perspectives
The key features influencing the performance of photothermal evaporation systems, including evaporation rate and energy conversion efficiency, depend on material selection and system optimization factors such as wettability, thermal conductivity, light absorption, durability, and minimal salt accumulation (Table 3). The design principles for photothermal materials focus on creating hot spots within the materials at the liquid–air interface and facilitating nonradiative thermal energy generation through two primary mechanisms: (i) the introduction of defects and (ii) the reduction of the bandgap, which enhances the plasmonic effects of metals. Various factors, including particle size, shape, dielectric environment, and material composition, can be tuned to optimize these effects. Improvements in light absorption efficiency can be achieved by increasing crystallinity, designing hierarchical porous nanostructures, and incorporating nanomaterials or organic materials. Salt resistance, essential for maintaining stable operational efficiency, can be attained by forming a leaning structure, creating thermal gradients, designing vertically aligned pore morphology, and establishing a Donnan effect within the materials. Furthermore, it is crucial to reduce thermal conductivity through defect engineering, vacancy creation, and enhancing floatability by lowering density while increasing porosity. Increasing wettability through surface roughness and hydrophilicity can also significantly improve the performance properties of photothermal materials.
| Materials | Applications | CA/WT | A (%) | TC (W m–1 k–1) | ER (kg m–2 h–1) | ECE | Ref. |
|---|---|---|---|---|---|---|---|
| RGO–CF | Desalination | 0.34 s | 100.00 | 0.07 | 1.62 | 94.00 | [177] |
| CP@PVA | Desalination + sewage + electricity generation (PD 1.04 W m–2) | 63.80 | 94.00 | 0.34 | 2.96 | 98.80 | [64] |
| CB/PAN//PVDF | Desalination | 23.00° | 98.60 | 0.08 | 1.20 | 82.00 | [115] |
| CNTs/PVA (CPGs) | Desalination | — | 95.00 | 0.43 | 2.06 | 90.50 | [178] |
| CU9S8/PVDFM | Vapor Generation | 75.00° | 91.70 | — | 1.17 | 80.20 | [41] |
| PQC | Desalination | 0° | 100.00 | — | 2.04 | 98.60 | [23] |
| CCFR | Desalination | 0.02 s | 91.70 | 0.03 | 5.62 | 89.30 | [99] |
| CNFA | Desalination | 0.10 s | 98.00 | 0.62 | 1.50 | 85.40 | [160] |
| cl–PVA/SA/PAAS | Desalination | 59.83° | 94.60 | 0.26 | 2.00 | 89.98 | [179] |
| CPC Fabric | Desalination + electricity generation (PD 1.2 W m–2 in 4 sun) | — | 91.70 | — | 1.38 | — | [180] |
| CNT/Na–citrate | Desalination | — | 90.00 | 0.19 | 2.63 | 96.00 | [157] |
| PVA/λ–Ti3O5 hydrogel | Desalination | 19.00° | 96.40 | 0.20 | 6.09 | 95.92 | [181] |
| Cl–PVA–SA–PAAS | Desalination | 24.84° | 94.60 | 0.26 | 2.20 | — | [179] |
| PPCA | Desalination | — | 96.00 | 0.04 | 1.70 | 90.00 | [182] |
| CNT/PVA | Desalination + electricity generation (PD 0.40 W m–2) | — | 90.00 | — | 5.00 | — | [183] |
| CPB–PVA–5 | Desalination | 19.10° | 95.00 | — | 3.50 | — | [184] |
| CPB | Desalination | 31.00° | 96.10 | 0.40 | 1.23 | 85.05 | [185] |
| 3D–SCS | Desalination + electricity generation (PD 0.48 W m–2) | 0.12 s | 96.00 | 0.56 | 3.54 | 95.86 | [186] |
| CPAF | Desalination | 0° | 90.00 | 0.03 | 1.49 | 87.86 | [187] |
| MBS–HPGC/LI PI | Desalination | 81.60° | 97.00 | 0.004 | 1.34 | 83.50 | [188] |
| LIG/NaOH | Desalination | 0° | 90.00 | — | 2.41 | 86.60 | [189] |
| rGO/CS | Desalination | — | 97.20 | 0.04 | 2.02 | 115.00 | [190] |
| ACLA–C_PEI | Desalination | — | 95.60 | — | 1.27 | 85.66 | [154] |
| NPG–28 | Desalination | 2.20 s | 94.60 | — | 1.53 | 96.28 | [191] |
| MC–10 | Vapor + electricity generation (PD 0.60 W m–2) | 0.08 s | 88.80 | 0.06 | 2.24 | 81.70 | [192] |
| CB/PDA@f–SF | Desalination | 0.81 s | 94.80 | 0.04 | 1.56 | 92.70 | [193] |
| C–Daikon | Desalination | 0.03 s | 93.50 | 0.05 | 1.57 | 85.90 | [194] |
| 3D–HCE | Desalination | 0.25 s | 97.00 | — | 7.60 | 178.60 | [195] |
| EuTTA–350 | Desalination | 85.40° | — | 0.20 | 1.44 | 97.90 | [196] |
| TiN/TiO2@CC–3 | Desalination + electricity generation (PD 2.74 W m–2) | 0.01 s | 99.7 | — | 2.02 | 92.18 | [197] |
| rGO@HCF | Desalination | 0.63 s | 90.86 | — | 3.42 | 96.90 | [198] |
| 3D Ti3C2Tx/Ag@SiO2 gel | Desalination | 34.00° | 97.60 | 0.66 | 1.42 | 98.00 | [199] |
| Ag–PDA@wood | Sewage treatment + desalination | — | 96.00 | 0.40 | 1.58 | 88.60 | [200] |
| Double–layered CCS | Desalination | 21.90° | — | 0.05 | 1.76 | 91.30 | [201] |
| TiN/MoO3–x | Desalination | 0.40 s | — | 0.50 | 2.05 | 106.70 | [38] |
- Abbreviations: A: light absorption; CA, contact angle (°); ECE, energy conversion efficiency (1 sun); ER, evaporation rate (1 sun); PD, power density; TC, thermal conductivity; WT, wettability time at 0°(s).
Advancing photothermal steam generator materials necessitates the development of multifunctional, smart materials that emphasize sustainability and scalability to facilitate widespread adoption. Future research should address key challenges affecting efficiency, environmental impact, and large–scale production, paving the way for practical applications in water purification, desalination, sterilization, waste water treatments, photothermal–electric generation and off–grid systems.
Exploring different metal nanoparticles, carbon–based materials (e.g., graphene, GO, CNTs, and MXene), and MOFs can enhance light absorption across a broader spectrum. Emphasis should be placed on using eco–friendly and biodegradable materials, such as organic polymers, biochar, and cellulose–based materials, to reduce environmental impact. Engineering surface defects or creating vacancies in lattice structures can lower thermal conductivity, allowing for localized heating. Additionally, bio–inspired designs, such as those mimicking plant or water lily structures, can further improve efficiency by optimizing heat localization, water transport, and salt resistance.
Materials with hierarchical structures featuring open and closed pores can enhance light absorption through multiple scattering. Closed pores contribute to the floatability of structures without external support. Synthesizing methods significantly impact the crystal structure of materials, influencing their light absorption properties and the efficiency of heat generation at the liquid–air interface. Photothermal materials should exhibit self–floating capabilities, achievable through 3D design or buoyant supports, to maintain high performance. Ensuring continuous water flow to the heated zones is critical for maximizing evaporation rates, and materials with enhanced wettability, particularly hydrophilic materials or those with hydrophilic coatings, are well–suited for this purpose.
To prevent salt accumulation, vertical pore channels combined with semi–permeable membranes utilizing the Donnan effect may be effective. Simplifying the synthesis process and enhancing the cost–effectiveness and scalability of production are essential for broader implementation. Integrating multifunctional applications with photothermal steam generators could further enhance their effectiveness. Additionally, leveraging artificial intelligence (AI) and machine learning can accelerate the discovery of new photothermal materials by predicting material properties and optimizing designs through simulations. AI–driven real–time monitoring and optimization of solar desalination systems will enable adaptive control of material properties and configurations, ensuring optimal performance under varying environmental conditions.
The future of photothermal materials design for solar desalination is promising, with a strong focus on enhancing efficiency, scalability, and sustainability. Innovations in nanotechnology, defect engineering, bio–inspired designs, hybrid systems, AI–driven approaches, and the development of eco–friendly materials will continue to propel this field forward, providing innovative solutions to address the global water crisis.
Research should also address the correlation between floatability and performance, including the development of floatability engineering techniques at micro– to millimeter scales, or shape–related design optimizations. There is a need for a comprehensive evaluation guide based on material ratios, porosity, density, and the balance between closed and open pores.
Key principles in photothermal material design include the creation of localized hot spots through nonradiative thermal energy generation, either by introducing defects or reducing the bandgap. The effectiveness of plasmonic metals depends on several factors, including particle size, shape, dielectric environment, and material composition, which should be optimized to achieve superior performance in photothermal applications.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea (Grant RS-2024-00487321). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Grant RS-2024-00457356).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




