Quality of Life of Male Breast Cancer Survivors: A Systematic Review
Abstract
Introduction: Breast cancer treatments significantly influence the quality of life of cancer survivors. While most studies have focused on female breast cancer survivors, data on the quality of life of men who have undergone medical, surgical or radiological treatments for breast cancer are limited.
Objective: To evaluate the quality of life of male breast cancer survivors.
Methods: A systematic review was conducted according to the PRISMA statement with a search in four databases: PubMed, Embase, Cochrane and Web of Science. We screened and extracted data and assessed the methodological quality of the studies via the Cochrane RoB-2 and ROBINS-I tools for randomised clinical trials and follow-up studies, respectively. The data were presented in a narrative synthesis format.
Results: In total, 993 records were identified, of which three studies were included for analysis: two randomised clinical trials and one cohort study. The interventions in the randomised clinical trials involved evaluating the impact of endocrine therapy and the implementation of online physical training. Although there was a reduction in the overall quality of life during cancer treatment, patients who received tamoxifen monotherapy did not report a clinically significant change in their sexual function. Additionally, moderate exercise was shown to improve physical health and enhance social well-being. In the cohort study, quality of life and symptom burden were evaluated in men with breast cancer treated with endocrine therapies at the time of diagnosis via symptom assessment scales.
Conclusions: Male breast cancer survivors experience a decrease in quality of life during and after treatment. While tamoxifen monotherapy preserves sexual function, moderate-intensity physical rehabilitation significantly improves both quality of life and social functioning. The limited number of studies included in this review highlights the need for further research to address the physical, psychological and social needs of male breast cancer survivors.
1. Introduction
In the 21st century, the number of cancer diagnoses has increased despite advances in health [1]. Breast cancer (BC) is now the most common type of cancer diagnosed [2], becoming a global health problem and a leading cause of morbidity and mortality, especially in women. Since 1990, the incidence of BC has increased 1.6-fold, while global mortality has increased by 0.23% annually [3]. BC in men (BCM) accounts for 0.5%–1% of diagnoses and has a 19% higher mortality rate than it does in women [4]. Moreover, the net survival in BC patients also increased from 84% to 88% in 2019, owing to early detection, new treatments and improved surgical techniques [5]. However, prolonged treatment often leads to physical and emotional deterioration [6], which negatively impacts patients’ quality of life (QoL). As a result, QoL assessments are essential, offering valuable insights into the physical, emotional, social and spiritual dimensions of survivorship and guiding strategies for improved outcomes [7].
The concept of ‘cancer survivorship’ was first defined by Dr. Mullan [8], who identified three phases of survival: the acute phase, focused on active treatment and managing immediate effects; the extended phase, where monitoring for recurrence and managing lingering side effects takes priority after treatment ends; and the permanent phase, which emphasises long-term survival and addresses the late effects of cancer and its treatment. The definition of cancer survivorship remains controversial, but specific steps can ensure comprehensive patient care beyond the 5-year mark after diagnosis. This posttreatment period has been less studied, although survivors often face ongoing physical, psychological and social effects that impact their QoL [9]. Therefore, comprehensive cancer care for survivors [10], including the consideration of sex-specific needs and differences, is crucial.
Three main lines of treatment are considered for male BC [5], and treatment strategies vary depending on the stage of the disease. In early, localised or operable cases, surgery is the primary treatment and is sometimes combined with radiation therapy, and adjuvant therapies may be added to reduce the risk of recurrence. For locally advanced BC, the approach typically involves the use of neoadjuvant chemotherapy to shrink the tumour before surgical excision, followed by radiation therapy to target any remaining cancer cells. In metastatic cases that are hormone receptor-positive, endocrine therapy is used, often involving an aromatase inhibitor (AI) in combination with a gonadotropin-releasing hormone (GnRH) agonist to manage the spread of the disease.
While treatment approaches for BCM are largely based on studies of women and small clinical cases, most research has focused on women’s QoL. Studies on female BC survivors have shown a correlation between psychosocial factors and women’s QoL; however, the results remain inconclusive [11]. A systematic review revealed that multimodal interventions are particularly effective in enhancing women’s QoL [12]. In contrast, men, often perceived as emotionally detached and resilient in difficult situations, face a significant impact on their identity when diagnosed with BC [12]. Historically, the impact of BC on men has been underestimated, as research has focused primarily on women [13]. This disparity highlights the need to better understand the unique ways in which BC affects men’s QoL, especially given the limited focus on their experiences. Therefore, this systematic review aimed to evaluate the QoL of male BC survivors.
2. Methods
2.1. Types of Study
A systematic review of the scientific literature was conducted on the basis of the Cochrane Handbook for Systematic Reviews of Interventions [14] to describe the impact of cancer treatments on the QoL of men diagnosed with BC. The protocol was registered on the PROSPERO international registry platform for systematic reviews with the following identification: CRD42024510200. The Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) statement [15] was used to structure the description of the results of this review.
2.2. Search Strategy
We conducted literature searches in the following four databases, PubMed, Embase, the Cochrane Library and Web of Science, to identify and retrieve studies on the effects of treatments on QoL in men with BC. Initially, searches were performed to determine the relevant MeSH terms and keywords related to the components of the research question (population, intervention and outcomes), adapting the strategy to the functionality of each database. The Boolean operators ‘OR’ and ‘AND’ were used to combine intra- and intercategory searches, respectively. No language or publication date restrictions were applied. However, titles and abstracts were filtered, restricting the retrieval of search terms to these sections in the articles. The detailed search strategies developed from March to April 2024 for each database are presented in Tables 1, 2, 3, 4.
| Pubmed | |
|---|---|
| Total items: 310 | |
| Search date: 5/03/2024 | |
| PIO question | Search strategy |
| Population #1 | ((Breast neoplasms, male [title/Abstract] OR male breast cancer [Title/Abstract] OR male breast carcinoma [title/Abstract]) |
| Intervention #2 | (Mastectomy OR chemotherapy, adjuvant OR immunotherapy OR radiotherapy OR tamoxifen OR drug-related side effects and adverse Reactions)) |
| Outcome #3 | (Quality of life OR health-related quality of life OR quality adjusted life OR years OR healthy lifestyle OR mental health OR adjusted life years OR quality adjusted life years OR life year, quality-adjusted OR life years, quality-adjusted OR healthy lifestyle OR physical well-being OR physical health OR psychological well-being OR psychological health OR social well-being OR social welfare OR spiritual well-being OR spiritual health) |
| Combination | Population (#1) AND intervention (#2) AND outcome (#3) |
| Embase | |
|---|---|
| Total items: 40 | |
| Search date: 2/04/2024 | |
| PIO question | Search strategy |
| Population #1 | ‘Male breast cancer’/exp |
| Intervention #2 | (‘Breast surgery’/exp OR ‘cancer chemotherapy’/exp OR ‘cancer immunotherapy’/exp OR ‘tamoxifen’/exp OR ‘cancer radiotherapy’/exp) |
| Outcome #3 | (‘Health care quality’/exp OR ‘health’/exp OR ‘mental health’/exp OR ‘spiritual health’/exp OR ‘social evolution’/exp) |
| Type of studies #4 | (‘Randomised controlled trial’/exp OR ‘cohort analysis’/exp) |
| Combination | Population (#1) AND intervention (#2) AND outcome (#3) AND type of studies (#4) |
| Cochrane | ||
|---|---|---|
| Total items: 2 | ||
| Search date: 5/03/2024 | ||
| Population | #1 | MeSH descriptor: [Breast neoplasms, male] explode all trees |
| #2 | #1 | |
| Intervention | #3 | MeSH descriptor: [Mastectomy] explode all trees |
| #4 | MeSH descriptor: [Antineoplastic agents] explode all trees | |
| #5 | MeSH descriptor: [Radiotherapy] explode all trees | |
| #6 | MeSH descriptor: [Immunotherapy] explode all trees | |
| #7 | MeSH descriptor: [Tamoxifen] explode all trees | |
| #8 | Mastectomy OR antineoplastic agents OR radiotherapy OR immunotherapy OR tamoxifen | |
| #9 | #3 OR #4 OR #5 OR #6 OR #7 | |
| Outcome | #10 | MeSH descriptor: [Quality of life] explode all trees |
| #11 | MeSH descriptor: [Mental health] explode all trees | |
| #12 | MeSH descriptor: [Spiritual therapies] explode all trees | |
| #13 | MeSH descriptor: [Physical appearance, body] explode all trees | |
| #14 | MeSH descriptor: [Social status] explode all trees | |
| #15 | #10 OR #11 OR #12 OR #13 OR #14 | |
| Combination | #16 | #2 AND #9 AND #15 |
| Filters | Trials | |
| Web of science | |
|---|---|
| Total items: 310 | |
| Search date: 2/04/2024 | |
| PIO question | Search strategy |
| Population #1 | AB = (breast neoplasms, male OR male breast cancer OR male breast carcinoma) |
| Intervention #2 | AB = (mastectomy OR chemotherapy, adjuvant OR immunotherapy OR radiotherapy OR tamoxifen OR drug-related side effects and adverse reactions) |
| Outcome #3 | AB = (quality of life OR health-related quality of life OR quality adjusted life OR years OR mental health OR well-being OR adjusted life years OR physical health OR mental health OR psychological well-being OR psychological health OR social well-being OR social welfare OR spiritual well-being OR spiritual health) |
| Combination | Population (#1) AND intervention (#2) AND outcome (#3) |
2.3. Inclusion and Exclusion Criteria
Studies were included on the basis of the following criteria: (a) studies involving male patients diagnosed with BC who were over 18 years of age; (b) studies that included men diagnosed with BC who underwent chemotherapy, radiotherapy, endocrine therapy, surgery or immunotherapy as part of their treatment or interventions related to their health and treatment; (c) studies that measured the QoL after the end of treatment in male patients diagnosed with BC; and (d) randomised controlled trials (RCTs) for their ability to minimise bias and establish causality or observational cohort studies for their feasibility in real-world settings. In addition, no limits were set regarding the language of the publications.
The exclusion criteria were as follows: (a) studies conducted with both male and female BC patients who did not explicitly report outcomes for men; (b) studies involving men diagnosed with BC who underwent alternative treatments; (c) studies measuring QoL without using a validated questionnaire; and (d) literature reviews, conference abstracts, letters, editorials and grey literature.
2.4. Study Selection Process
We identified studies available in databases (Tables 1, 2, 3, 4), downloaded the search results and exported them to the Covidence online platform. On this platform, duplicate studies and those deemed ineligible by automated tools were removed. Study selection was performed by analysing titles and abstracts and applying the inclusion and exclusion criteria. Full-text evaluations were conducted for records that met the initial screening criteria. Studies that addressed the research question were included in the systematic review.
The selection of records was performed independently by two researchers (first and second authors). In cases of discrepancies, a third expert investigator (last author) was consulted to resolve disagreements. Data from each article were extracted and recorded independently.
2.5. Data Extraction
- •
General information: authors, year of publication, purpose of the study, country of origin and journal.
- •
Specific details about the participants (sociodemographic data): age, type of treatment and clinical stage of cancer.
- •
Study characteristics: design, distribution of participants in each trial group, sample size, follow-up period of participants, description of treatment/intervention and inclusion/exclusion criteria for study participation.
- •
Outcome measures: results or variables assessed, measurement tools used (scales, questionnaires, etc.) and statistical analysis.
2.6. Risk of Bias Assessment
The quality of the RCTs included in this review was assessed via the Cochrane Risk of Bias Tool for Randomised Trials (RoB 2) [16], which is recommended for use in Cochrane reviews and includes the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases.
To assess the risk of bias in observational cohort studies, we used the ROBINS-I tool for follow-up studies [17], which is used in systematic reviews that include nonrandomised studies. Finally, judgements about the risk of bias were formulated with the following possible response options: Yes (Y); Probably Yes (PY); Probably Not (PN), No (N) and No information (NI).
2.7. Strategy for Data Synthesis
Owing to heterogeneity between the included studies, we presented a narrative summary of the data. Depending on the design of the study, the different results of the selected articles were analysed separately. RCTs and observational cohort studies were the priority studies for evidence analysis.
3. Results
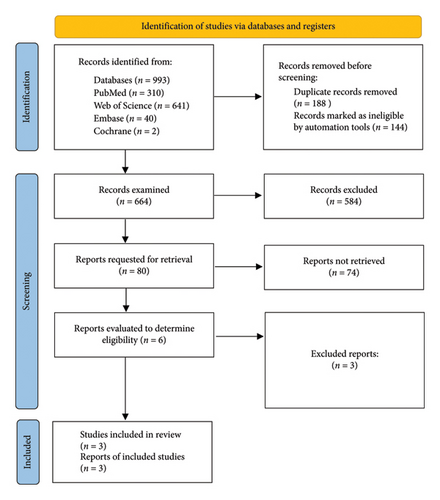
In total, 993 records were retrieved from the database searches. After 188 duplicates were removed, 141 references were marked as ineligible by the automatic Covidence tool, leaving 664 records for further screening by title and abstract. Of these, 584 were excluded. We retrieved the full texts of 80 studies, which were reviewed on the basis of the inclusion and exclusion criteria. Seventy-four studies were excluded, and six reports were evaluated for eligibility. Finally, three studies [18–20] were selected for inclusion in this review (Figure 1).
3.1. Risk of Bias Assessment for RCTs
The study by Reinisch et al. [18] received a satisfactory overall evaluation, with a low risk of bias in each of the domains analysed. This was not the case for the trial conducted by Schultz et al. [20], which was assessed as having ‘some concerns’ in domain 2 because both patients and assessors were aware of the intervention. Similarly, it was classified as having some concerns in domain 4, as the therapist may have had unconscious biases when adjusting the workouts for each participant, potentially influencing the study’s outcomes. Furthermore, the therapist’s knowledge of the protocol could have led to a biased interpretation of the data collected during the study (Table 5).
| Studies | Bias domains | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | General | |
| Reinisch et al., [18] | + | + | + | + | + | + |
| Schultz et al., [20] | + | ! | + | ! | + | ! |
- Note: D1: Bias arising from the randomisation process. D2: Bias due to deviations from planned interventions. D3: Bias due to lack of outcome data. D4: Bias in outcome measurement. D5: Bias in the selection of the reported outcome. +: Low risk of bias. !: Some Concerns. -: High risk of bias.
3.2. Risk of Bias Assessment for Nonrandomised Follow-Up Studies
In the study by Schröder et al. [19], demographic characteristics such as age, sex, ethnicity, socioeconomic status and general health status could have influenced QoL outcomes, along with factors such as the severity of BC, treatment received, other interventions, and psychological and social factors. Some participants were excluded because of a lack of data on intervention status. Additionally, it was not specified whether the evaluators were blinded, leading to possible variability in how the intervention was implemented across different centres or investigators, which could have affected the outcome measurements (Table 6).
| Studies | Bias domains | |||||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | General | |
| Schröder et al. (2023) | ! | + | ! | + | ! | - | + | - |
- Note: D1: Bias due to confounding. D2: Bias in the selection of study participants. D3: Bias in the classification of interventions. D4: Bias due to deviations from planned interventions. D5: Bias due to lack of data. D6: Bias in the measurement of outcomes. D7: Bias in the selection of the reported outcome. +: Low risk of bias. !: Some Concerns. -: High risk of bias.
3.3. General Characteristics of the Included Studies and Participants
Table 7 presents the general information of the three included studies. The year of publication ranged from 2021 to 2023, with 66.6% (n = 2) published in 2023 and 33.3% (n = 1) in 2021. Regarding the country of origin, two studies were conducted in Germany [18, 20] and one was conducted in the Netherlands [19].
| Authors | Reinisch et al. [18] | Schultz et al. [20] | Schröder et al. [19] |
|---|---|---|---|
| Objective of the study |
|
|
|
| Country of origin | Germany | Germany | Netherlands |
| Journal | Oncología JAMA | Breast care | The oncologist |
| Design | Multicentre, randomised, phase 2 clinical trial | RCT, crossover | Prospective cohort study |
In terms of study design, one was a multicentre, randomised phase 2 clinical trial [18], another was a crossover RCT [20] and the third was a prospective cohort study [19]. All the articles were published in English.
Table 8 presents the main characteristics of the studies and specific details of the participants. Each study had a designated follow-up period, ranging from 3 to 41 months. Similarly, all the authors defined specific inclusion and exclusion criteria, with a common factor being the inclusion of male patients over 18 years of age who were diagnosed with BC. However, in the study by Schröder et al. [19], no exclusion criteria were specified. The sample sizes varied from 22 to 363.
| Characteristics | Reinisch et al. [18] | Schultz et al. [20] | Schröder et al. [19] |
|---|---|---|---|
| Distribution of participants in each trial group | Three randomly distributed treatment groups were created | Using a randomisation scheme, patients were divided into two groups: One for intensive exercise and the other for moderate exercise | Male breast cancer survivors were compared with a normative population of men in six European general population studies |
| Sample size (n) | n = 52 | n = 22 | n = 363 |
| Participant follow-up period (months) | 3 and 6 | 7 | 41 |
| Description of the intervention | 1st group: Tamoxifen; 2nd group: Tamoxifen + GnRHa; 3rd group: AI + GnRHa | Online training program with resistance and strength exercises over 3 months, followed by different models. | Not applicable |
| Inclusion/Exclusion criteria | Inclusion: Male patients with HRE positive CM, age > 18, Karnofsky index ≥ 60, no history of prostate cancer, normal renal, hepatic and cardiac functions; exclusion: Female patients, having received endocrine therapy life expectancy < 6 months, history of hypersensitivity reactions. | Inclusion: Men after primary BC diagnosis, age ≥ 18, treatment of BC within 6 months, statement of medical fitness/medical clearance to participate, Internet access; exclusion: Men with metastatic BC, acute orthopaedic conditions. | Inclusion: Men with histologically proven BC, age ≥ 18, who recently presented to a participating centre or within 3 months prior to centre activation and who are enrolled in the international MBC Program’s prospective registry. Exclusion: Nonreferrals. |
| Age, mean (SD), range of participants | 37–83 years | 32.6–92.3 years | 61.34 ± 9.14 |
| Cancer stage |
|
Nonreferrals | M0: 253, M1: 28, Mx: 82 |
| Type of treatment | Endocrine | Surgery, chemotherapy, radiation, endocrine therapy | Surgery, chemotherapy, endocrine, targeted therapy |
| Variables evaluated | Age, treatment modality, Karnofsky index, TNM classification, sexual function, QoL | Age, BMI, heart rate, oxygen consumption, global health, physical/emotional functioning | Time from diagnosis, TNM classification, treatments, global health, cognitive/social functioning |
| Instruments used | IIEF, AMS questionnaire | EORTC-QLQ-C30, MFI-20, AMS symptom scales | EORTC-QLQ-C30 symptom scales, EORTC-QLQ-BR23 |
| Main results |
|
|
|
- Note: T1 (includes T1a, T1b and T1c): The tumour is 3/4 inch (2 cm) or less across. T2: The tumour is larger than 2 cm but no larger than 5 cm (2 inches) across. T3: The tumour is more than 5 cm across. T4 (including T4a, T4b, T4c and T4d): A tumour of any size that grows into the chest wall or skin. This includes inflammatory CMs. N0: The cancer has not spread to nearby lymph nodes. N1: Cancer has spread to one to three axillary (armpit) lymph nodes, and/or cancer is found in the internal breast lymph nodes (those near the breastbone) on sentinel lymph node biopsy. M0: No distant spread is found on X-rays (or other imaging tests) or physical examination. M1: The cancer has spread to distant organs (most often to the bones, lungs, brain or liver), as seen on imaging tests or by physical examination, and/or a biopsy of one of these areas shows that the cancer has spread and is larger than 0.2 mm. Source: Authors’ own creation.
- ∗Mean.
The cancer stage was not reported in one of the studies [20], where not all stages were detailed, with only the presence of metastases being mentioned. In the other two studies [18, 19], cancer was classified according to the TNM system (T: size of the primary tumour; N: regional lymph node involvement; M: presence of distant metastases) [21].
In terms of treatment types, one clinical trial [18] evaluated endocrine therapy, whereas the other studies involved multiple modalities, including surgery, radiotherapy, chemotherapy, antihormone therapy and targeted therapy [18, 20].
3.4. Characteristics of the Interventions
The two clinical trials included in this review involved heterogeneous interventions, one related to endocrine treatments and the other focused on exercise. In the Reinisch et al. [18] study, eligible patients were divided into three arms to evaluate changes in oestradiol levels in male patients with BC after 3 and 6 months of therapy. The treatment groups received tamoxifen, tamoxifen plus a GnRHa analogue, or an AI combined with a GnRHa. The treatment was administered for 6 months in neoadjuvant, adjuvant or metastatic settings. Blood samples and questionnaire responses were collected at study entry, before the start of treatment and after 3 and 6 months of therapy.
In the Schultz et al. [20] trial, a randomisation scheme was used to divide patients who had previously received chemotherapy and radiotherapy into two groups: one for intensive exercise and the other for moderate exercise. The authors chose a crossover design to enhance internal validity, given the low incidence of the disease and the small number of patients. This design allowed participants to serve as their own control group. An online training program was implemented due to the geographical dispersion of patients, and the interactive platform EmotionNet was used to track physical activity. Two online diaries and guidance from a sports therapist facilitated the collection of information on resistance, strength and intensive training over a 3-month period, during which patients recorded their subjective perceptions of effort. After a 4-week break from any exercise intervention, the participants switched to the other training model. The exercises included squats, sit-ups, diagonal leg and arm raises (in a prone position), dynamic knee curls, dynamic tiptoe exercises and deadlifts with weights. To be included in the data analysis, participants were required to complete at least 80% of the recommended exercises.
3.5. Quality of Life
In the trial by Reinisch et al. [18], QoL was measured via the AMS Questionnaire, which assesses elements related to androgen decline in ageing men, with a score of 27 or higher indicating androgen deficiency. The study revealed that patients’ QoL did not significantly deteriorate over the course of treatment (p < 0.001). Although the QoL reduction at baseline was 59.6%, it increased to 75% at 3 months and slightly decreased to 67.4% at 6 months, indicating no significant deterioration over time (p < 0.001).
In the other two studies [19, 20], the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) was used. This is one of the most widely used patient-reported outcome (PRO) measures in cancer research [22, 23] for assessing patient QoL.
Both studies also used the EORTC QLQ-BR23, a validated 23-item questionnaire designed to complement the EORTC QLQ-C30, providing additional information on QoL and symptoms specific to BC patients. Schultz et al. [20] reported significant improvements in ‘physical function’ (p = 0.037) and ‘social function’ (p = 0.016) after moderate training. With respect to the QoL assessment results, Schultz et al. [20] reported that global QoL improved with moderate exercise, whereas intense exercise led to a decline in QoL.
In the study by Schröder et al. [19], the average global health score on a 100-point scale was 73. The most frequently reported symptoms among men were fatigue (mean score: 22), insomnia (mean score: 21) and pain (mean score: 16). Additionally, four men aged 40 years or younger reported higher levels of fatigue, nausea/vomiting, pain, loss of appetite, constipation and financial difficulties than did older men. The study also revealed that there were no significant differences in QoL between men with early-stage disease and those with advanced disease.
The Multidimensional Fatigue Inventory (MFI-20) [20], a tool that differentiates between various dimensions of fatigue (e.g., emotional and general), was used to assess self-reported fatigue. The results revealed no change in fatigue levels (p = 0.306) following the training interventions, likely because of the small sample size.
3.6. Sexual Function
Sexual function was assessed via the International Index of Erectile Function (IIEF) in the studies by Reinisch and Schultz [18, 20]. The IIEF evaluates dimensions such as erectile function, orgasmic function, sexual desire, sexual satisfaction and overall satisfaction. A score of 21 or lower indicates the potential for erectile dysfunction.
In the study by Reinisch et al. [18], patients reported an improvement in sexual function over the course of treatment, although those treated with tamoxifen alone did not report a clinically significant change. Schultz et al. [20] reported no significant difference in sexual functioning between the moderate and intensive training groups.
The study by Schröder et al. [19] used the EORTC-PR25 scale, a 25-item modular supplement developed and validated for prostate cancer patients, to assess sexual function related to hormonal treatment. Eleven items from this scale were used to complement the EORTC QLQ-C30. Schröder and collaborators reported that the mean sexual activity score, on a 100-point scale, was 31, whereas the mean sexual function score was 80 in those who were sexually active.
4. Discussion
This review examined the current evidence from RCTs and nonrandomised follow-up studies and assessed the impact of cancer treatments on the QoL of men diagnosed with BC. This finding was also reached by Duma et al. [24], who analysed 426 clinical trials on BC and reported that 65% of the included studies excluded male patients with BC, whereas 7% failed to include male patients in the inclusion and exclusion criteria. Similarly, male-specific treatment options are poorly addressed in studies, which may be due to the low incidence and perception of BC in men, differences in treatment responses between men and women and the high costs of clinical trials required to evaluate new therapies [25].
In the included studies, two types of scales were used to assess the QoL of male patients with BC: the AMS questionnaire [18] (33.3%) and the EORTC-QLQ-C30 Symptom Scale [19, 20] (66.6%). Despite its ability to collect data on physical conditions due to the decrease in androgens typical of ageing, it is limited to a part of the male population since BC can be diagnosed at any age. It does not have sufficient parameters to address all the adverse effects that may arise from cancer treatment. Although androgen deficiencies have been shown to exist in patients with male breast carcinoma [26], other affected patients are not yet represented. However, a study by Reinisch et al. [18] demonstrated a reduction in global QoL during cancer treatment. At the start of the study, a decrease was reported in 59.6% of patients, a figure that increased to 75% at 3 months. These data reflect the rapid and progressive deterioration faced by male patients.
In the study conducted by Schröder et al. [19], parameters from two additional scales were integrated with the EORTC QLQ-C30, specifically an adapted version of the EORTC QLQ-BR23 and selected elements from the QLQ-PR25. The findings showed that the overall QoL was greater than that of both women with BC and the healthy male reference population. However, it is important to highlight a critical aspect of this study: QoL assessments were conducted at the time of diagnosis rather than during long-term cancer survival. This limitation could lead to potential misinterpretations when the results are compared with those of other studies.
One study included in this review revealed that moderate exercise interventions may help alleviate specific side effects associated with cancer treatment in men, with no adverse effects reported during the intervention. In women, a nurse-led exercise rehabilitation program lasting 4 weeks demonstrated a significant increase in global health status, physical functioning and role functioning scores (p = 0.001) [27]. Therefore, moderate exercise interventions are strongly recommended for individuals with BC, both men and women, as they provide significant benefits in improving QoL as well as physical and social functioning [27]. These findings support the integration of moderate exercise programs into cancer care for both genders, emphasising the critical role of physical activity as a core component of treatment and rehabilitation [27].
With respect to sexual function, this parameter was evaluated in all three studies. Reinisch et al. [18] reported that tamoxifen monotherapy did not result in a clinically significant change in sexual function in men with BC. Because there is no specific validated scale to assess the sexual function of these patients, some items were taken from the EORTC QLQ-PR25 [19], which was designed specifically for prostate cancer survivors undergoing active treatment [28]. Another study that evaluated QoL and symptoms in men with BC [29] used an Expanded Prostate Cancer Index Composite (EPIC) hormonal and sexual symptom scale to assess sexual function and hormone levels in men diagnosed with BC. Among the participants, 40% reported poor sexual function in 4 weeks prior to the survey. Sexual function is a significant concern for men with BC. While tamoxifen monotherapy does not appear to substantially worsen this problem, poor sexual function remains a common problem within this population. The reliance on assessment scales not specifically validated for men with BC highlights the urgent need to develop and validate appropriate tools that accurately capture the impact of the disease and its treatments on sexual function and other aspects of QoL in men. Such advancements would enable more precise assessments and facilitate the development of targeted interventions to improve the QoL of men with BC.
In the mental, emotional and social domains, limited data have been collected for male patients, emphasising the need to develop and implement new assessment tools. It is also crucial to address the psychological and social impact of cancer, particularly in men with BC. Kowalski et al. [30] demonstrated that health-related QoL was significantly lower in male BC patients than in reference populations, particularly in role functioning. This study highlights the importance of early psychological interventions targeting both physical and emotional role functioning to improve the overall QoL in this population. A scoping review [11] focussing on women BC survivors identified social support, depression and future outlook as the most studied psychosocial determinants. It also demonstrated that greater social support was correlated with lower levels of depression, which in turn was associated with improved QoL. However, a significant limitation of this study was the absence of an evaluation of these psychosocial determinants in men with BC. This omission further underscores the urgent need for research that addresses the unique psychological and social challenges faced by male BC patients, as highlighted by Kowalski et al. [30], to ensure a comprehensive understanding and better care for this underrepresented group.
A final aspect to highlight is the precise relationship between the type of treatment used and its impact on the QoL of each patient. The degree of radicalisation, intensity and duration of treatment can have both positive and negative effects on patient survival and QoL. However, this information is notably lacking in the reviewed studies. Camejo et al. [31] found that female patients who underwent breast-conserving surgery reported significantly better physical and emotional functioning (p < 0.005). This finding further underscores the pressing need for similar evaluations in male patients, as their unique experiences and outcomes remain underexplored.
4.1. Strengths and Limitations
This review offers valuable insights into the impact of screening on QoL in male BC survivors after the end of treatment. It provides a foundation for future research aimed at identifying gaps in patient follow-up and supporting the development of more inclusive and compassionate health policies for this population. However, several limitations must be considered when interpreting the findings.
While a thorough search was conducted, only three studies [18–20] met the inclusion criteria, limiting the generalisability of our findings. However, the review was conducted with methodological rigour, including a comprehensive search across multiple databases to ensure a thorough assessment of the available literature.
Another key limitation is the heterogeneity among the included studies. The three studies—two RCTs and one cohort—varied in their use of QoL instruments (AMS Questionnaire, EORTC QLQ-C30, EORTC QLQ-BR23, and IIEF) and interventions (endocrine therapy vs. exercise), making direct comparisons difficult. Although the combined sample comprised 437 participants, most studies were conducted in European countries, and detailed information on clinical stage and specific treatments was insufficient. This variability contributed to heterogeneous findings. Furthermore, the risk of bias assessments using RoB-2 and ROBINS-I identified ‘some concerns’ or high risk in areas such as outcome measurement and confounding, suggesting caution in interpreting these results.
Moreover, the limited follow-up periods in the included studies pose another constraint. Survivors were followed up between 3 and 41 months after treatment, meaning the identified QoL impacts are confined to this timeframe. As a result, the long-term effects of screening remain unclear, highlighting a critical knowledge gap.
Finally, the lack of validated QoL instruments specifically designed for male BC survivors may have further restricted the number of eligible studies, underscoring the need for more tailored assessment tools in future research.
5. Conclusions
Male BC survivors often experience a progressive decline in QoL during and after treatment. In patients with hormone receptor-positive BC, tamoxifen monotherapy did not result in a significant reduction in sexual function, suggesting that this treatment may preserve certain aspects of well-being. However, moderate-intensity physical rehabilitation significantly improved QoL, enhancing both physical and social functioning in male BC survivors. These findings highlight the need for more tailored QoL assessment tools specifically designed for men with BC, considering not only physical challenges but also psychological and emotional dimensions. Future research should prioritise both the validation of QoL instruments for male BC survivors and the extension of follow-up beyond 5 years to better capture late-emerging effects of treatment.
Given the increasing incidence of male BC, particularly among men at risk of developing breast tissue due to hormone therapy, more robust preventive measures and targeted screening strategies could facilitate earlier diagnosis and improve patient outcomes.
Finally, the limited number of studies on male BC survivors may stem from the shortage of validated assessment instruments. To enhance the comparability of findings and develop inclusive, evidence-based care guidelines for this often-overlooked population, future research must incorporate broader, more diverse samples and standardised measurement tools. Expanding study populations to include male BC survivors from varied backgrounds and extended survival phases would provide more generalisable insights, particularly regarding the long-term effects of treatment on QoL. Given the scarcity of studies and their diverse methodologies, careful interpretation of the findings is essential. Nonetheless, this review highlights critical gaps in our understanding of QoL in male BC survivors and emphasises key directions for future research aimed at strengthening the evidence base.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Carolina Foundation [Graduate Scholarships 2023, Spain].
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.




