Probability of Tumor Lysis Syndrome in Electrochemotherapy of Large Solid Tumors: A Pilot Study
Abstract
Background: Electrochemotherapy (ECT) has emerged as a promising targeted therapy to enhance drug delivery and minimize systemic side effects in cancer patients. However, concerns about tumor lysis syndrome (TLS) and other complications persist, particularly in patients with large solid tumors.
Methods: This study evaluated the clinical outcomes, adverse effects including TLS occurrence and survival rates of ECT in 15 patients with advanced or metastatic solid tumors at Emam Khomeini Hospital from November 2023 to November 2024.
Results: The study included 15 patients (60% female, 40% male) with a median age of 55 years. Tumor types included sarcoma (20%), squamous cell carcinoma (40%), invasive ductal carcinoma (33%), and angiosarcoma (7%). Eleven patients achieved complete remission, while four showed partial response. No cases of stable disease or progression were observed. The mean tumor area reduction was 90%, with an 84% overall survival rate for 3 months. No patients developed TLS, and adverse effects were minimal, with only four patients experiencing transient acidosis post-ECT. There was no significant difference between disease-free survival (DFS) of various tumor types, while recurrent tumors had better DFS in comparison with nonrecurrent ones.
Conclusion: This study confirms ECT as an effective and safe treatment for large solid tumors, with significant tumor regression and no serious complications such as TLS. These findings support ECT as a viable alternative method for patients with primary tumors, inoperable or treatment-resistant ones.
1. Introduction
Cancer is still one of the major challenges and imposes a heavy burden on the health care system [1]. The World Health Organization (WHO)’s cancer agency estimated 20 million new cases and 9.7 million deaths in 2022 [2]. Despite impressive advances in conventional cancer therapeutic approaches, the disease has high morbidity and mortality [3]. By this opinion, state-of-the-art cancer therapy has been shifting toward targeted therapy to address the low response and high side effects of systematic treatments including gastrointestinal complications, hepatotoxicity, acute renal failure (ARF), chemotherapy-associated thrombotic microangiopathy (TMA), and tumor lysis syndrome (TLS) [4–6].
TLS, as a life-threatening condition, usually occur after antineoplastic therapy in patients with large solid tumors and high-grade hematological malignancies [7, 8]. This event results from the rapid death of a vast majority of malignant cells, which releases intracellular ions and metabolic byproducts into the bloodstream, leading to hyperkalemia, hyperuricemia, hyperphosphatemia, and hypocalcemia [9]. In this regard, rapid indiscriminate distribution of cancer cells in the body is one of the main concern in developing the new therapeutic methods such as photothermal therapy (PTT) and electrochemotherapy (ECT) [10, 11].
ECT is a novel intraoperative therapeutic method that enhances the local delivery of anticancer drugs [12]. The tumor microenvironment and abnormal blood flow, as significant obstacles to the efficacy of chemotherapy, lead to uneven drug distribution [13]. ECT has been developed with an electric pulse generator and some needle electrodes, allowing the use of lipophobic drugs with a narrow therapeutic index by permeabilizing electric pulses with appropriate amplitude and waveforms [14]. This method is utilized in the management of large deep-seated tumors and cutaneous neoplasms and the palliation of extensive skin metastases [12, 15].
Since the primary goal of targeted therapies is to affect cancer cells with more precision, ECT proved to be a feasible technological technique with well treatment effectiveness even in tumors resistant to the traditional approaches. In this study we applied ECT for patients with various types of large malignant tumors and then monitored all complications of them like acidosis and TLS occurrence.
2. Methods
2.1. Study Design and Participants
This is pilot study, which was carried out according to the STROBE checklist [16]. In this research, patients with different types of solid tumors who were candidates for ECT at the referral cancer center of Emam Khomeini hospital were recruited between November 2023 and November 2024. All the test protocols and methods were performed in accordance with the relevant guidelines and regulations of the institutional review board of Tehran University of Medical Science which approved the study (IR. TUMS.IKHC. REC.1401.125). The written informed consent was obtained from all the patients.
The inclusion criteria were patients older than 18 years old with measurable solid tumors. Patients with clinically demonstrated arrhythmia, epilepsy, lung cystic fibrosis, sepsis, allergy to bleomycin, severe kidney dysfunctions, patients with incomplete preclinical data, and patients with previous administration to bleomycin exceeding the maximum cumulative dose of 400,000 IU were excluded. A multidisciplinary oncology team consisting of pathologists, surgeons, oncologists, radiologists, and dermatologists was involved in the selection of the 15 patients included in this study. All the patients have complete demographic information and preclinical evaluations before and after intervention such as tumor histopathology and staging, tumor dimension (measured manually with calipers), a complete hematological profile (complete blood count, biochemical profile, inflammatory markers, and electrolytes), venous blood gas (VBG), urine analysis, and diagnostic imaging (magnified resonance imaging and/or total body computed tomography). The tumor site was classified as head and neck for tumors involved the parotid glands or tongue, soft tissue tumors for the one located in the upper or lower limbs, and upper trunk tumors for the one involved breasts and chest wall. The patients with recurrence cancers had been treated by routine therapeutic approaches including surgery, chemotherapy, and radiotherapy previously. Each patient was following up continuously and at each visit the local response, complications, and toxicity were monitored. The follow up protocol for every patient is demonstrated in Table 1. The RECIST criteria was utilized for assessing the local response after the ECT treatment protocol at 1 month follow up: progressive disease (PD) ≥ 20% increase in tumor diameter, stable disease (SD) < 30% reduction of tumor diameter or < 20% increase of tumor diameter, partial remission (PR) as ≥ 30% reduction in tumor diameter, and complete remission (CR) as total resolution of the tumor [17]. A grading system described by Lowe et al. for evaluation of toxicity after the intervention was used at the first week of clinical follow-up [18]. The local toxicity after ECT is typically graded on a scale, often from 0 to 5, where 0 represents no toxicity and 5 represents the highest level of toxicity. We have calculated the median survival time (MST) from the first ECT session to the death of the patient or to the end of the study (November 2024). The progression free survival (PFS) was accounted from the time of the first ECT session to the first recurrence or to the death of the patient. In alive patients with no recurrence, we alternatively right censored the length of the PFS to the end of the study.
| Days | 0 | 3 | 7 | 30 | 60 | 90 | 120 | 150 | 210 | 240 |
|---|---|---|---|---|---|---|---|---|---|---|
| Session | 1st | — | — | 2nd (optional) | — | 3rd (optional) | — | — | — | — |
| Follow-up | × | × | × | × | × | × | X (optional) | X (optional) | X (optional) | X (optional) |
2.2. Electroporation Operating Procedure and Patients’ Management
In this case series, the bleomycin was administered intravenously (15,000 IU/m2 body surface area, dissolved in sterile water with a concentration of 1000 IU/mL) for 60 s, 8 min before the application of electric pulses (8 pulses with the frequency of 5 kHz and time duration of 100 μs). The permeability of the ablated cells to the bleomycin increases to 1000-fold further than conventional drug injection [19]. The ECT procedure was carried out based on the ESOPE guidelines [20]. The electroporation device (OncoPore, Iran) (Figure 1) was applied for the treatment and procedures were performed under general anesthesia. Two types of needle electrodes utilized with different settings for the electrical parameters mainly based on the size and localization of the tumor. The 5 patients with heterogenous tumor morphology or tumor depth more than 3 cm, were treated with a decorated single electrode array, a specialized configuration for deep and irregularly shaped tumors, (electrical parameters: A train of 8 pulses for 80–150 times, frequency 5000 Hz, amplitude 1000 V, length 100 μs); and the other 10 patients with tumor depth less than 3 cm or homogenous morphology were treated with a hexagonal probe (electrical parameters: A train of 8 pulses for 80 times, frequency 5000 Hz, amplitude 600–1000 V, length 100 μs). After the intervention, all patients received 24-h intravenous fluid resuscitation using isotonic saline (2-3 L/day) for hydration, antibiotic therapy (Ciprofloxacin 500 mg every 12 h, Cephalexin 500 mg every 8 h), analgesic protocol (Gabapentin 300 mg per day, Acetaminophen 500 mg every 12 h), and Allopurinol (10 mg/kg every 12 h) for 5 days. In addition, anti-inflammatory drugs were administered for the patients from the seventh day after the procedure up to 5 days (Betamethasone LA 0.3 mg/kg two times with 4 days interval, and Naproxen 5 mg/kg every 12 h per day). Furthermore, in patients who revealed toxicity-related events and necrotic tissues, symptomatic treatment was performed accompanied with debridement of the necrotic area with sterile saline solution.

2.3. Statistical Analysis
The results were analyzed by using SPSS software (ver.26). Moreover, the positive-predictable value of the study was calculated utilizing SPSS. A p value lower than 0.05 was considered statistically significant. The Kaplan–Meier method was used for MST analysis. The log-rank test was applied for differences between groups in MST.
3. Results
3.1. Patients’ Characteristics
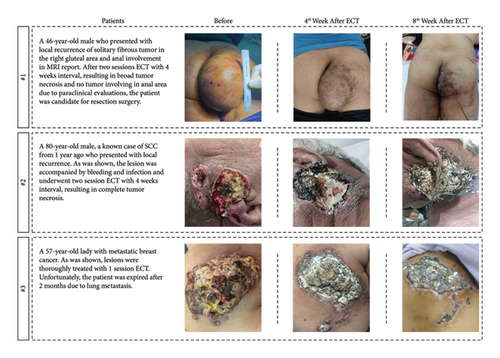
In this study, six (40%) patients were male, and the remaining nine (60%) were female. The median age was 55 years (range: 24–86 years). ECT was done for 3 patients with sarcoma of upper and lower limbs (20%), 6 patients with squamous cell carcinoma (SCC) of head and neck (40%), 5 patients with invasive ductal carcinoma (IDC) of breast (33%), and one patient with chest wall angiosarcoma. From 15 patients with advanced (11/15) or metastatic (4/15) cancer; six of them were primary and nine were recurrent. Eight patients had undergone ECT to palliate their symptoms, since others had inoperable tumors and entered the study due to surgery being possible. The patients’ characteristics, tumor histotype, tumor size, TNM staging, ECT toxicity grade, electrical parameters, ECT session numbers, remission rate (%), and RECIST criteria are shown in Table 2. Regarding the staging of the patients: Six patients were stage III, while there were four each for stages IV and II, and one were stage I. Ten patients suffered from large tumors (≥ 5 cm in the largest diameter) (77%), while the remaining five (33%) had medium ones (between 3 and 5 cm). As follows, three examples of patients who had undergone ECT are shown in Figure 2.
| N | Age | Gender | Tumor site | Tumor histotype | Size | TNM | TR | ET | Tox | ECT session | RECIST | Size reduction | PFS (days) | MST (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Male | Right gluteal area | Solitary fibrous tumor | L | T3N0M0 | N | DSA | 3 | 2 | PR | 90 | N | 90 |
| 2 | 62 | Male | Right shin | Spindle & pleomorphic sarcoma | L | T3N0M0 | N | DSA | 4 | 2 | CR | 100 | N | 120 |
| 3 | 42 | Female | Left arm | Fibromatosis | L | T3N0M0 | Y | DSA | 3 | 2 | PR | 40 | Y (90) | 210 |
| 4 | 33 | Female | Tongue | SCC | M | T2N0M0 | Y | HE | 5 | 1 | CR | 100 | N | 150 |
| 5 | 24 | Male | Tongue | SCC | L | T3N0M0 | N | HE | 4 | 2 | CR | 100 | N | 90 |
| 6 | 80 | Male | Left parotid | SCC | M | T2N1M1 | Y | HE | 4 | 2 | CR | 100 | N | 90 |
| 7 | 86 | Male | Left parotid | SCC | M | T2N0M0 | N | HE | 4 | 2 | CR | 100 | N | 90 |
| 8 | 45 | Female | Chest wall | SCC | L | T3N1M1 | Y | DSA | 5 | 2 | CR | 100 | N | 90 |
| 9 | 86 | Male | Right parotid | SCC | M | T1N0M0 | N | HE | 4 | 2 | CR | 100 | N | 90 |
| 10 | 47 | Female | Left breast | IDC | L | T4N2M2 | Y | HE | 4 | 1 | PR | 60 | N | 60 |
| 11 | 57 | Female | Left breast | IDC | L | T4N3M3 | Y | HE | 4 | 1 | CR | 100 | N | 60 |
| 12 | 66 | Female | Right chest wall | Angiosarcoma | L | T3N0M1 | Y | HE | 4 | 3 | CR | 100 | N | 240 |
| 13 | 50 | Female | Left breast | IDC | L | T4N1M2 | Y | DSA | 5 | 1 | CR | 100 | N | 90 |
| 14 | 55 | Female | Right breast | IDC | M | T2N1M1 | N | HE | 5 | 2 | CR | 100 | N | 120 |
| 15 | 47 | Female | Right breast | IDC | L | T4N2M2 | Y | HE | 4 | 1 | PR | 60 | N | 60 |
- Note: L, large tumor, > 5 cm; M, medium tumor, 2–5 cm; S, small tumor < 2 cm; Y, yes; N, no; HE, hexagonal probe; Tox, local toxicity grade; (the three patients number 10, 11, and 15 who suffered from pulmonary metastasis of breast cancer died after 60 days).
- Abbreviations: CR, complete remission; DSA, Decorated single array; ET, electrode type; IDC, invasive ductal carcinoma; MST, median survival time; PFS, progression free survival; PR, partial remission; SCC, squamous cell carcinoma; TR, tumor recurrence.
3.2. Tumor Response
In total, 15 patients with solid tumors more than 2 cm were treated. Nine patients undergone second electrochemotherapy around 30–40 days after the first session, one patient needed 3 times, and the others had received one session of treatment. The mean decrease in the area involved was 90%. Regarding the RECIST criteria parameters, 11 patients experienced complete clinical response, four patients experienced partial response, and none of them had stable disease or faced progression (Table 2).
3.3. Adverse Effects
Hematological blood test, VBG, and urine analysis were taken to assess the side effects of ECT before treatment and every week thereafter. The results are reported in Table 3. There were not any symptoms of TLS during the study in the patients. No patients had shown dehydration signs and oliguria. Two patients with tongue SCC and one patient in each other group had acidosis 24 h after the first session of ECT.
| Laboratory findings (n) | Head and neck tumor (n = 6) | Soft tissue tumor (n = 3) | Upper trunk tumor (n = 6) |
|---|---|---|---|
| Oliguria | 0 | 0 | 0 |
| Metabolic acidosis | 2 | 1 | 1 |
| ESR rising | 3 | 2 | 2 |
| CRP rising | 2 | 2 | 3 |
| Hyperuricemia | 0 | 0 | 0 |
| Hyperkalemia | 0 | 0 | 0 |
| Hyperphosphatemia | 0 | 0 | 0 |
| Hypocalcemia | 0 | 0 | 0 |
3.4. Disease FS (DFS) and Overall Survival
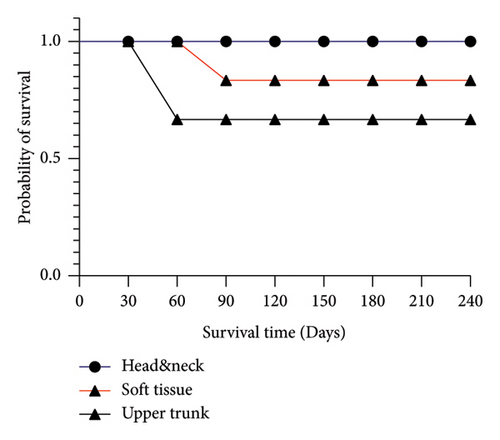
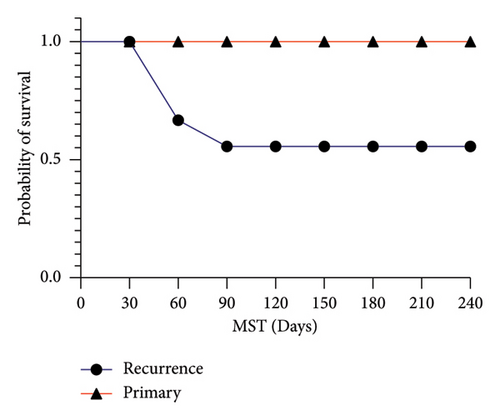
The follow-up time of patients was 110 days (range: 60–240). One of them underwent resection surgery after 1 month. Three patients who suffered from pulmonary metastasis of breast cancer died after mean 2 months, and the other are still alive at the time of writing of this manuscript. Only one patient with fibromatosis of left arm had experienced recurrence after 90 days. The DFS analysis did not show any significant differences among 3 divers tumor localization categories for survival time (p value = 0.15) (Figure 3(a)). While there was a significant difference between patients with primary tumors in comparison with recurrent ones (p value = 0.03) (Figure 3(b)).
4. Discussion
This case series demonstrated treatment results of 15 cancer patients with ECT, which evaluates their complications, treatment responses, survival time, and local recurrence rate. As previously mentioned in the study, ECT is an alternative therapeutic approach in cases of conventional methods failure or where surgery or thermal ablation is limited [19, 21, 22]. ECT specifically targets active tumor cells with low-dose chemotherapeutic agents and does not damage the extracellular matrix, making it an effective method for patients with tumors in challenging locations [23, 24]. Based on scientific evidence, bleomycin-based ECT is a practical approach for managing various types of tumors, including head and neck, breast, chest wall, and soft tissue, with significant antitumor activity [25–27]. Inconsistent with previous studies, our obtained results confirmed significant ECT’s effectiveness in tumor size reduction and acceptable 3 months overall survival, 84% and 80%, respectively [22, 28–30]. In addition, despite no significant difference between DFS of various tumor types, recurrent tumors had better DFS in comparison with nonrecurrent ones which could highlight the substantial role of ECT in the therapeutic approach of primary patients.
Regarding complications of impressive cancer therapeutic methods like ECT, TLS is still the primary concern in management of large solid tumors due to rapid extensive tumor cells destruction and releasing into the blood circulation [31, 32]. This oncological emergency is responsible for increasing the morbidity and mortality of cancer patients due to severe complications, including cardiac arrhythmia, seizures, AKI, and death [33]. The patients could be classified into three groups; high, intermediate, and low risk for developing TLS [34]. Patients with more sensitive tumors to chemotherapeutic agents, as well as those with pre-existing renal dysfunction, are at a higher risk of occurring TLS [34–36]. This syndrome has diverse clinical signs including fluid overload, uremia, and electrolyte imbalances [34, 37]. Therefore, it is important to monitor the patients with TLS diagnostic approaches, including laboratory findings and clinical symptoms, before, during and after the intervention [34, 35, 38]. It is recommended to monitor biological values every 4–6 h after initiating intervention for high-risk patients, every 8–12 h for those at intermediate risk, and daily check-up for low-risk ones [9]. In this case series, all patients received full medical care after intervention every 24 h up to 1 week.
Taking into consideration the high morbidity and mortality rate of TLS, immediate intensive monitoring and management of this urgent condition have great value and must be considered in at risk patients to prohibit following multiple organ failure progressions [39, 40]. TLS prophylaxis and treatment strategies include intravenous hydration, administering sodium bicarbonate and hypouricemic drugs such as allopurinol and rasburicase, cardiac monitoring, regular measurements of urine output and blood contents (creatinine, electrolytes, and uric acid), and even hemodialysis [34, 38, 41]. Remarkably, none of our patients proceed to TLS occurrence based on clinical signs and symptoms, which could emphasize on no serious ECT-associated adverse effects [42, 43].
5. Conclusion
Analysis of these studies approved ECT as an effective treatment method for large solid tumors. Our study emphasized findings in previous studies that have no serious adverse effects such as TLS occurrence related to ECT, and it could be a controllable and minimally invasive technique.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
A.L.: investigation, writing–reviewing and editing, project administration; S.M.Y.: investigation, methodology; F.R.P.: investigation, data gathering; N.M.: investigation, data gathering; M.B.: writing–reviewing and editing, data curation, software; Sama Mansouri: investigation, data gathering; N.S.: investigation, data gathering; Sepideh Mansouri: investigation, data gathering; O.N.: investigation, supervision; F.M.: investigation, supervision; H.M.: investigation, supervision; S.R.M.: investigation, supervision; M.A.: conceptualization, methodology, supervision, resources, project administration, data curation, writing–reviewing and editing, software.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors received no financial support for the research authorship and/or publication of this article.
Open Research
Data Availability Statement
The authors declare that all data are available from the corresponding authors upon a reasonable request.




