Recent Advances in Electrocatalytic Transesterification: Enhancing Biodiesel Synthesis for Sustainable Energy
Abstract
The depletion of fossil fuels and the escalating environmental degradation have intensified the global search for sustainable and renewable energy alternatives. Biodiesel, either used directly or blended with petrodiesel, presents a more environmentally favorable option for diesel engines, owing to its superior combustion efficiency, biodegradability, low sulfur content, high cetane number, and high flash point. However, traditional biodiesel production methods, such as acid-catalyzed transesterification, which requires significant acid consumption, and supercritical transesterification, which operates at elevated temperatures, are limited by their complexity and high production costs. In contrast, electrocatalytic transesterification represents a promising and bio-based approach for biodiesel synthesis. This method utilizes electrochemical mechanisms to drive the transesterification reaction under milder conditions, with or without the use of a catalyst, significantly enhancing reaction efficiency and selectivity. Electrocatalytic processes have demonstrated efficiency across various feedstocks, including palm oil, lemon seed oil, sunflower oil, corn oil, and alternative resources such as waste cooking oil (WCO), chicken fat, vegetable oil refinery waste, and algal oil, WCO, chitosan gel, plastic waste, and biomass-derived biochar incorporated with metal or metal–oxide nanoparticles. This review critically explores the electrocatalytic synthesis of biodiesel from a range of raw materials, emphasizing its potential for advancing sustainable energy production. Furthermore, this approach not only offers a cleaner energy solution but also contributes to environmental protection by reducing greenhouse gas emissions. The review emphasizes the critical role of electrocatalytic transesterification in advancing the development of biodiesel as a sustainable and energy-efficient alternative in addressing global energy challenges.
1. Introduction
The growing interest in sustainable and renewable energy sources is driven by the twin challenges of fossil fuel depletion and the environmental consequences of greenhouse gas emissions [1–3]. Within the range of renewable energy sources, biofuels have emerged as a promising alternative. Specifically, biodiesel has garnered significant attention due to its compatibility with existing diesel engines and its ability to reduce carbon emissions [4–6]. Moreover, bioethanol and biodiesel, when compared to other forms of biofuels, serve as viable alternatives for renewable fuel within the transportation sector [7, 8]. Biodiesel can either be utilized directly or blended with petrodiesel for use in diesel engines [9]. The combustion of biodiesel results in reduced global warming effects in comparison to traditional fossil fuels [10]. Noteworthy advantages of biodiesel include its high combustion efficiency, biodegradability, and low sulfur content as well as its high cetane number and flash point, thus, attracting significant attention from researchers for large-scale production [11]. Typically, the conventional diesel fuel exhibits a cetane number falling within the range of 40–55. Conversely, biodiesel commonly showcases an elevated cetane number, frequently varying from 50 to 70, contingent upon the feedstock material and production process. Seen as a clean energy source, biodiesel plays a role in environmental protection by minimizing the direct and indirect emission of greenhouse gases like carbon dioxide (CO2), carbon monoxide (CO), sulfur dioxide (SO2), and hydrocarbons (HCs) [12]. Biodiesel can be derived from feedstocks containing triglycerides (TGs) [13]. Primary feedstocks encompass sunflower oil, palm oil, peanut oil, soybean oil, et cetera, while secondary feedstocks include linseed oil, rubberseed oil, Jatropha curcas oil, and castor oil. Tertiary feedstocks like waste cooking oil (WCO), chicken fat, vegetable oil refinery waste, and algal oil are also utilized in biodiesel production [14–17].
Moreover, the WCO represents a notable byproduct of the global food industry. Estimations regarding the quantity of WCO generated worldwide vary, although there is a general consensus that a substantial amount is produced annually. The European Union, for example, is estimated to produce between 700,000 and 1 million tons of WCO each year. Germany, as another illustration, generates approximately 150,000 tons annually. In the United States, the production of WCO amounts to around 2.5 billion gallons (equivalent to about 9.5 million tons) annually. China stands out as one of the leading producers of WCO, with estimates ranging from 3 to 4 million tons per year. Japan, on the other hand, generates about 400,000 tons of WCO on an annual basis. As of 2022, reports indicated that roughly 3.7 billion gallons (around 14 million metric tons) of WCO were collected globally. This number is projected to more than double to approximately 31 million metric tons by 2030, primarily due to increased collection in Asia and other regions driven by the growing demand for biofuels [18]. One of the primary applications of recycled cooking oil is in the production of biodiesel, a process that involves collecting the used oil, purifying it to eliminate impurities, and then, converting it through a chemical process known as transesterification [12]. Biodiesel serves as a cleaner-burning alternative to petroleum diesel, effectively reducing harmful emissions and offering an environmentally friendly fuel choice. Apart from biodiesel, used cooking oil can be repurposed into various industrial and consumer goods. For example, it can be utilized in the production of soaps, detergents, fertilizers, and even animal feed [19, 20]. Moreover, certain specialized recycling methods enable the creation of biolubricants and other bio-based items, including cosmetics. Furthermore, in the scope of energy and fuel oils, processed, dehydrated, and filtered cooking oil can function as a burner fuel. It can also undergo further refinement to yield light fuel oils utilized in industrial contexts. The recycling procedure frequently involves stages like demineralization and distillation to refine the oil and render it suitable for these applications. Nonetheless, recycling used cooking oil presents numerous challenges, including processing equipment and the intricacy of ensuring consistent quality due to varying levels of contaminants. Furthermore, market restrictions restrict the profitability of recycled goods, while inadequate handling and storage can result in environmental and health risks.
R─COOH: carboxylic acid (FFA)
R′─OH: alcohol (methanol/ethanol)
R─COO─R′: TG (fat/oil)
R″─COO─R: ester (biodiesel)
R′─OH: glycerol.
Through the combined application of esterification and transesterification, a broader array of raw materials can be harnessed for biodiesel synthesis, enhancing the comprehensive efficiency and sustainability of the procedure. Nonetheless, esterification, a process aimed at reducing FFAs in raw materials like waste oils, frequently faces challenges associated with impurities hindering the efficiency of the reaction [25]. Moreover, the utilization of acid catalysts in this process may lead to equipment corrosion and necessitate cautious handling. Transesterification, the primary method for transforming TGs into biodiesel, is vulnerable to the presence of water and FFAs, resulting in the formation of soap that diminishes the overall yield [28, 30–32]. Furthermore, this process produces a considerable amount of glycerol by-products, presenting difficulties in disposal and utilization. Both reactions require precise conditions for optimal performance, consequently escalating energy consumption and operational intricacy. Advancements in catalyst technology, optimization of operational procedures, and the implementation of sustainable practices in feedstock management are crucial for overcoming these obstacles and improving the efficacy and viability of biodiesel production [33–36].
Additionally, research investigations into acid-catalyzed transesterification, supercritical transesterification, and acid–base-catalyzed transesterification reveal considerable obstacles. These techniques generally necessitate elevated thermal conditions and the employment of feedstocks characterized by FFA concentrations, thereby mandating significant quantities of H2SO4 for the conversion of FFAs into biodiesel. This method amplifies the complexity of the process, resulting in escalated costs, increased energy requirements, prolonged reaction durations, and more elaborate processing procedures. To overcome these challenges, research is exploring innovative strategies for biodiesel synthesis, particularly through the application of electrocatalytic processes for converting biomass-based waste oils into biodiesel [37]. Electrochemical processes offer benefits including lower temperature requirements and the flexibility to operate with or without a catalyst, making them a favorable approach for sustainable biodiesel production from biomass [38, 39]. Electrocatalysis involves the utilization of electrical energy to facilitate chemical reactions and electrocatalysts to enhance reaction efficiency and selectivity. Furthermore, electrocatalytic processes can be powered by renewable electricity sources such as solar and wind power, thereby enhancing their sustainability aspects [40]. A key challenge in advancing this technology is the extraction of reaction products, particularly the efficient separation of biodiesel from unreacted oil, glycerol, and other byproducts. Traditional separation techniques, including phase separation and washing, may prove to be inefficient and economically impractical when applied at an industrial scale. Consequently, the innovation of energy-efficient and economically viable separation methodologies, such as membrane-based separation or continuous-flow processing, has the potential to significantly improve scalability. While, electrochemical transesterification offers a promising approach for biodiesel production, the resolution of these scalability challenges through process intensification, strategies for cost reduction, and enhanced separation techniques will be crucial for its commercial feasibility. Moreover, the electrocatalytic conversion of biomass bases waste oils into biodiesel typically involves several key stages: Pretreatment of biomass to break down complex polymers like cellulose, hemicellulose, and lignin into simpler molecules, which can be achieved through mechanical, chemical, or biological methods depending on the type of biomass and desired end products [41]. The pretreated biomass is converted into tiny molecules, including simple sugars, syngas, or bio-oils, serving as intermediates that can be further processed into biodiesel. These tiny molecules undergo electrocatalytic reactions to produce biodiesel, which may include electroreduction, the reduction of CO2 or syngas to HCs or alcohols, and electrooxidation, the conversion of bio-oils into FAMEs, the key components of biodiesel [42]. The utilization of the electrocatalytic approach presents numerous advantages compared to traditional techniques. Electrocatalysts can be tailored to selectively target specific reactions, thus, potentially enhancing yield and decreasing the formation of unwanted by-products. Through the utilization of sustainable electricity sources and biomass, this approach diminishes dependency on nonrenewable resources and reduces the environmental impact of biodiesel manufacturing [42–44]. Electrochemical processes typically function under milder conditions, leading to reduced energy consumption and operational expenditures.
Moreover, in the field of electrocatalytic biodiesel production, Figure 1 presents the key parameters that require monitoring, including electrocatalyst activity, current density, cell potential, feedstock composition, and reaction temperature. The electrocatalyst activity, quantified through turnover frequency, directly influences the pace of the transesterification reaction and the overall efficiency of the process. Current density, which denotes the electric current per unit area of the electrode, plays a pivotal role in shaping the speed of electrochemical reactions and must be fine-tuned to strike a balance between reaction velocity and energy utilization. Cell potential, which signifies the voltage applied across the electrodes, impacts the kinetics of the reaction and the energy efficiency, demanding meticulous regulation to minimize losses due to overpotential. The composition of the feedstock, specifically the TG content and the presence of impurities, dictates the caliber and quantity of the biodiesel that is generated. The reaction temperature stands as another critical factor, exerting influence on the reaction kinetics and the stability of the catalyst. Sustaining an optimal temperature range becomes imperative in order to enhance the yield of biodiesel while reducing energy consumption and averting the deterioration of the electrocatalyst.
In electrocatalytic biodiesel synthesis, numerous research studies are constrained to validate the efficacy of the resulting biodiesel quality [45]. Fundamental parameters to analyze the conversion rate, gauging the effectiveness of the transesterification process, and the yield, denoting the volume of biodiesel acquired concerning the initial feedstock utilized [46]. Moreover, viscosity and density play a pivotal role in delineating the fluidic characteristics of the fuel and its compatibility with various engines. In addition, the acid number serves as a crucial gauge for evaluating the existence of FFAs and potential corrosive attributes, while the proportion of FFAs influences the stability and caliber of the fuel. Further, vigilant monitoring of water content is essential, as an excess of moisture can precipitate microbial production and fuel decomposition [47]. However, adherence to established standards for these parameters is paramount in generating superior-grade biodiesel that exhibits dependable performance in diesel engines, complies with regulatory directives, and mitigates environmental repercussions [48]. Table 1 outlines the properties, advantages, and key differences between traditional transesterification and esterification with the electrocatalytic process. An in-depth analysis and improvement of these factors are crucial for the advancement of electrocatalytic biodiesel technology, as will be discussed in the subsequent sections, utilizing different feedstocks in electrocatalytic biodiesel synthesis.
| Parameter | Conventional thermocatalytic transesterification | Electrochemical esterification |
|---|---|---|
| Reaction mechanism | Uses high temperature and a catalyst (acid/base) to drive transesterification | Utilizes electrochemical activation (i.e., voltage) to enhance esterification efficiency |
| Catalyst type | Homogeneous (NaOH, KOH, H2SO4) or heterogeneous (CaO, MgO, ZnO) | Involves electroactive catalysts or electrode materials |
| Operating temperature | Typically 50–70°C, which requires external heating | Can occur at room temperature to milder temperatures to electrochemical activation |
| Reaction time | Requires several hours, depending on catalyst and conditions | Potentially faster, safer due to electric field-assisted reaction kinetics |
| Energy requirement | High energy consumption due to heating and stirring | Lower energy consumption, since voltage replaces thermal activation |
| Byproducts and purification | Requires extensive washing to remove catalysts and soap formation (in base-catalyzed reactions) | Potentially fewer side reactions and easier separation of biodiesel |
| Catalyst reusability | Homogeneous catalysts are difficult to recover; heterogeneous catalysts can be reused | Electrochemical catalysts and electrodes can be reused multiple times |
| Scalability | Well-established and widely used in industry | Still in the research phase, with scalability challenges |
| Environmental impact | Generates chemical waste and requires hazardous catalyst handling | Potentially greener process with fewer hazardous chemicals |
2. Electrolysis Method for Biodiesel Production Using Ionic Liquids (ILs)
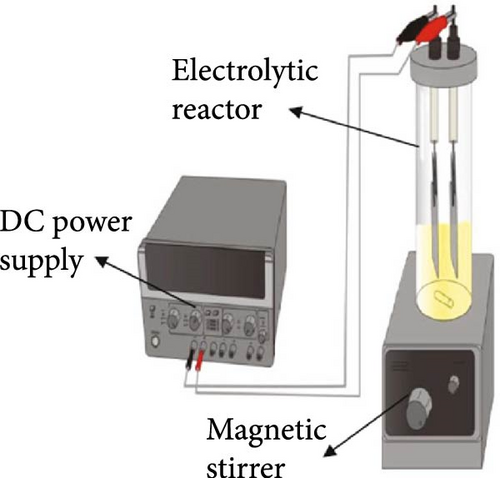
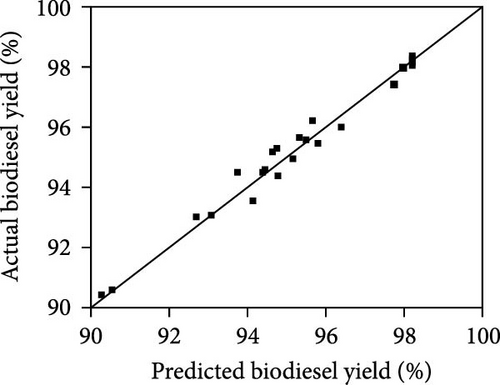
In recent times, electrolysis has garnered significant attention as a promising method for biodiesel production due to its environmentally friendly characteristics and its resistance to FFA and water content in oils. Electrolysis involves electrochemical reactions that result in the generation of OH− ions at the cathode, thereby aiding in the transesterification of oil into biodiesel. Various approaches have been developed to improve reaction efficiency in electrolysis, such as incorporating a catalyst (e.g., H2SO4, KOH, and NaOH) into the reaction mixture or utilizing diverse electrode materials. Another effective strategy to enhance reaction efficiency is by introducing additional electrolytes to boost the electrical conductivity of the reaction mixture. The use of NaCl as a supporting electrolyte is common and has been shown to accelerate the reaction rate. Despite the potential of supporting electrolytes in boosting reaction rates in electrolysis, there is a scarcity of relevant studies. Hence, it is imperative to explore and develop new supporting electrolytes for electrolysis applications. ILs have emerged as a viable option for sustainable biodiesel production. When compared to traditional homogeneous and heterogeneous catalysts, ILs exhibit superior dissolving capacity, immiscibility with organic solvents, high chemical stability, and recyclability. The utilization of ILs streamlines the reaction and purification processes while enhancing economic viability. ILs serve as catalysts for transesterification reactions and are characterized as heat-stable, nonflammable organic salt solutions containing both cations and anions, making them ideal for eco-friendly biodiesel production. The cations and anions present in ILs indirectly impact physicochemical properties such as viscosity, melting point, density, conductivity, and refractive index. ILs are categorized into acid ILs (AILs) and basic ILs (BILs) based on their physicochemical properties. To address the high cost, challenges in product separation, and catalyst recovery issues associated with ILs, they are often used in conjunction with conventional catalysts to enhance catalytic performance in biodiesel production. ILs play a crucial role in influencing the catalytic activity during biodiesel conversion, being liquid organic salts comprising both organic/inorganic anions and organic cations. Their application has extended beyond biodiesel production, finding use as a sustainable alternative to traditional organic solvents in various fields like material sciences, biological sciences, environmental sciences, and medicine. Notably, ILs are characterized by their high electrical conductivity and electrochemical stability, rendering them a highly promising medium or electrolyte across a variety of applications, including the electrochemical reduction of CO2. The utilization of ILs as electrolytes in water electrolysis has been proven to effectively facilitate hydrogen production. This phenomenon can be attributed to their ability to facilitate electron transfers, promote intermolecular interactions, modify surface accessibility, and regulate the local concentration of reactants on the electrode surface. Given the advantages of ILs, the present study introduces a novel electrolysis procedure for biodiesel synthesis that leverages ILs as supporting electrolytes without the need for additional catalysts. In the field of biodiesel production, ILs bring forth a variety of benefits, such as expediting reaction speeds, shortening reaction times, and minimizing the requirement for harmful substances, thereby developing a technique that is both environmentally conscious and productive. Recently, Aregawi et al. [49] demonstrated in their research study, the unveiling of a revolutionary catalyst-free electrolysis technique for biodiesel production utilizing an IL, [Emim]Cl, as a supporting electrolyte, as illustrated in Figure 2. This pioneering research obviates the necessity for conventional catalysts, thus, presenting a more environmentally friendly and effective approach to biodiesel production. The schematic representation of the electrolysis set-up for biodiesel is illustrated in Figure 2a. The determination of the optimal conditions leading to the highest biodiesel yield is calculated based on the amount of biodiesel produced relative to the initial amount of oil used in the transesterification process. This is typically expressed as a percentage. The researchers optimized the electrolysis process using response surface methodology, which involves statistical techniques to determine the optimal conditions for maximizing biodiesel yield. Equations (3)–(5) in systematically varying the parameters to find the best combination for yield improvement. The maximum biodiesel yield achieved was reported as 97.76%, which indicates that nearly all of the oil was converted into biodiesel under the optimal conditions as shown in Figure 2b. The specific conditions for achieving this yield included an electrolysis voltage of 19.42 V, an [Emim]Cl amount of 4.43% (w/w), a water content of 1.62% (w/w), a methanol to oil molar ratio of 26.38:1, and a reaction time of 1 h. The properties of the biodiesel produced were also evaluated to ensure compliance with established standards (EN and ASTM), which further supports the reliability of the yield calculations (Equations (3) and (4)) and the quality of the biodiesel conversion produced (Equation (5)).
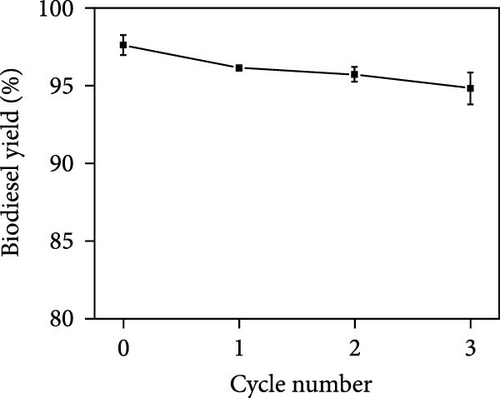
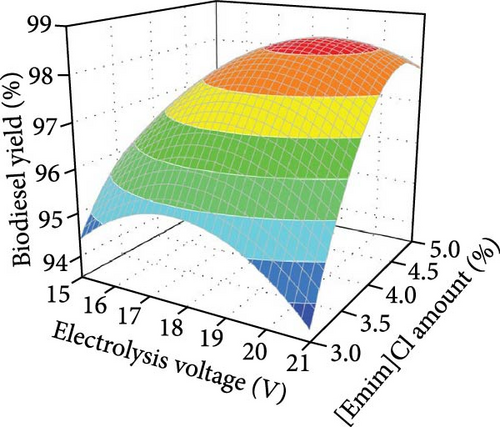
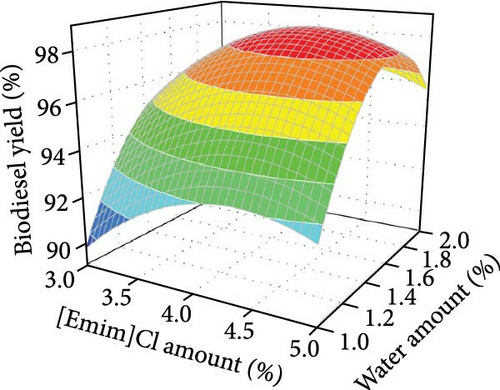
To improve commercial feasibility, the reusability of [Emim]Cl in biodiesel production was evaluated. Electrolysis under optimal conditions showed a slight decrease in biodiesel yield from 97.61% to 94.81% after three reuses (Figure 2c), likely due to [Emim]Cl loss during recovery. Supplementing [Emim]Cl can maintain reaction efficiency, as it is easily recoverable. Figure 2d illustrates the combined effects of electrolysis voltage and [Emim]Cl amount on biodiesel yield, with other factors held constant. At low [Emim]Cl levels, electrolysis voltage had minimal impact on biodiesel production. However, at higher [Emim]Cl concentrations, an increase in electrolysis voltage led to a significant rise in yield. Likewise, at low voltage, [Emim]Cl amount showed little effect, but at high voltage, higher [Emim]Cl levels improved biodiesel yield. This result highlights the positive impact of both electrolysis voltage and [Emim]Cl on the process, with higher voltage and [Emim]Cl increasing conductivity, current, and ion formation, ultimately enhancing the reaction rate and biodiesel yield. Figure 2e shows the combined effects of [Emim]Cl amount and water content on biodiesel yield, with other factors constant. At a given [Emim]Cl level, increasing water content enhanced biodiesel yield, as water is essential for reactions on the anode and cathode.
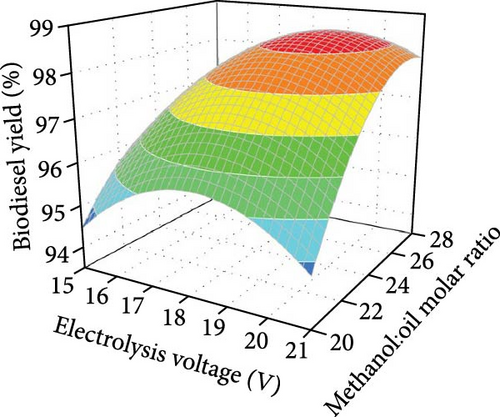
Moreover, water is electrolyzed to produce OH− ions, which react with methanol to form CH3O−, facilitating the transesterification reaction (Figure 2a). Figure 2f illustrates the combined effects of electrolysis voltage and methanol-to-oil molar ratio on biodiesel yield, with other factors constant. At any electrolysis voltage, biodiesel yield increased to a peak before stabilizing as the methanol-to-oil ratio increased. This is due to transesterification being an equilibrium reaction, where a higher methanol level drives the reaction towards biodiesel production. This phenomenon underscores the sustainability and cost efficiency of the electrolysis procedure, thereby contributing to the advancement of more sustainable approaches to biodiesel manufacturing.
3. Electrochemical Transforming Oils Into Biodiesel
The electrochemical production of biodiesel from oils signifies a promising progression in sustainable energy technology, providing an effective and eco-friendly substitute to traditional chemical approaches. This method harnesses electrochemical processes to convert TGs located in oils into FAMEs, which form the fundamental building blocks of biodiesel. The typical procedure entails the use of an electrochemical cell where the oil undergoes transesterification, a process facilitated by an electric current instead of conventional chemical catalysts like NaOH or KOH. This technique not only simplifies the recovery of catalysts but also diminishes the formation of soap, a typical side product in conventional biodiesel production. The benefits of electrochemical synthesis encompass decreased energy consumption and the possibility of local production, leading to substantial reductions in transportation and logistical expenses. Additionally, this approach can be adjusted to function at room temperatures and pressures, further amplifying its energy efficiency. The utilization of sustainable sources of electricity, such as solar or wind power, can heighten the overall sustainability of the entire process, aligning with global endeavors to minimize carbon footprints and reduce reliance on fossil fuels. Research studies in this field have showcased comparable, biodiesel yields through electrochemical routes in comparison to traditional methods. Moreover, the adaptability of this strategy in utilizing different raw materials, including used cooking oils, which are often more complex to process due to impurities. This flexibility not only widens the array of viable feedstocks but also contributes to initiatives in waste management and recycling. As this technology advances, the electrochemical synthesis of biodiesel could have a pivotal role in the shift towards more sustainable energy frameworks.
3.1. Electrocatalytic Biodiesel Synthesis Using Palm Oil
Electrocatalytic biodiesel production using palm oil involves utilizing electrolysis to enhance the esterification–transesterification process. Studies have shown that electrolysis with platinum electrodes can significantly impact biodiesel yield and physicochemical properties, with the highest yield achieved at 6 V [50]. Additionally, the removal of water during transesterification using electrodes can influence fuel characteristics, such as kinematic viscosity and water content, with double-pair electrodes being more effective in reducing acid value and water content compared to single-pair electrodes [51]. Furthermore, the synthesis of biodiesel from palm oil using a magnetic solid base catalyst has demonstrated high conversion rates above 92% and properties meeting standard requirements [52]. Electrocoagulation techniques have also been explored for the purification of biodiesel, resulting in efficient glycerol removal and high purity levels of up to 98.89% [53]. These findings collectively highlight the potential of electrocatalytic methods in enhancing biodiesel production from palm oil.
Plasma electrocatalysis offers a promising approach for more efficient and sustainable biodiesel production. Istadi et al. [54] elucidated the reaction mechanism observed in the plasma electrocatalysis system for the production of biodiesel from palm oil as nonthermal pyrolysis rather than transesterification. This determination was made utilizing gas chromatography–mass spectrometer (GC–MS) and Fourier transform infra-red (FT-IR) analyses of the biodiesel products over a period of time. The plasma electrocatalysis system exhibited promising outcomes, necessitating a brief reaction duration, absence of catalyst, absence of soap formation, and absence of glycerol by-products. It managed to attain a FAME yield of 75.65% within a span of 120 s, in addition to generating other substances such as alkynes, alkanes, esters, carboxylic acid, and aldehydes. The research underscored the complexity of controlling the reaction mechanisms within the plasma electrocatalysis system due to the presence of highly energetic electrons. Further exploration is imperative to devise strategies for selectively regulating the reaction mechanisms within this system. The plasma electrocatalysis procedure triggered diverse reactions due to the interaction of energetic electrons with liquid bulk molecules, resulting in excitation, dissociation, ionization, and the formation of atoms and metastable compounds. This process yielded a blend of compounds with varied compositions, thereby showcasing the distinctive characteristics of plasma reactions. Recently, Junior et al. [55] conducted a study aimed at synthesizing biodiesel from palm oil through the utilization of cathodic plasma electrolysis. The raw materials employed in this process included palm oil, methanol, and KOH catalyst. A remarkable biodiesel yield of 98.76% was obtained, with optimal conditions being a cathode depth of 3.5 cm and an oil:methanol molar ratio of 1:24. Notably, the lowest specific energy consumption was 605 J/mL, achieved by maintaining a cathode depth of 3.5 cm along with an oil:methanol molar ratio of 1:12. Their findings emphasize the high process efficiency achieved through cathodic plasma electrolysis, highlighting its effectiveness in biodiesel synthesis. The research demonstrates that cathodic plasma electrolysis is a promising approach for efficient biodiesel production from palm oil, showcasing high yields and minimal energy consumption rates.
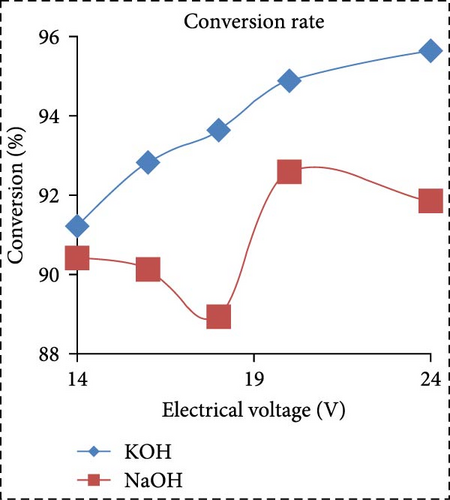
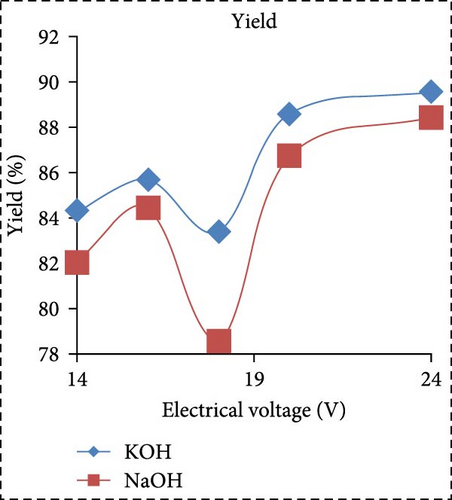
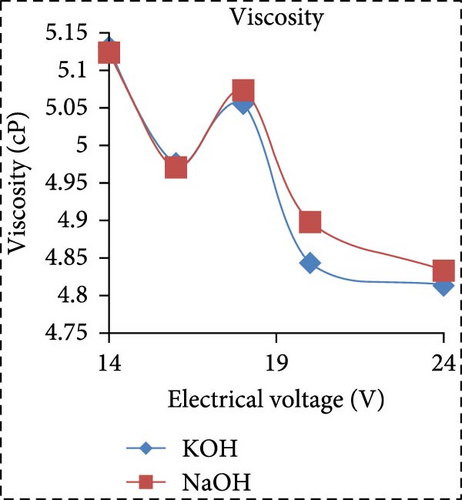
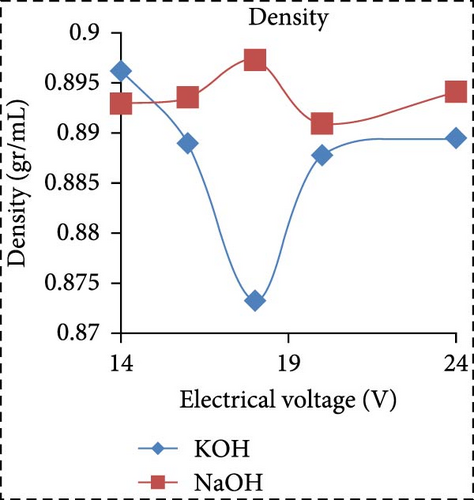
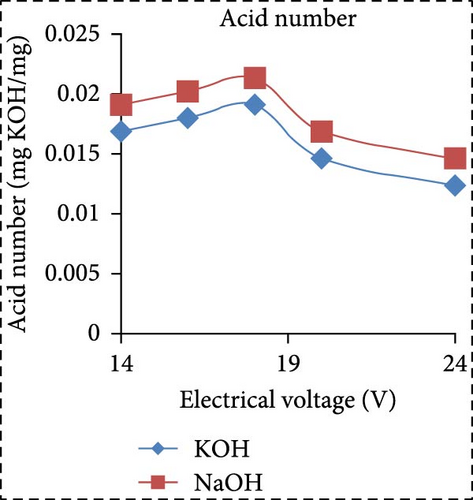
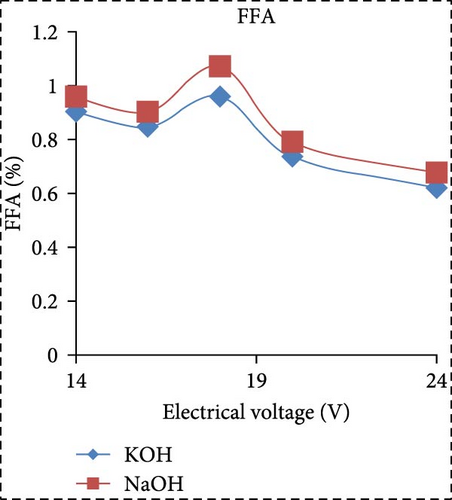
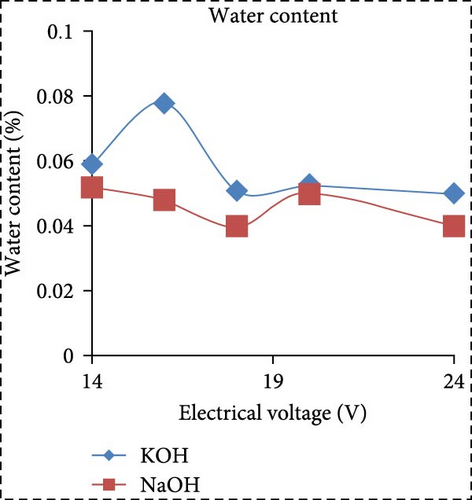
In a separate study, Saksono et al. [56] explored biodiesel synthesis using contact glow discharge electrolysis (GCDE), employing a blend of crude palm oil (CPO) and methanol at a molar ratio of 1:24, in combination with NaOH and KOH catalysts at concentration of 1 wt%. The production of biodiesel was facilitated through transesterification initiated by radical methoxide, with superior outcomes observed with KOH compared to NaOH in terms of biodiesel yield and energy consumption. The optimal biodiesel yield of 92% was achieved after a 30-min synthesis duration utilizing 1 wt% KOH, with an energy consumption of 1.32 kJ/mL. Furthermore, Rachman et al. [37] conducted research that concentrated on the application of the electrolysis technique for the synthesis of biodiesel, utilizing CPO and WCO as raw materials, as depicted in Figure 3. The electrocatalytic device for biodiesel production is presented in Figure 3a. As depicted in Figure 3b,c, the transformation rates exhibit variability and the biodiesel yield from CPO rises with the escalation of electrical voltage. The maximum transformation rate accomplished was 95.6% at a voltage of 24 V, employing KOH as a catalyst, while the highest yield was 89.55% at the same voltage and catalyst. Figure 3d illustrates that the viscosity initially declines with an increase in electrical voltage up to 16 V, but then ascends at 18 V before descending once again up to 24 V. This pattern can be ascribed to the heightened tension, which boosts the evaporation mechanism in the solution blend, consequently enhancing the viscosity of the product. The minimum viscosity documented was 4.77 cSt for biodiesel synthesized utilizing an 18 V configuration with NaOH as the catalyst. All biodiesel variants derived from CPO conformed to the kinematic viscosity criteria of SNI standard 7182:2015, which spans from 2.3 to 6.0 cSt. According to Figure 3e, the biodiesel density from CPO remains relatively constant with fluctuating electrical voltages, except at 18 V with KOH as the catalyst, where it registered the lowest value of 0.873 g/mL. Figure 3f exhibits the acid number of biodiesel generated from CPO. Each CPO-sourced biodiesel specimen demonstrated minimal acid numbers, with measurements ranging from 0.024684 mg KOH/g at electrical potentials of 16–24 V. These readings fall well below the SNI 7182:2015 upper limit of 0.5 mg KOH/g, signifying high-quality biodiesel. The content of FFA in the original CPO material was measured at 1.24%, as illustrated in Figure 3g. The utilization of NaOH and KOH as catalysts proved to be effective in reducing the FFA levels during the transesterification process, leading to increased biodiesel yields and conversions. Initial water content in the raw materials was relatively high, amounting to 1.33% for CPO and 0.98% for WCO. Following the electrolysis procedure, certain biodiesel samples displayed fluctuating levels of water content. The application of KOH at 16 V resulted in elevated water content, potentially attributed to inadequate heating during the purification phase. Extended heating could be considered as an alternative method to decrease water content in the final biodiesel output. Analysis through GC delineated distinguishable fatty acid compositions between CPO and WCO, exhibiting varying proportions of FFA and water content (Figure 3h). The study also encompassed biodiesel purification through evaporation to eliminate water and methanol, followed by a thorough examination utilizing GC, a pycnometer, and a viscometer to evaluate the composition, water content, and density.

3.2. Electrocatalytic Biodiesel Synthesis From Waste Amalfi Coast Lemon Seed Oil
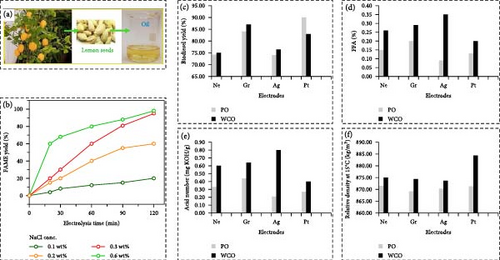
Electrocatalytic biodiesel synthesis from waste Amalfi Coast lemon seed oil presents an innovative approach to sustainable energy. Utilizing waste lemon seed oil, a byproduct of the Amalfi Coast’s lemon industry, this method harnesses electrochemical reactions to convert TGs into FAMEs, the primary components of biodiesel. This process is facilitated by electrocatalysts, which enhance the efficiency and selectivity of the transesterification reaction under mild conditions. Utilizing waste lemon seed oil not only enhances the value of a typically discarded byproduct but also supports waste management strategies and promotes circular economy practices. Additionally, the electrocatalytic approach reduces the need for traditional chemical catalysts, minimizing environmental impact and simplifying purification. Recently, Sarno and Ponticorvo [43] introduced an innovative electrocatalytic approach utilizing a PtIrRu nanocatalyst for the production of biodiesel from lemon seed oil as schematically illustrated in Figure 4a. This electrocatalytic technique functions without the use of harmful cosolvents or extra-active dissolved substances, resulting in the formation of methyl esters. Various parameters such as applied voltage, temperature, NaCl concentration, methanol–oil ratio, and water content were investigated to enhance the yield of methyl esters. Figure 4b depicts the FAME yield, which peaked at 89.79% with a NaCl level of 0.6 wt% within 1.5 h, and escalated to 98.20% after 2 h with an electric current of around 0.052 A. When NaCl concentration is at 0.3 wt%, the FAME yield progression is more linear, reaching up to 95.12% after 2 h with an electric current of approximately 0.034 A. Notably, the conversion rates of lemon seed oil are influenced by key process parameters like applied voltage, temperature, NaCl concentration, methanol/oil ratio, reaction duration, and water content. Their research studies reveal that a high biodiesel yield was achieved at ambient temperature and low voltage, attributed to the exceptional activity and stability demonstrated by the suggested nanoalloy. The properties of the produced biodiesel align with the standards set by European regulations.
3.3. Electrocatalytic Biodiesel Synthesis From Soybean Oil
Electrochemical production of biodiesel from soybean oil represents an emerging approach that presents numerous benefits in comparison to traditional biodiesel manufacturing processes. Soybean oil, a widely abundant and economically feasible raw material, is commonly transformed into biodiesel via a method known as transesterification. During this process, TGs undergo a reaction with alcohol in the presence of a catalyst to yield FAMEs and glycerol. In electrochemical synthesis, the function of the catalyst is performed by an electric current, which triggers the necessary chemical transformations. This technique eliminates the necessity for typical chemical catalysts like NaOH or KOH, thus, streamlining the procedure and decreasing the production of waste byproducts. The electrochemical approach operates under gentler conditions, often at room temperature and pressure, enhancing its energy efficiency and potentially lowering costs. Furthermore, this method can be sustained by renewable energy sources, further strengthening its environmental friendliness.
Darwin et al. [57] recently conducted a comprehensive investigation into the impact of diverse electrode materials on the electrolysis-assisted transesterification process of palm oil and WCO for the purposes of biodiesel synthesis. Their results indicated that the incorporation of electrocatalysts remarkably improved the yield of biodiesel. Among the various electrodes examined, graphite emerged as the most effective material for the transesterification of WCO, achieving a biodiesel yield of approximately 87%, whereas platinum was identified as the most suitable electrode for the transesterification of palm oil as shown in Figure 4c. Moreover, the electrocatalytic process was found to significantly diminish the FFA content in the resultant biodiesel, implying its viability as an efficient method for processing feedstocks characterized by elevated fatty acid and moisture levels. Within the evaluated electrode materials, the platinum electrodes enabled the production of biodiesel from WCO with the least FFA content (0.20%), in contrast to other electrode materials (0.30%–0.35%) and the noncatalyzed procedure (0.26%; Figure 4d). In addition, the acid number of biodiesel sourced from palm oil (0.21–0.44 mg KOH/g) was observed to be lower than that of biodiesel generated from WCO (0.4–0.8 mg KOH/g). Among the electrode materials investigated, silver demonstrated remarkable efficacy in reducing the acid content of palm oil biodiesel, whereas platinum proved to be the most effective electrode for diminishing the acid number in biodiesel obtained from WCO (Figure 4e). Density constitutes a vital parameter for fuel injection and combustion [58]. The research indicated that biodiesel produced from WCO exhibited a higher density (875–884 kg/m3) compared to that derived from palm oil (870 kg/m3). The integration of electrocatalysts within the transesterification process influenced the density of biodiesel, with platinum electrodes elevating the density of biodiesel from WCO to 884 kg/m3, whereas alternative electrode materials resulted in biodiesel densities within the range of 873–875 kg/m3 (Figure 4f). The introduction of electrocatalysts during the transesterification process significantly influenced the viscosity of the generated biodiesel. The biodiesel obtained from WCO using silver electrodes demonstrated the lowest viscosity (3.20 mm2/s), followed by graphite (4.0 mm2/s) and platinum (4.90 mm2/s). The superior efficiency of silver electrodes in reducing viscosity may be ascribed to their heightened reactivity and participation in redox reactions. Additionally, the application of electrocatalysts in the transesterification of palm oil effectively diminished the viscosity from 11 mm2/s to an approximate value of 2.40 mm2/s, thereby ensuring adherence to ASTM standards (1.9–6.0 mm2/s). The influence of electrocatalysts on the electrical conductivity of biodiesel demonstrated the empirical findings revealed that biodiesel synthesized from palm oil exhibited a baseline conductivity of 5000 pS/m, which escalated to 12,000 and 11,000 pS/m upon the employment of graphite and platinum electrodes, respectively. Conversely, the incorporation of silver electrodes led to a reduction in biodiesel conductivity to 4000 pS/m during the transesterification process involving palm oil. Furthermore, the application of electrocatalysts had a significant effect on the flash point of biodiesel. The biodiesel generated from WCO utilizing graphite and silver electrodes demonstrated higher flash points (120°C) when compared to that produced with platinum electrodes (110°C) or in the absence of electrocatalysts (112°C). In a similar vein, biodiesel derived from WCO with the assistance of electrocatalysts exhibited an elevated flash point (118°C) relative to the noncatalyzed process (112°C). The reported flash point measurements remained within the bounds of the ASTM and European biodiesel standards (101–130°C) [59]. Moreover, the integration of graphite and platinum electrodes within the electrolysis-assisted transesterification of both palm oil and WCO markedly improved biodiesel yield, attaining values ranging from 85% to 90%. Interestingly, the introduction of electrocatalysts substantially decreased the viscosity of biodiesel derived from palm oil (2–2.7 mm2/s) in comparison to the noncatalyzed approach (6 mm2/s). Additionally, biodiesel produced utilizing graphite and silver electrodes displayed higher flash points (>115°C) than the other samples examined (100–115°C), further substantiating the benefits of electrocatalytic transesterification for biodiesel synthesis. Moreover, this technique can facilitate the establishment of on-site biodiesel production facilities, especially in regions where soybean oil is abundant. This dispersed approach has the potential to decrease transportation expenses and the carbon footprint linked to biodiesel production. As advancements in technology continue, the electrochemical synthesis of biodiesel from soybean oil holds great promise in supporting the global transition towards more sustainable and eco-friendly energy sources.
3.4. Electrocatalytic Biodiesel Synthesis From Sunflower Oil
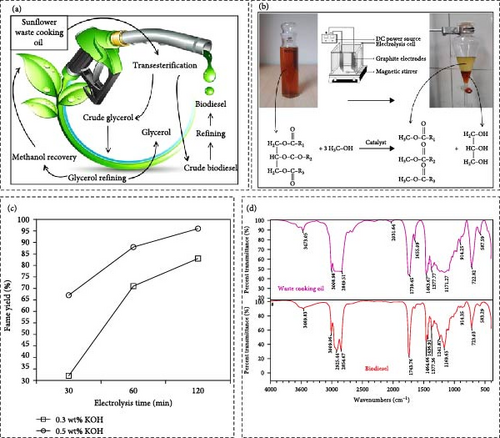
Electrocatalytic biodiesel synthesis from sunflower oil offers an innovative and sustainable method for generating renewable energy. Utilizing sunflower oil, a widely available and high-yielding feedstock, this process employs electrocatalysts to facilitate the transesterification reaction that converts TGs into FAMEs, the main components of biodiesel. Electrocatalysis enhances reaction efficiency and selectivity, often operating under mild conditions, which reduces energy consumption and simplifies the production process. This method eliminates the need for conventional chemical catalysts, thereby minimizing environmental impact and avoiding the generation of undesirable byproducts. The use of renewable electricity sources, such as solar or wind power, can further enhance the sustainability of this process. Research indicates that electrocatalytic biodiesel production from sunflower oil can achieve high yields and quality, making it a promising alternative to traditional biodiesel synthesis methods. Recently, the liquid sunflower WCO, sourced from the Oyla Company in Iran, was collected from a restaurant in Tehran. The materials used by Fereidooni et al. [60] included methanol (99.5%), KOH, sodium sulfate (97%), and acetone (99%). Biodiesel is typically produced through a transesterification process, where TGs from plant and animal-derived feedstocks are reacted with light alcohols like methanol in the presence of a catalyst, yielding FAMEs and glycerin. Notably, for every 10 kg of biodiesel produced, 1 kg of unrefined glycerin is generated as a byproduct. Figure 5a depicts the fatty acid chains, which include both single and double carbon bonds, that are central to the biodiesel synthesis process. Methanol and ethanol are commonly used alcohols in transesterification, with methanol being preferable due to its cost-effectiveness and favorable physical and chemical properties. In electrochemical transesterification, OH− ions are generated at the cathode and react with alcohol to produce nucleophile methoxide ions, which attack the TG carbon, producing biodiesel. The stoichiometric requirement for transesterification is three alcohol molecules per TG molecule. To drive the reaction towards biodiesel and glycerol production, an excess of alcohol is used. The electrolysis cell utilized for biodiesel synthesis is shown in Figure 5b. The biodiesel yield stabilizes at 96% due to the reaction’s equilibrium nature, highlighting the importance of optimizing reaction time. In this study, a catalyst amount of 0.5 wt% was identified as optimal for achieving this yield (Figure 5c). FT-IR spectroscopy was employed to identify the main functional groups in both the optimum biodiesel sample and its parent WCO, revealing similar spectra with minor changes due to the transesterification process (Figure 5d). Utilizing WCO and an inexpensive KOH catalyst in the electrolysis process resulted in high biodiesel yields. Such innovative methods reduce production costs and improve the environmental quality of the biodiesel produced. Given that raw material costs constitute a significant portion of biodiesel production expenses, using WCO helps mitigate these costs. The primary advantage of this method is that it allows for the production of biodiesel from WCO with high fatty acid content under ambient temperature and environmental conditions. This approach supports the development of a circular economy by utilizing renewable resources and promoting cleaner energy production.
3.5. Electrocatalytic Biodiesel Synthesis From Corn Oil
Electrocatalytic biodiesel synthesis utilizing corn oil demonstrates a promising strategy for sustainable energy. This technique exploits electrochemical catalysts to improve the transesterification process, leading to a more efficient conversion of corn oil into biodiesel compared to conventional methods.
The methodology involves the application of an electric current to a catalyst, thereby facilitating the chemical reaction between corn oil and alcohol (typically methanol), resulting in the production of biodiesel and glycerol as a secondary product. Most importantly, electrocatalysis has the potential to significantly decrease reaction time and operational temperatures, thus, enhancing the energy efficiency and cost-effectiveness of the process. However, this approach has the ability to reduce the necessity for excessive chemical catalysts, thereby mitigating the environmental impact. Corn oil, as a renewable and easily accessible feedstock, further strengthens the sustainability of this biodiesel production technique. The inclusion of electrocatalytic processes in biodiesel production could have a critical role in the advancement of green energy technologies and the reduction of dependency on fossil fuels. The exploration of electrocatalytic biodiesel production from corn oil has encompassed a variety of innovative methodologies. Research has emphasis into the utilization of catalyst-free electrolysis in combination with ILs such as [Emim]Cl to enhance transesterification efficiency [49]. Furthermore, the advancement of organobase functionalized magnetic nanocatalysts, for instance, Peganum harmala spice seed extract modified GO-CuFe2O4, has exhibited promising outcomes in biodiesel synthesis from corn oil [61]. Additionally, the application of mixed oxide nanocatalysts like ZrO2–SrO2 and ZrO2–CuO has displayed efficient catalytic performance in biodiesel production from corn oil, underscoring the significance of optimal molar ratios and reaction conditions [62].
Interestingly, the electrocatalytic biodiesel production was exemplified by Guan and Kusakabe [63] utilizing corn oil alongside reagent-grade, dehydrated methanol, dehydrated tetrahydrofuran (THF), and sodium chloride. The WCO originated from a biodiesel production facility. As depicted in Figure 6a, an electrolytic cell comprises two Pt plate electrodes (20 mm × 20 mm) separated by a 12 mm distance. The electrolysis cell was filled with a 30 mL reaction mixture consisting of methanol, oil, THF, water, and NaCl as a supporting electrolyte. During the electrolysis process, hydroxide ions are continuously generated at the cathode and may promptly interact with adjacent methanol, leading to the transesterification reaction occurring in the methanol phase. Consequently, transesterification predominantly advanced with the concentrated hydroxide ion proximal to the cathode. Meanwhile, the FAME yield rose with the escalation in the electrolysis voltage. Figure 6b demonstrated that the FAME yield achieved 88.7%, even with a reduction in the electrolysis voltage to 9.5 V from 18.6 V in the presence of 0.56 wt% NaCl. Their results collectively emphasize the array of strategies and materials deployed to achieve sustainable and environmentally friendly biodiesel production from corn oil through electrocatalytic processes.
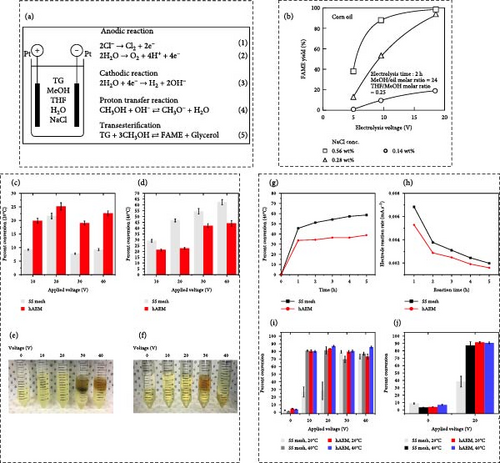
3.6. Electrocatalytic Biodiesel Synthesis From Canola Oil
Electrocatalytic biodiesel production from canola oil epitomizes a forward-thinking strategy within the field of renewable energy. This innovative technique involves the utilization of an electric current alongside a specialized catalyst to expedite the transesterification process. Moreover, the process entails the conversion of canola oil into biodiesel and glycerol through its reaction with methanol. The integration of electrocatalysis in this chemical transformation significantly boosts efficiency, thereby markedly reducing both the duration of the reaction and the amount of energy consumed in comparison to conventional approaches. Moreover, the electrocatalytic procedure functions at lower temperatures and pressures, rendering it more energy-efficient and cost-effective. Importantly, this method diminishes the necessity for chemical catalysts, consequently mitigating the environmental implications linked to their manufacturing and disposal. Due to its abundant presence and high oil content, canola oil emerges as a prime candidate for biodiesel production. Opting for canola oil, not only is a sustainable source of raw material guaranteed but also a strengthening of agricultural sectors and a decrease in the dependency on nonrenewable energy sources. The benefits of electrocatalytic biodiesel production transcend environmental advantages, as the technique can be meticulously regulated and scaled to suit both small-scale and industrial settings. Furthermore, the generation of biodiesel from canola oil holds the potential to safeguard energy security by broadening the array of fuel sources and reducing the reliance on petroleum-derived fuels.
Allioux et al. [64] conducted a study concentrating on the electrocatalytic conversion of canola oil into biodiesel utilizing various electrode materials and reaction conditions. The electrocatalytic conversion of canola oil within a methanolic reaction blend was analyzed under differing cell voltages at temperatures of either 20 or 40°C. The percentage of canola oil converted into methyl esters following 1 h of reaction time is illustrated in Figure 6c,d. Figure 6c,d reveal that at 10 V, the conversion rate was 19.9% ± 0.1% at 20°C and 21.2% ± 0.9% at 40°C when employing the hAEM electrode. Similarly, at a cell voltage of 20 V, the conversion rates at 20 or 40°C were analogous. The emergence of a brownish and turbid phase, as depicted in the images in Figure 6e,f, signified the occurrence of side reactions like hydrogenation and saponification at higher voltages. Hence, the electrocatalytic conversion of canola oil was more pronounced at 40°C when utilizing the plain SS mesh electrode. The conversion rate of canola oil was subsequently examined in relation to reaction duration at 20 V and 40°C for both the SS mesh and hAEM electrodes (Figure 6g). Figure 6h showcases the electrode reaction rates in milliamperes per second corresponding to the reaction duration.
The quantitative conversion rates of canola oil as a function of applied voltage and reaction temperature in methanolic and ethanolic reaction blends are explained in Figure 6i,j, respectively. Using the hAEM electrode, the conversion rate was found to be consistent at both 20 and 40°C, covering around 80% across all cell voltages. Subsequent investigations and advancements in the fields of electrocatalytic biodiesel synthesis from Canola oil are primarily focused on enhancing the effectiveness, durability, and specificity of the electrocatalyst materials. Furthermore, there is a focus on improving the scalability of the procedure to render it economically feasible. Through tackling these obstacles, the electrocatalytic production of biodiesel from canola oil holds the potential to transform the field of sustainable energy, offering a more environmentally friendly and enduring substitute to traditional fossil fuels. This progression could have a substantial impact on alleviating climate change and fostering sustainable energy methodologies on a global scale.
3.7. Biodiesel Synthesis From Cooking Oil and Locally Used Frying Oil (UFO)
Electrocatalytic approaches present a promising strategy for the synthesis of biodiesel from residual cooking oil, effectively addressing environmental and economic considerations. Research efforts have explored the optimization of biodiesel generation from locally sourced UFO via electrocatalysis as investigated by Aulia [65]. Various factors such as electric potentials, methanol-to-oil molar ratios, concentrations of KOH catalyst, and duration of electrolysis were addressed to assess their impact on biodiesel output. The maximum biodiesel efficiency attained was 95.3%, under specific conditions utilizing 30 V potential, 6:1 methanol-to-oil molar ratio, 1% w/w KOH catalyst concentration, and 120 min of electrolysis. A regression model was formulated to project the optimal parameters, resulting in a slightly increased biodiesel yield of 95.54% at 24.4 V potential, 5.8:1 methanol-to-oil molar ratio, 1% w/w KOH catalyst concentration, and 120 min of electrolysis. The economic viability of biodiesel production from local UFO was also verified, indicating a monthly net profit of IDR 738,000 for small-scale enterprises based on basic economic evaluations [65]. However, the research showcased the potential of electrocatalytic techniques in biodiesel synthesis from local UFO and emphasized the economic feasibility of such processes in small-scale setups. Shoelarta et al. [66] directed their attention towards employing plasma electrolysis for the conversion of WCO into biodiesel, examining various voltage ranges (400–500 V) and reactants (methanol and ethanol) to assess their influence on biodiesel output and attributes. The outcomes revealed that at 500 V using methanol as the reactant, the biodiesel yield stood at 85.73%, in compliance with the SNI 7128:2015 standard. The biodiesel properties encompassed a density of 888.36 Kg/m3, viscosity of 5.79 cSt, acid number of 0.39 mg KOH/g, ester content of 97.56%, and flash point of 117°C. The singular deviation observed was the water content at 0.373%. The energy demand specific to the synthesis process under these conditions was computed to be 1758.031 J/mL.
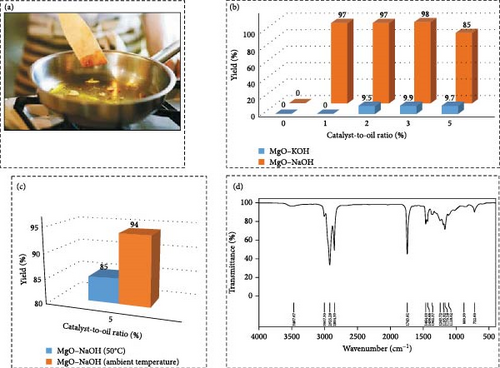
Rafati et al. [67] successfully exhibited the production of biodiesel via the electrolysis of WCO employing different heterogeneous nanocatalysts, including MgO–NaOH. Among the various catalysts examined, MgO–NaOH displayed the highest efficiency in biodiesel synthesis, suggesting its suitability for large-scale biodiesel manufacturing processes. Figure 7a illustrates the schematic representation of WCO. Figure 7b depicts the comparison of yields achieved utilizing MgO–KOH and MgO–NaOH catalysts at various catalyst-to-oil ratios (0.5, 1, 2, 3, and 5) at 50°C. An increase in the catalyst quantity from 3% to 5% results in a rise in the liberated caustic in the reaction medium, thereby inducing saponification and decreasing the yield. In comparison to MgO–NaOH, MgO–KOH demonstrates a lower yield. The electrolytic reaction generates the methoxide ion, initiating an esterification reaction and the production of biodiesel. Upon elevating the MgO–NaOH quantity to 5%, saponification occurs, leading to a reduction in the biodiesel yield. Figure 7c contrasts the yields obtained using a 5% MgO–NaOH catalyst-to-oil ratio at both ambient temperature and 50°C. It highlights that reducing the temperature reduces the transesterification reaction, consequently enhancing the yield. Furthermore, elevating the temperature during the electrolysis reaction can sometimes yield an opposing outcome due to molecular motion and reduced conductivity. Performing the reaction at ambient temperature results in a comparatively high biodiesel yield, making temperature elevation unnecessary, particularly given the increased energy demands associated with scale-up. Consequently, temperatures above 50°C were not investigated. Figure 7d displays the FT-IR spectrum of the synthesized biodiesel. The peaks at 1195 and 1436 cm−1 correspond to the bending and oscillating vibrations of the ─OCH3 group. The utilization of WCOs in this study for biodiesel synthesis was motivated by their cost-effectiveness and widespread availability. Employing homogeneous NaOH or KOH catalysts in the industrial-scale electrolysis method for biodiesel generation is viewed economically impractical.
To facilitate the production of biodiesel on an industrial scale through electrocatalysis using recycled cooking oil while mitigating adverse effects on the environment and minimizing health risks. Saputro et al. [68] examine the viability of incorporating used cooking oil as a key ingredient in the manufacturing of biodiesel emphasizing the considerable cost benefits that can be realized through the replacement of CPO with used cooking oil. Their study analyzes different approaches to biodiesel production, such as microemulsion, pyrolysis, and transesterification. Specifically, the authors highlight the merits of transesterification with a homogeneous catalyst, including its cost-effectiveness, reduced processing duration, and enhanced biodiesel output. Nonetheless, they also acknowledge the complexity of the separation process associated with this method. Through a thorough review of existing literature, the study aims to offer valuable insights into the biodiesel manufacturing process, thereby assisting in the selection of appropriate procedures for biodiesel plants, particularly those utilizing used cooking oil as a primary source.
Wicaksono et al. [69] demonstrated a study that utilized waste concrete as a catalyst for biodiesel synthesis from WCO through an electrochemical technique without requiring a cosolvent. The waste concrete exhibited superior catalytic performance compared to hydrated cement in the generation of FAME from WCO. The biodiesel manufacturing process accomplished a substantial FAME yield of up to 92.92% under ambient conditions utilizing 0.5 wt% of catalyst, 24:1 methanol/oil molar ratio, and 0.56 wt% of NaCl (wt% of oil) at a constant voltage of 9.6 V for a 2-h reaction. The results illustrate the potential of waste concrete as an economical and efficient catalyst for biodiesel production, emphasizing the viability of utilizing waste materials in sustainable energy generation. In separate research, Moeksin et al. [70] achieved the highest biodiesel yield of 38.3% at an electrolysis voltage of 12 V with the incorporation of 20 mL of methanol. This outcome highlights the efficiency of the electrolysis technique in the production of biodiesel from recycled frying oil. The lowest recorded cetane number was 80.3, which was observed at an electrolysis voltage of 9 V with the addition of 20 mL of methanol. This value surpasses the standard biodiesel cetane number of 51, indicating the necessity for further enhancements to meet regulatory specifications. The sole biodiesel density figure that conformed to the standard was 877 g/cm3, identified at an electrolysis voltage of 9 V with either 20 or 40 mL of methanol. This underlines the influential role of methanol supplementation in shaping the characteristics of the resulting biodiesel. Their study emphasizes the imperative for more extensive investigations and the incorporation of additional variables to elevate the caliber of biodiesel derived from utilized frying oil to align with national directives. Consequently, there is a clear indication of the essentiality of further exploration to refine the electrolysis process for optimal biodiesel synthesis.
4. Electrocatalytic Biodiesel Production With Chitosan Gel
Electrocatalytic biodiesel production utilizing chitosan gel represents an emerging and ecological method in the field of biofuel technology. Chitosan, a biopolymer extracted from chitin present in the exoskeletons of crustaceans, is renowned for its exceptional catalytic characteristics and compatibility with living organisms. Utilized in gel form, chitosan can effectively serve as a foundational structure for electrocatalysts, thereby enhancing the process of transesterification that transforms vegetable oils or animal fats into biodiesel. Their approach exploits the gel’s expansive surface area and porosity, which facilitate improved interactions among the oil, alcohol, and catalyst, consequently enhancing reaction efficiency and output. Furthermore, chitosan gel is biodegradable and harmless, aligning with the principles of sustainable and environmentally friendly chemistry. Integration of chitosan gel in electrocatalysis not only reduces operational expenses but also diminishes the environmental footprint of biodiesel production.
Recent research on electrocatalytic biodiesel production using chitosan-derived materials has shown promising results. Research studies on the fabrication of chitosan–polyvinyl pyrrolidone K-30 membranes as substrates for catalytic assistance in transesterification reactions, resulting in notable biodiesel conversion efficiencies [71]. Moreover, chitosan that has been altered with ethylenediamine and cross-linked using epichlorohydrin displayed effective performance in biodiesel synthesis, with optimum parameters recognized for the generation of methyl esters [72]. Additionally, electrospun nanofibers based on chitosan have been effectively employed for the adsorption of impurities from industrial glycerol, offering an environmentally friendly and effective substitute for glycerol purification [73]. Furthermore, a comprehensive treatment procedure incorporating acidification, electrochemical oxidation, and chitosan adsorption phases exhibited substantial decreases in pollutants, COD, BOD, and FOG in biodiesel effluents, guaranteeing adherence to discharge regulations [74]. These discoveries collectively underscore the potential of chitosan-based materials in augmenting the efficacy and sustainability of electrocatalytic biodiesel manufacturing processes. Research conducted by Putra et al. [42] focused on the production of biodiesel from used cooking oil through the enhancement of the electrolysis process with chitosan gel (comprising hydrogel and xerogel) as a fundamental catalyst. The yield of biodiesel was influenced by the ratio of chitosan gel to oil mass (0, 5, 10, and 15 wt%) as well as electrolysis duration (30, 60, 90, and 120 min). Methyl ester yields were assessed using GC–MS based on these operational variables. Findings indicated that biodiesel conversion rates rose with an increase in the chitosan gel-to-oil ratio, yielding 98.0% for 15 wt% hydrogel, and 94.5% for the equivalent mass ratio of chitosan xerogel. Conversely, results demonstrated that prolonged electrolysis times led to reduced biodiesel conversions, resulting in yields of 99.4%, 98.5%, 97.1%, and 98.2% for 30, 60, 90, and 120 min, respectively. Subsequent application of optimized processes, such as 30 min and 15 wt% catalyst, successfully converted biodiesel from used cooking oil, yielding 90.3% for chitosan hydrogel and 93.1% for chitosan xerogel as the foundational catalyst. Future studies are aimed at refining the properties of chitosan gel and upscaling the process to attain commercial feasibility, thereby making a substantial contribution to renewable energy solutions.
5. Electrocatalytic Biodiesel Synthesis From Plastic Wastes
Electrocatalytic biodiesel synthesis from plastic wastes embodies an innovative and sustainable strategy for combatting plastic pollution while generating renewable energy. The procedure entails the transformation of plastic wastes, specifically polyethylene (PE) and polypropylene (PP), into biodiesel via an electrocatalytic process. Through the application of an electric current and the utilization of a specialized catalyst, plastic polymers undergo degradation into smaller HCs, subsequently undergoing transesterification with alcohol to yield biodiesel. This approach confers numerous advantages; it tackles the escalating environmental issue of plastic waste, diminishes dependence on fossil fuels and presents a fresh outlet for renewable energy. Recently, Sutapa et al. [75] exemplify the utilization of PET plastic waste for the production of terephthalic acid organic linker employed in the solvothermal synthesis of Cu-1,4-benzene dicarboxylate metal–organic frameworks (Cu-BDC MOFs). Subsequently, these Cu-BDC MOFs were utilized as catalysts in the esterification reaction for the synthesis of biodiesel from coconut oil. The esterification process exhibited a conversion rate of 43.75% for FFAs, whereas the transesterification process yielded a conversion rate of 68.90%. Examination through GC–MS analysis revealed the presence of eight peaks, with the predominant areas corresponding to FAMEs. Specifically, the main constituents identified were methyl laurate (31.17%), methyl myristate (18.77%), methyl palmitate (12.50%), methyl caprylate (10.76%), methyl elaidate (10.07%), methyl caprate (7.34%), methyl stearate (5.67%), and methyl linoleate (2.26%). The quality assessment of the biodiesel entailed determining a density of 842.5 kg/m3, a viscosity of 2.9572 cSt, and a water content of 0.09%. Furthermore, the acid number was quantified at 0.2799 mg KOH/g. The electrocatalytic mechanism proves to be effective, functioning at reduced temperatures and pressures in comparison to conventional techniques, thereby curbing energy consumption and expenses. Furthermore, this strategy diminishes the necessity for harsh chemicals, rendering it more ecologically sound. Subsequent research endeavors strive to fine-tune the catalysts and reaction parameters to amplify the efficacy and scalability of this process, rendering it a feasible commercial remedy for sustainable energy generation.
6. Electrocatalytic Biodiesel Synthesis From Biochar
Electrocatalytic biodiesel synthesis utilizing biochar offers an innovative and environmentally sustainable strategy for the production of renewable energy. Biochar, a carbon-enriched substance obtained from biomass via pyrolysis, functions effectively as a catalyst support owing to its substantial surface area, porosity, and conductive properties. Within this framework, biochar is modified with electrocatalysts to enable the conversion of oils and fats into biodiesel through the process of transesterification. The utilization of electrocatalysis enhances the efficiency of the reaction through the application of an electric current, thereby expediting the conversion process and leading to heightened biodiesel yields at reduced temperatures and pressures in contrast to traditional methodologies. The incorporation of biochar not only enhances the sustainability of biodiesel manufacturing but also enhances the valorization of agricultural residues and diminishes carbon footprints. Furthermore, this technique reduces the support of harsh chemicals, aligning with the fundamental principles of green chemistry. Prospective investigations are directed towards refining the characteristics of biochar and the efficacy of catalysts to optimize efficiency and scalability, possibly metamorphosing biochar from waste material into a valuable asset for the sustainable generation of energy. Maleki et al. [45] demonstrated a straightforward one-step hydrothermal approach to synthesize MnFe2O4/biochar composite. The various stages of nanocatalyst synthesis are depicted in Figure 8a. Figure 8b illustrates the mechanism of the transesterification reaction facilitated by MnFe2O4@biochar. The initial attack of the methoxide ion on the TG molecule, attached to the catalyst surface, generates a tetrahedral intermediate. This intermediate is subsequently reconfigured to produce a mole of diglyceride anion and methyl ester. The charged anion is stabilized by the proton from the catalyst surface to form diglyceride while simultaneously renewing the catalyst. Upon the complete attack of methoxide ions on the carbonyl carbon of the three TGs, 1 mol of glycerol, and 3 mol of methyl ester are produced. The reusability of MnFe2O4@biochar nanoparticles was assessed over seven cycles under optimized operational conditions: 3.5 wt% catalyst dosage, an 11:1 methanol to edible waste oil (EWO) molar ratio, a reaction time of 50 min, and a reaction temperature of 60°C (Figure 8c). In each experiment, the MnFe2O4@biochar was magnetically separated, as shown in Figure 8d, washed with methanol to remove organic contaminants, and dried at 110°C in a hot air oven. The MnFe2O4@biochar catalyst demonstrated superior reusability and biodiesel yield compared to MnFe2O4 alone, maintaining a yield greater than 90% up to the seventh cycle. A slight reduction in yield after each cycle may be attributed to the loss of active sites on the MnFe2O4@biochar.
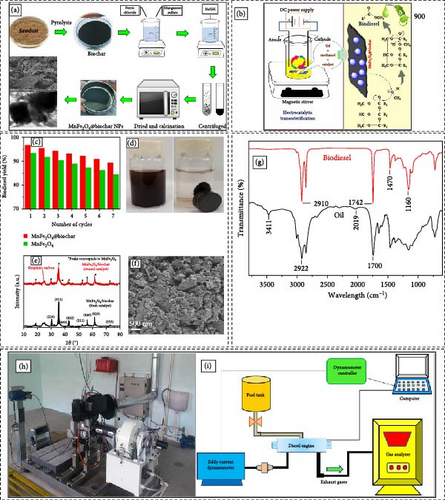
Figure 8e shows the PXRD pattern of the MnFe2O4/biochar catalyst after seven cycles, compared with the fresh catalyst. The unchanged PXRD pattern indicates the structural integrity of the catalyst. Additionally, flake-like assemblies in the MnFe2O4/biochar catalyst observed after biodiesel production (Figure 8f) demonstrate its robust nature. Approximately 99.5% of the MnFe2O4/biochar catalyst was recovered after each cycle using an external magnet (Figure 8d). The FT-IR spectra of EWO and its methyl ester (EWOME) are depicted in Figure 8g. The transition from EWO to EWOME is indicated by the disappearance of the peak at 1700 cm−1 in EWO and the presence of a peak at 1742 cm−1 in EWOME. The peak at 1743 cm−1 suggests the presence of ester, specifically the carbonyl group (C═O), indicating unsaturated groups. The absence of carboxylic acid peaks between 3000 and 3300 cm−1 (O─H stretching) reveals the lack of moisture in EWOME. Figure 8h displays the schematic of the engine used in these experiments, while Figure 8i outlines the general process of evaluating diesel engine performance. A CI diesel engine (Lombardini) was employed to study the effects of various variables on engine performance and pollutant emissions. The variables included different concentrations of MnFe2O4@biochar nanoparticles (40–120 ppm) in the fuel, various engine loads (20%–100%) and two different proportions of diesel and biodiesel (B0 and B20). The test fuels were denoted as B20-Cat40 (20% biodiesel + 80% diesel + 40 ppm MnFe2O4@biochar), B20-Cat80 (20% biodiesel + 80% diesel + 80 ppm MnFe2O4@biochar), and B20-Cat120 (20% biodiesel + 80% diesel + 120 ppm MnFe2O4@biochar). Emissions such as CO, CO2, UHC, and NOx, along with engine parameters including BSFC, BTE, and EGT, were measured to determine the optimal concentration of nanoparticles, engine loads, and fuel ratio. Hence, it can be inferred that the utilization of the MnFe2O4@sawdust-derived biochar nanocatalyst is highly advisable in industrial settings owing to its significant reusability, exceptional catalytic performance, and impressive biodiesel production. Table 2 presents a comparison of electrocatalytic esterification of WCO and plant-based oil at room temperature with traditional biodiesel esterification, which requires high temperatures. The data for both processes are provided in the table.
| Sr. no. | Oil | Catalyst | Cosolvent | Electrolyte | Biodiesel yield | Electrochemical reaction conditions | Temp | Applied voltage (V) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Waste cooking oil | NaOH and KOH | Acetone | NaOH + H2O |
|
Catalyst 1 wt%, acetone 10 wt%, methanol- to-oil molar ratio 1:7.5, distance between electrodes 1 cm, water 2.0 wt%, stirring rate 300 rpm, duration 2 h | RT | 40 V | [76] |
| 2 | Amalfi Coast waste lemon-seed oil | Absence of catalyst | Absence of solvent | NaCl | 98.2 | Oil-to-methanol molar ratio 10:1, NaCl 0.3 wt%, water 2 wt%, duration 2 h | RT | 15 V | [43] |
| 3 | Canola oil | Without addition of external catalyst | Absence of solvent | NaCl |
|
Oil-to-methanol molar ratio 1:10, NaCl 0.3 wt%, deionized water 1 wt%, duration 1 h | 40°C | 20 V | [66] |
| 4 | Waste oil | Zeolite | THF | KOH | 90 | Catalyst 2 wt%, oil-to-methanol molar ratio 1:9, THF 10 wt%, duration 4 h | RT | 10–40 V | [77] |
| 5 | Waste cooking oil | Waste concrete | Free | Methanol/NaCl | 78.51% | Reaction time 30 min | RT | 14.4 V | [78] |
| 6 | Soybean oil | CaO-900-600 | Absence of solvent | NA | 97.35 | Catalyst 20 wt%, oil-to-methanol molar ratio 1:6, duration 1 h | RT | 18.2 V | [79] |
| 7 | Used cooking oil | CaO-900-600 | Absence of solvent | NA | 98.58 | Catalyst 20 wt%, oil-to-methanol molar ratio 1:6, duration 1 h | RT | 18.2 V | [80] |
| 8 | Used cooking oil | Chitosan | Tween 80, THF | NaCl | 59.1 (without cosolvent) | Catalyst 10 wt%, duration 4 h, water 2 wt%, cosolvent- to-methanol molar ratio (100:1) | RT | 18 V | [81] |
| 9 | Waste cooking oil (WCO) | Zeolite/chitosan/KOH | Acetone | NA | 93 | Catalyst 1 wt%, oil-to-alcohol ratio 1:7, water 2 wt%, stirring rate 100 rpm, duration 3 h | RT | 40 V | [82] |
| 10 | Crude Jatropha oil | CaO | Ethanol and diethyl ether | NA | 95.8 | Catalyst ratio 0.02:1 (w/w); reaction time 133.1 min; oil/methanol molar ratio 1:5.15; stirring rate 500 rpm | 65°C | — | [83] |
| 11 | Soybean oil and poultry fat | CaO (nanocrystalline-1) | Methanol | NA | 100 | Catalyst amount 1 mmol; time 6 h; methanol 10 mL | 25°C | — | [84] |
| 12 | Fatty acid | CaO/CaN, CaO/SS | Glycerol | NA | 93 (CaO/CaN) 96 (CaO/SS) | Catalyst 8 wt%; oil/methanol ratio 1:12; time 6 h | 65°C | — | [85] |
| 13 | Karanja and Jatropha oils | Li–CaO | Hexane/ethyl acetate/acetic acid | NA | >99 | Oil/methanol molar ratio 1:12; catalyst 5 wt%; time 2 h | 65°C | — | [86] |
| 14 | Recycled cooking oil | CaO–MgO | Ester | NA | 98.95 | Oil/methanol molar ratio 1:7; catalyst 1.2 g (CaO, 0.7 g; Mgo, 0.5 g); time 6 h | 75°C | — | [87] |
| 15 | Waste frying oil | KF/CaO | Methanol | NA | 96.8 | Oil/methanol molar ratio 1:12; catalyst 4 wt%; time 2.5 h | 65°C | — | [88] |
| 16 | Soybean oil | ZrO2–C4H4O6HK (Zirconia-loaded potassium bitartrate) | Methanol | NA | 98.03 | Oil/methanol ratio 16:1; catalyst 6 wt%, time 2 h | 60°C | — | [89] |
| 17 | Waste edible oil | CoFe2O4@GO | CDCl3 | NA | 98.17% | Achieved at time of 55.75 min. | 65°C | — | [90] |
| 18 | Waste cooking oil | Absence of catalyst | THF | NaCl | >97 | Oil-to-methanol molar ratio 1:24, duration 2 h, THF/methanol molar ratio 0.25, NaCl (>1.1 < 1.2) | 65°C | — | [91] |
7. Conclusions and Future Perspectives
Recent research on electrocatalytic biodiesel synthesis from waste oil and biochar highlights significant advancements in sustainable energy technologies. Conversion of bio-oils to FAMEs emerges as a promising technique for biodiesel manufacturing, utilizing diverse factors such as electrolysis voltage, duration, cosolvent quantity, water content, and oil-to-methanol molar ratio. The enhancement of electrodes with high catalytic activity is essential, with continuous exploration of novel materials. Particularly, nanostructured materials have exhibited substantial results due to their distinctive mechanical, electrical, and optical characteristics. The utilization of ILs in biodiesel creation has broadened the spectrum of raw materials, encompassing palm oil, lemon seed oil, soybean oil, sunflower oil, corn oil, canola oil, WCO, chitosan gel, plastic waste, and biomass-derived biochar incorporated with metal or metal–oxide nanoparticles. These materials, characterized by lower toxicity and eco-friendliness, hold promise for transforming biodiesel production by enhancing its physical and chemical attributes. The current emphasis is on researching electrocatalytic processing at ambient temperature and determining optimal electrode materials.
Nevertheless, despite these benefits, various technical and economic obstacles impede widespread implementation. Tackling these issues is vital for enhancing efficiency, decreasing expenditures, and ensuring sustainability. A significant challenge resides in the advancement of efficient and stable electrocatalysts. Numerous catalysts exhibit degradation over time, possess low selectivity, or depend on costly noble metals. The formulation of economical, durable alternatives is imperative for scalability. Another pivotal consideration is the dependence on relatively expensive components, such as ILs, which function as catalysts or cosolvents within the electrochemical transesterification process. While ILs provide advantages including heightened catalytic efficiency, minimal volatility, and recyclability, their extensive utilization is constrained by elevated synthesis costs and possible environmental implications. To mitigate this issue, research efforts should concentrate on the development of low-cost and biodegradable IL alternatives, the optimization of catalyst recovery and recycling methodologies, and the investigation of hybrid catalytic systems that minimize IL usage without sacrificing efficiency. Another critical concern involves the separation and purification of biodiesel from unreacted oil, glycerol, and other byproducts. Existing separation methodologies may be energy-intensive and ineffective, thereby increasing production costs. Innovative purification techniques, such as electro-assisted separation or membrane filtration, require further exploration and refinement. Furthermore, feedstock variability, particularly in WCO, presents difficulties due to impurities such as FFAs and moisture. These contaminants adversely influence reaction efficiency and catalyst lifespan, thereby necessitating effective pretreatment methods. Research endeavors should prioritize the development of cost-effective electrocatalysts, the enhancement of separation processes, and the improvement of pretreatment techniques. Progressive electrocatalytic optimization strategies can increase efficiency, while the utilization of renewable energy sources (like solar or wind) can support sustainability. By addressing these issues, electrocatalytic biodiesel production has the potential to evolve into a viable and environmentally sustainable alternative, thereby contributing to a more sustainable future in biofuel production.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Masimukku Srinivaas: conceptualization, methodology, writing – review and editing. Yen-Yi Lee: funding acquisition. Noora Al-Qahtani: resources supervision. Manohar Reddy Busireedy: formal analysis. Brindha Devi S.: data curation. Ramyakrishna Pothu: formal analysis. Fatemah M. Barakat: validation. Rajender Boddula: conceptualization, methodology, writing – review and editing. Samuel Lalthazuala Rokhum: resources supervision. Chien-Hsing Wu: conceptualization, methodology. Bo-Wun Huang: formal analysis. I-Cheng Li: data curation. Guo-Ping Chang-Chien: visualization, investigation.
Funding
This research was funded by the Center for Environment Toxin and Emerging Contaminant Research, the Center for Conservation and Research, the Ministry of Education Taiwan (ROC), under the Higher Education Sprout Project (2023–2028), and Cheng Shiu University, Taiwan (ROC). Open access funding provided by Qatar National Library.
Open Research
Data Availability Statement
The data will be made available from the corresponding authors upon request.




