Quality of Life and Patient-Reported Outcomes in Patients With Osteosarcoma: A Systematic Review
Abstract
Patients diagnosed with osteosarcoma undergo intensive multimodality treatment that can lead to long-term adverse effects, significantly impacting various aspects of daily living. To objectively assess the Health-Related Quality of Life (HR-QoL) in pediatric and adult populations, several Patient-Reported Outcome Measurements (PROMs) are available. However, these questionnaires often exhibit substantial variability in the domains and items they encompass, frequently failing to address aspects that are particularly important after osteosarcoma treatment. A systematic review was conducted to identify the most frequently used questionnaires concerning QoL in pediatric and adult patients with osteosarcoma and to examine the diverse domains and subdomains of QoL assessed by these questionnaires to identify gaps in their coverage, to recommend suitable instruments for an upcoming European trial within the Fighting Osteosarcoma Through European Research (FOSTER) Consortium. English-language literature published since 1980 in PubMed was reviewed. One hundred twenty-eight articles were initially screened for eligibility. Sixty-three original articles were included in the qualitative synthesis. An overview from review articles was given. Selected studies displayed substantial heterogeneity in terms of their objectives, target populations, age ranges, follow-up time, and number of patients included. None of the questionnaires covered all age groups and addressed all important aspects following osteosarcoma treatment. To comprehensively address as many relevant aspects as possible, a combination of questionnaires is suggested. For the adult population, it is recommended to use the EORTC-QLQ-C30 questionnaire together with the Body Image Scale (BIS), while for pediatric patients, the PedsQl-generic and PedsQl-cancer-specific questionnaires and BIS (> 16 years) are suggested. The use of Patient-Reported Outcome Measurement Information Systems (PROMIS) can provide a comprehensive assessment of symptoms such as anxiety, pain, and fatigue. The development of new bone sarcoma-specific, pediatric and adult self-reported questionnaires, or the validation and translation of existing bone sarcoma-specific questionnaires, along with the utilization of new digital possibilities, holds great value for upcoming trials.
1. Introduction
Osteosarcoma, although a rare cancer, is the most frequent primary malignant tumor arising in the bone, with an incidence peak in adolescents and young adults (AYAs) reaching 6.7 per million individuals [1]. Osteosarcomas mostly arise in the metaphysis of the long bones near the growth plates, with the distal femur as the most frequent location. However, they can occur in any other bone location with different surgical implications [1]. The treatment of osteosarcomas is multidisciplinary and involves surgery and systemic treatment with multi-agent chemotherapy. Radiotherapy might be an option in certain scenarios. With the introduction of multi-agent chemotherapy, the 5-year event free survival (EFS) has improved from less than 20% to around 60% in the last decades [2]; however, multimodality treatment implicates long-term adverse effects and influences the quality of life (QoL).
QoL is an overall assessment of one’s satisfaction with all the essential aspects of life compared to a self-perceived ideal [3]. Health-Related QoL (HR-QoL) is how the disease and its treatment influence QoL [4]. Besides diagnosis and treatment aspects, there are also personal and environmental factors, as well as aspects like functional limitation, disability, and personal identity, which include body image and self-concept, that can influence QoL [5]. Patient-Reported Outcomes (PROs) are indicators that come directly from the patient and include different aspects and outcomes concerning HR-QoL without interpretation by a clinician [6, 7]. In general, two types of Patient-Reported Outcome Measurements (PROMs) can be distinguished to measure the PROs: the first one is disease-specific, and the second one concerns generic measurements that can also be used within healthy populations. Often, both types are used, however, the former having greater face validity and credibility, and the latter allowing comparisons across different conditions [8].
PROMs could be used in different contexts. Firstly, PROMS collected in clinical trials can offer valuable evidence regarding the risks and benefits of treatment from a patient perspective, helping to shape regulatory approvals, clinical guidelines, and healthcare policies [9]. To evaluate and facilitate improved decision-making regarding the value of anticancer therapies, the ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) [10, 11] was designed and it is taken into account when approving new drugs by the regulatory agencies. Secondly, at an individual patient level, PROs can be used to facilitate shared decision-making, screen or monitor symptoms, and provide timely care tailored to individual needs and screen for long-term effects in survivors after treatment of osteosarcoma [9, 12].
Treatment and local therapy affect daily functioning across various areas: physical, psychosocial, and HR-QoL. In relation to cancer treatment, different domains have been described. In a systematic review by Anthony et al. regarding QoL and PROMS in pediatric oncology patients, four major domains and eleven subdomains were identified [13]. The four significant domains cover physical health (subdomains: function and symptoms), psychological health (which includes body image, self-esteem, positive psychological functioning, behavior, cognitive and emotional distress as subdomains), social health (subdomains: relationship and social functioning), and general health (subdomain: health perception). Many questionnaires have been designed to measure QoL in both pediatric and adult populations. However, items and domains included in these questionnaires are very variable and often do not ask specifically about aspects important after the treatment of bone sarcomas. Moreover, not all questionnaires have been validated and translated into different languages, and most are directed only to either the adult or pediatric population. These hurdles hamper the generalization of a single questionnaire when evaluating QoL in the population treated for bone sarcoma.
This study aims to give an overview of PROMs that have been used in more than one study concerning QoL and/or HR-QoL (often used interchangeably in literature) in pediatric and adult patients with osteosarcoma. It also aims to give an overview of the different domains and subdomains of QoL used in the different questionnaires in current literature to identify gaps recommending suitable questionnaires for an upcoming European trial within the Fighting Osteosarcoma Through European Research (FOSTER) Consortium (https://www.fosterconsortium.org) to cover the different QoL aspects important for patients with osteosarcoma. Within this trial, the FOSTER-CabOs project will be opened (ATTRACT CSET EU CT No: 2023-505575-69-00) early 2025. This is a phase III randomized controlled trial that will evaluate the effectiveness of a year of maintenance therapy with cabozantinib, a tyrosine kinase inhibitor, after the standard treatment in pediatric and adult osteosarcoma patients compared to placebo. In this trial, EFS will be the primary objective. Among secondary objectives, one concerns the measurements of QoL during and after treatment. Thus, the selected PROMS will be used to evaluate the upcoming trial and to assess QoL of individual patients.
2. Methods
2.1. Data Sources and Searches
“Osteosarcoma”[Mesh] OR osteosarcoma ∗[tiab] OR Ewing sarcoma ∗[tiab] OR (bone[ti] AND cancer[ti]) OR (bone[ti] AND tumor[ti]) OR (bone[ti] AND tumour[ti])) AND (“Quality of life”[MAJR] OR “Surveys and Questionnaires”[MAJR] OR psychology[sh] OR “Health status”[MAJR] OR “Health status indicators”[MAJR] OR “Activities of daily living”[Mesh] OR “Health surveys”[MAJR] OR “Quality adjusted life years”[Mesh] OR “Psychometrics”[MAJR] OR “quality of life”[tiab] OR QOL[tiab] OR HRQL[tiab] OR HRQOL[tiab] OR “quality adjusted life year”[tiab] OR “quality adjusted life years”[tiab] OR QALY ∗[tiab] OR “health state”[ti] OR “health states”[ti] OR wellbeing[tiab] OR “well-being”[tiab] OR “daily living”[tiab] OR ADL[tiab] OR “short form 36”[tiab] OR “SF 36”[tiab] OR SF36[tiab] OR “short form 12”[tiab] OR “SF 12”[tiab] OR SF12[tiab] OR euroqol[tiab] OR “EQ 5D”[tiab] OR “health utilities”[tiab] OR “health utility”[tiab] OR “time trade off”[tiab] OR “standard gamble”[tiab] OR “patient reported”[tiab] OR “patients reported”[tiab] OR “reported outcome”[tiab] OR “reported outcomes”[tiab] OR “reported experience”[tiab] OR “reported experiences”[tiab] OR PROM[tiab] OR PREM[tiab] OR PROMS[tiab] OR PREMS[tiab]
The selected articles include different aspects of QoL discussed concerning at least one of the determined domains or subdomains (physical health (function, symptoms), psychological health (body image, self-esteem, positive psychological functioning, behavior, cognitive and emotional distress), social health (relationship, functioning), and general health (health perception), as mentioned by Anthony et al. [13]. Retrieval was limited to full publications only; abstracts or meeting presentations were excluded.
2.2. Study Selection
The inclusion criteria for article selection were as follows: i) articles including bone sarcoma patients with at least ten patients diagnosed with osteosarcoma, and ii) pediatric, adolescents, and/or adult population. Exclusion criteria included: i) no QoL assessment, ii) assessment of only physical function, iii) determining QoL after one specific surgical technique, iv) development and validation studies, and v) exclusively focus on functional outcome. This last exclusion criterion was applied because another substudy within the FOSTER consortium is specifically focused on functional outcomes after the treatment of osteosarcoma.
The software Rayyan (https://www.Rayyan.ai) was used to select the articles in PubMed. Studies were selected in a stepwise approach. First, eligible papers were identified based on their title and abstract by two independent authors (A.S. and F.T.) and analyzed in a blind fashion. When there were discrepancies, those were resolved between both authors or in some cases, and the decision to include the article was decided by a third author (L.M.H.). Second, the full texts of studies identified as relevant the authors rated eligible papers independently against the inclusion and exclusion criteria. All publications considered to be relevant were selected by both authors. In the next step, the full texts of studies identified as relevant were reevaluated in detail by two of the authors (A.S., C.B., F.T., M.D.T., N.F., L.M.H.). In the Supporting Information (available here), the PRISMA 2020 Checklist for systematic reviews can be found.
A total of 102 studies were selected after evaluating titles and abstracts. An additional 26 studies were identified through reference lists, bringing the total to 128 studies. The full texts of these 128 studies were evaluated for eligibility by one of the authors. The final analysis included 73 studies, comprising 63 original articles and 10 review articles.
2.3. Data Extraction and Synthesis
Target data were selected by all authors based on the endpoint of this study. Study aim, study design, study population, number of included patients and number of included osteosarcoma patients, age group, inclusion of a control group, used PROMs, and outcomes were extracted from included articles. The selected articles were also screened for the various domains and subdomains onto the conceptual model of Anthony et al. using an Excel sheet by two of the reviewers (A.S., C.B., F.T., M.D.T., N.F., L.M.H.).
In general, for International Clinical Studies, PROMs should ideally meet the criteria of validation, suitability for the target population, translation into multiple languages, and efficiency in terms of patient time [14]. For the upcoming trial, also the use in current or recently used European pediatric and adult sarcoma treatment protocols (osteosarcoma (https://fosterconsortium.org), Ewing sarcoma (Euro-Ewing Consortium: https://www.ucl.ac.uk/cancer/research/centres-and-networks/euro-ewing-consortium/euro-ewing-consortium), and rhabdomyosarcoma trials (https://www.epssgassociation.it)) and the availability of the PROMs for different age groups are relevant. Therefore, to assist in the final selection of questionnaires for the upcoming trial an overview has been made of the PROMs which have been used in more than one of the selected articles, concerning the available languages of the questionnaires, the time it takes to complete each questionnaire (as described by the publisher), and whether the PROMs were used in previous sarcoma trials. The final selection of PROMs was made using the Delphi method within the “Quality of Life” subgroup of the FOSTER consortium.
3. Results
3.1. Selected studies
A total of 1377 studies were identified. Figure 1 shows the flow diagram of the selected studies and the reasons for eligibility and noneligibility. After screening titles and abstracts, 102 articles were selected. By screening the reference lists of selected studies, a total of 26 articles were added. So, in total, 128 full-text articles were assessed for eligibility. Fifty-five articles were excluded from the review. The main reason was that articles did not use QoL assessments, give only a general overview (n = 23), or included less than ten osteosarcoma patients in their study (n = 13). Finally, 73 articles were included in the synthesis: 63 original articles and 10 systematic reviews, which were discussed separately.
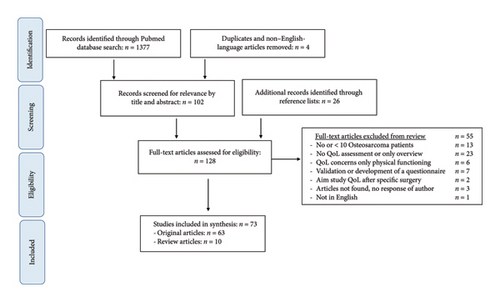
The 63 original included studies were characterized by heterogeneity. Most of the included articles were cross-sectional studies (n = 48), and 4 of them were partly qualitative also using (semi)structured interviews. Eight articles were qualitative studies (6 single-center, 2 multicenter) using (semi)structured interviews. Seven studies were prospective longitudinal studies (4 single-center, 3 multicenter), using measurement time points ranging from two to six. From the 48 cross-sectional studies, 23 studies were single-center retrospective studies and one study a single-center prospective study, and 24 multicenter studies, of which 17 were retrospective and 7 were prospective. Of the 48 cross-sectional studies, 19 studies used a control group (mostly siblings/healthy controls, while 29 articles did not use a control group.
Also, the age of the target population exhibited considerable variation. In most articles, “children” refers to an age below 14 years, “adolescents” to ages 14–18, and “adults” to those over 18 years. However, this is not consistent across all the included studies. The term “young adults” also varies among articles regarding the age range included. Seven articles exclusively enrolled pediatric patients (< 14 years), while 4 articles encompassed both pediatric and adolescent patients (0–18 years). One paper specifically concentrated on adolescent patients, and 2 articles incorporated adolescent patients alongside adult patients. Additionally, 23 papers included both pediatric (up 18 years) and adult patients (< 18 years), while 22 papers exclusively focused on adult patients. Notably, four papers centered on adult patients who were pediatric or adolescents at the time of their initial diagnosis.
Furthermore, the study populations across these articles displayed heterogeneity. Of the 63 original selected articles, 20 articles specifically focused on osteosarcoma patients [15–34], with the majority of studies (n = 33) included patients with malignant bone tumors/bone sarcoma [5, 35–66]. Additionally, 10 studies extended their inclusion criteria to incorporate also individuals with soft tissue sarcomas [67–76]. The majority of the studies included patients with tumors in various anatomical locations; however, some studies exclusively enrolled patients with tumors localized to specific areas, primarily in the lower extremities. In these studies, the primary research question often focuses on assessing QoL along with functional abilities and/or physical activity for malignant bone sarcoma in the lower extremities [18, 37, 38, 45, 47, 51, 55, 56, 61, 65, 66, 72, 75]. Sometimes, these studies involve comparison between different surgical interventions [21, 27, 45, 46, 73, 77, 78].
With the exception of 11 cross-sectional, multicenter retrospective cohort studies [23, 27, 50, 51, 62, 70–72, 74, 76, 79], 1 cross-sectional, single-center retrospective cohort study [58], and 2 longitudinal prospective phase 3 trials [29, 35], most of the included articles evaluated small cohorts of patients. Looking at the 49 remaining papers, the studies included a mean of 48 patients (ranging from 10 to 202); however, these subjects had not all osteosarcoma.
3.2. Used Questionnaires in selected Articles
In total, 39 different questionnaires were identified in the selected articles to assess QoL or HR-QoL. In some studies, these terms are used interchangeable. The majority of the studies used various PROMs. However, 19 studies used one PROM [15, 20–22, 30, 32, 36, 39, 50, 53, 54, 57, 64, 67, 71, 73, 74, 79, 80], 18 studies used 2 PROMs [16, 18, 19, 23, 25, 27, 33, 35, 37, 38, 42, 46, 51, 52, 56, 58, 70, 81], 16 studies used 3 PROMS [17, 26, 28, 29, 34, 41, 43, 48, 49, 55, 61, 63, 66, 69, 72, 76], 5 studies used 4 PROMS [40, 47, 60, 65, 77], and 5 studies used 5 PROMS [24, 31, 44, 68, 82]. Table 1 presents an overview detailing the PROM that have been used in more than one study, encompassing aspects as the questionnaire’s target population, various major domains and subdomains covered, availability in languages, completion time, and its previous application in clinical sarcoma trials.
| Patient-Reported Outcome Measurements | Domains and item numbers | No. of articles using PROM | Target population Onc ∗/BS ∗∗/general (age range) |
Languages | Time to complete questionnaire | Used in earlier sarcoma trials |
|---|---|---|---|---|---|---|
| The Short Form (36) Health Survey (SF-36) |
|
30 |
|
English, Spanish, French, Swedish, Korean, German, Dutch, Portuguese, Chinese, Finnish, Danish, African, Hungarian, Hebrew, Italian, Japanese, Norwegian, Polish, Romanian, Slovak, Russian, Czech | 5–10 min | No |
| European Organization for the Research and Treatment of Cancer-Core Module (EORTC QLQ-C30) |
|
10 |
|
> 110 | 5–10 min | Yes: Euramos trial, Ewing 2008, InterEwing1, iEuroEwing, EpSSG2005, FaRRMS |
| TNO-AZL Questionnaire for adult´s Quality of Life (TAAQQL) |
|
6 |
|
English, Dutch | No | |
| TNO-AZL-Child Quality-Of-Life (TACQOL) | 5 domains: Physical complaints or physical ailments, motor skills, independence or autonomy, social functioning, cognition | 5 |
|
English, Dutch, French, Italian, Spanish, Vietnamese, Koreans, Russian, Bulgarian | 10–15 min | No |
| Quality of Life for Cancer-Specific (QOL-CS) | 4 domains: Physical, psychological, social, spiritual well-being | 5 |
|
5–10 min | No | |
| Brief Symptom Inventory (BSI) |
|
5 |
|
English, French, Spanish | 8–10 min | No |
| Pediatric Quality of Life Inventory (PedsQL) (3.0/4.0) |
|
4 |
|
> 125 | < 4 min | Yes: Euramos-1 trial, Ewing 2008, InterEwing1, iEuroEwing, EpSSG2005, FaRRMS |
| PedsQl-cancer module (PCQL) |
|
4 |
|
English, Spanish, French, Italian, Spanish, Thai, Swedish, Brazilian, Chinese, Japanese, Pakistani, German | < 4 min | Yes |
| DUX Questionnaire for lower extremity bone tumor (Bt-DUX) |
|
3 |
|
English, Dutch, German, Italian | 5 min | No |
| Impact of Event Scale (IES-R) |
|
3 | General and persons after traumatic events | English, Spanish, French, Chinese, Japanese, and German, Dutch | 5–10 min | No |
| Symptom Distress Scale (SDS) |
|
3 | Oncology | Dutch, English, Italian, Spanish, Swedish, Taiwanese | 5–10 min | No |
| Childhood cancer survivor study (CCSS) |
|
3 | Oncology | English | 10–15 min | No |
| Body Image Scale (BIS) |
|
2 |
|
English, German, Dutch, Italian, Greek, Portuguese, French, Spanish, | 5 min | No |
| The Posttraumatic Growth Inventory (PTGI) |
|
2 | General and persons after traumatic events; > 18 years and pediatric version |
|
5–10 min | No |
| Child Health Questionnaire (CHQ) |
|
2 |
|
21 languages | 15 min | No |
| Positive and Negative Affect Scale (PANAS), Mishel’s Uncertainty in Illness Scale-Community Form (MUIS), Kidscreen-52, Self-Perception-Profile of Adolescents (SPPA), Central Sensitization Inventory (CSI), 15 dimensions (15-D), Amputee Body Image Scale (ABIS), Beck depression Inventory (BDI), Short-Form 8/12 (SF8, SF12), EQ5D, Hospital Anxiety and Depression Scale (HADS), Ego-Resiliency Scale (RSI), Hopkins Symptom Checklist (HSCL), Mental adjustment to cancer (MAC), Brief Pain Inventory (BPI), Global Severity Index (GSI), Bern Questionnaire on adolescents Wellbeing (BFW), State Trait Anxiety Inventory (STAI), Impact of event scale-revised (IES-R), Body Image Instrument (BII) General health questionnaire (GHQ-38), Future expectations Scale for adolescents (FESA), Minneapolis-Manchester Quality of Life Instrument (MMQL), Multidimensional Fatigue Inventory (MFI) | 1 | |||||
- ∗Cancer-specific.
- ∗∗BS = bone sarcoma-specific.
The most commonly utilized questionnaire was the Short Form 36 (SF-36), which is designed as a multipurpose health survey instrument for patients aged 16 and older [83]. It assessed 8 different domains with 36 items, is validated, and is available in multiple languages. However, it was not used in earlier International sarcoma trials. Also, the European Organization for the Research and Treatment of Cancer-Core Module (EORTC QLQ-C30) [84] is frequently used. Initially designed for adult cancer patients, whether or not in remission, this questionnaire evaluates various aspects of QoL, including physical functioning, role functioning, emotional functioning, cognitive functioning, and social functioning. The PROM is a validated questionnaire translated into multiple languages, and utilized in various European pediatric and adult sarcoma treatment protocols, such as osteosarcoma trials (Euramos Protocol [85], Ewing trials (Ewing 2008 protocol [86], upcoming InterEwing-1 protocol (EudraCT number: 2021-005061-41), iEuroEwing protocol (EudraCT number: 2019-004153-93), and rhabdomyosarcoma trials (EpSSG2005 [87], FarRMS-protocol (NCT04625907)). The most used questionnaire was the Pediatric Quality of Life Inventory (PedsQL) or the PedsQl-Cancer module. The PedsQL [88] was developed to assess HR-QoL in children from the perspective of children and their parents and comprises 23 items divided into four different fields, which assess physical, emotional, social, and school functioning. Two summary scores are calculated: a physical health summary score and 1 psychosocial summary score. The questionnaire is age-specific (5–7 years (3-point Likert scale), 8–12 years, and 13–18 years (5-point Likert scale), and forms are available for patients and parents/caregivers. The PedsQL-Cancer module [89], a specific module of the PedsQl for patients being treated for cancer, is accessible for patients aged under 25, assessing physical, psychological, cognitive, and social functioning, and also inquiring items about body image and communication. Both the PedsQL and PedsQL-Cancer module are available in multiple languages and used in large previous and upcoming Ewing and rhabdomyosarcoma trials. Although the TACQOL was also used frequently, particularly in adults, it is a questionnaire that is not available in multiple languages. The Bt-Dux [49] is the only bone sarcoma-specific questionnaire and was developed to examine patients’ individual values of their life after a malignant bone tumor of the lower extremity at four domains: cosmetic, social, emotional, and functional.
3.3. Described Domains and Subdomains in Selected Studies
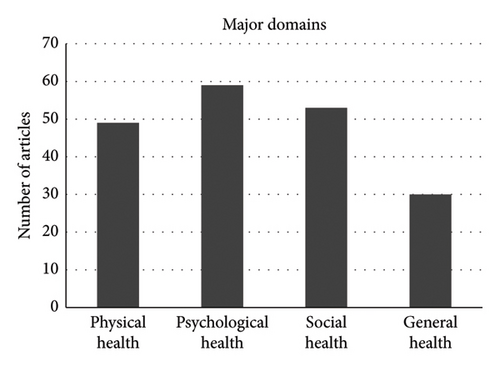
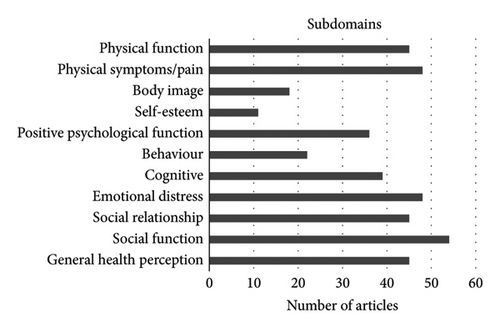
The domains evaluated in the selected articles displayed significant variability. Some articles focused solely on one of the (sub)domains outlined by Anthony et al. [13, 32, 50, 62]; however, most of the articles focused on 2-4 different primary domains. Among the primary domains defined by Anthony et al. [13], namely physical, psychological, social, and general health, general health was the least frequently domain addressed in the different analyzed studies. The most extensively assessed domain was psychological health, 56/63 (=89%) of the included studies evaluating at least one subdomain within this category, and general health was evaluated in only 21/63 (=33%) of the studies (Figure 2(a)). As for the specified subdomains, social function (47/63 = 75%) and emotional distress (47/63 = 75%) as well as physical function and symptoms (46/63 = 73%) were the most frequently examined aspects. Conversely, subdomains such as self-esteem (16/63 = 25%), body image 19/63 = 30%), and behavior (19/63 = 30%) were the least frequently evaluated subdomains (Figure 2(b)).
3.4. Systematic Reviews
Ten articles were systematic reviews [44, 45, 78, 81, 82, 90–94]; in two of them, a meta-analysis has been performed. Aims of the systematic reviews were very different. An overview of the aims and outcomes of the systematic reviews is shown in Table 2.
| References | Title | Authors | Year of publication | Study population | Aim of study | Outcomes |
|---|---|---|---|---|---|---|
| [45] | Quality of life, functional ability and physical activity after different surgical interventions for bone cancer of the leg: A systematic review. | Bekkering et al. [45] | 2012 | Pediatric and adults leg bone tumors after surgery | To review published studies comparing QOL, functional ability, and/or physical activity between different surgical interventions (ablative versus limb-sparing) due to a malignant bone tumor of the leg. | Twelve studies comparing outcomes of QoL, functional ability, and physical activity between limb-sparing and ablative surgery groups were identified, but no general conclusion on the advantage of either limb sparing or ablative surgery could be drawn, because of largely varying outcomes. |
| [90] | Unravelling the heterogeneity of soft tissue and bone sarcoma patients’ Health-Related Quality of Life: a Systematic literature review with focus on tumor location. | Den Hollander et al. [90] | 2020 | Adult sarcoma patients (soft tissue and bone sarcoma) | 1. To unravel the heterogeneity of Health-Related Quality of Life (HRQoL) of patients with sarcoma with regard to tumor location and 2. to summarize the used measures in research. |
|
| [91] | Systematic review and meta-analysis of objective and subjective quality of life among pediatric, adolescent, and young adult bone tumor survivors. | Stokke et al. [91] | 2015 | < 25 years treated for bone sarcoma (osteosarcoma, Ewing sarcoma, other sarcoma) |
|
|
| [44] | Quality of life in survivors of a primary bone tumor: a systematic review. | Eiser and Grimer [44] | 1999 | Pediatric and adults survivors of bone tumor |
|
|
| [78] | Functional outcomes and quality of life in patients with osteosarcoma treated with amputation versus limb-salvage surgery: a Systematic review and meta-analysis. | Mei et al. [78] | 2014 | OS patients with amputation or limb salvage surgery | To compare the functional outcomes and QoL experienced by OS patients in lower limbs who underwent amputation and limb-salvage surgeries |
|
| [92] | The need for improved patient-reported outcome measures in patients with extremity sarcoma: a Narrative review. | Blight and Choong [92] | 2021 | Upper and lower extremity sarcoma patients treated with surgery |
|
Seventy articles met eligibility criteria; six PROMs were identified. The Toronto Extremity Salvage Score, The Short-Form 36, The EORTC QLQ-C30, The Disabilities of the Arm, Shoulder and Hand questionnaire, the Reintegration to Normal Living index and the Patient-Reported Outcomes Measurement Information System. Most sarcoma patients score well in these tools, with bone sarcoma, and extent of resection being predictors of poor outcomes. The TESS is the only sarcoma-specific PROM, and though a valid assessment of functionality, it has difficulty differentiating patients with minor functional impairments. The absence of a disease-specific measure of health is concerning. |
| [93] | Patient-Reported Outcomes in Sarcoma: A scoping review. | Almeida, Martins, and Lima [93] | 2021 | patients with sarcoma > 18 years |
|
27 articles were selected. The most common PRO evaluated in the selected studies were Health-Related Quality of Life (HRQoL), followed by functional outcome, aspects of mental health, and specific symptoms. Generic HRQoL questionnaires were widely used. Quantitative studies usually applied more than one type of Patient-Reported Outcome Measures (PROMs) to measure different PROs. The PROMs used in the selected studies about sarcoma were not specific to sarcoma, a different new and specific measurement strategy should be considered. |
| [82] | Health-Related Quality of Life, psychosocial functioning, and unmet health needs in patients with sarcoma: A systematic review. | McDonough et al. [82] | 2019 | Adult sarcoma patients, STS and bone sarcoma | To systematically assess and synthesize literature in relation to HRQoL, psychosocial issues and unmet health needs for patients with sarcoma during diagnosis, treatment, and short-term follow-up (up to 5 years post-treatment) and what instruments are being used to assess HRQoL in patients with sarcoma. | 31 studies were included. Compared with healthy individuals, patients with sarcoma frequently scored lower in physical and psychological HRQoL domains and experienced higher rates of self-image issues, depression, and suicide. However, outcomes for patients with sarcoma were relatively comparable to those with other malignancies. Anxiety symptoms were more common in the diagnosis phase, while depressive symptoms were more common in the treatment phase. Patients who are older, female, and socially isolated often reported lower HRQoL. A sarcoma-specific HRQoL instrument is needed to accurately describe outcomes in this population. |
| [81] | A Critical Review of the Impact of Sarcoma on Psychosocial well-being | Storey et al. [81] | 2019 | Sarcoma patients both pediatric and adults | To describe the psychosocial impact of diagnosis and treatment on patients with all types of sarcoma using validated PRO measures. | 82 studies were included. Most (65%) were assessed of being of reasonable quality. The most common aspect of psychosocial well-being measured was quality of life (80%). It seems there is an improvement in the physical aspects of quality of life over time but not in psychosocial function or mental health. There are no differences according to the type of surgery patients receive, and psychosocial outcomes tend to be poorer than the general population. However, due to the heterogeneity of methods, outcomes, and populations, it was not possible to make definitive conclusions. |
| [94] | Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: What are the long-term implications? | Nagarajan et al. [94] | 2002 | Lower extremity bone tumors in pediatric patients | Description of both amputation and limb salvage techniques as a means for local control for lower-extremity bone tumors and their impact on both the physical function and general well-being of affected children. | Comparisons between studies are difficult because small numbers of patients and the use of varying research designs and methods have limited research in this area. Survival is equivalent between amputation and limb salvage. Complications occur more frequently in limb salvage. The long-term outcomes of those undergoing amputation and limb salvage have not been found to be substantially different in regard to quality of life. Prospective long-term follow-up of pediatric patients with lower limb tumors is needed to determine the long-term complications, quality of life, and functionality of this population. |
Four systematic reviews (Table 2, article nos. 2, 6, 7, 8) focused on the most utilized PROMs in sarcoma patients conform to the aim of our study. Concerning the research question about the use of different PROMs in osteosarcoma patients and bone sarcoma patients in general, all four review articles concluded that in most articles, no specific questionnaires tailored to sarcoma patients are used. They emphasized the necessity of developing a sarcoma-specific HR-QoL instrument that can also address the heterogeneity of sarcomas and their location-specific issues [82, 90, 92, 93].
4. Discussion
The treatment for osteosarcoma is often life changing and generally includes chemotherapy and local surgical treatment. Local surgical therapy often results in permanent changes in physical appearance and function, and chemotherapeutic treatment can lead to serious side effects in both the short term and long term. It is known that survivors of bone sarcoma (osteosarcoma, Ewing sarcoma) have the highest burden of severe late adverse events, namely 64% compared to 23.4% for all childhood cancer survivors [95]. The impact on daily functioning concerns physical functioning, psychosocial functioning, and HR-QoL. However, adult patients may prioritize different aspects compared to pediatric patients, and this also varies among infants and adolescents. Hence, age-appropriate questionnaires are needed. Aim of this systematic review was to identify relevant literature concerning HR-QoL in patients treated for osteosarcoma and to give an overview of the PROMs in both pediatric (until 18 years) and adult population (> 18 years), as well as to examine the various (sub)domains of the questionnaires to identify gaps to recommend suitable instruments for an upcoming European trial designed within the FOSTER consortium (CabOs trial). A total of 1377 articles were screened, and ultimately, 63 original articles were included in the qualitative synthesis. Selected studies showed a large heterogeneity concerning study aim, study design, and study population. Most of the studies focused on patients with bone sarcoma in general (osteosarcoma, Ewing sarcoma, and sometimes chondrosarcoma) and evaluated small cohorts of patients. Since similar QoL aspects are expected after the treatment of all bone sarcoma patients, the same questionnaires seem to be applicable. After a literature search, a total of 39 different questionnaires were identified as being used to assess QoL in bone sarcoma patients.
For the International FOSTER study, PROMs should ideally meet the criteria of validation, the translation into multiple languages, appropriateness for both pediatric and adult patients, compatibility with earlier studies, cover items, and aspects important for bone sarcoma patients and should be efficient in terms of patient time. A literature study was performed to see which PROMS were frequently used in studies to assess HR-QoL in osteosarcoma patients during and after treatment and which gaps could be identified in domains important to evaluate QoL in osteosarcoma patients. In the literature, the most frequently used questionnaire in adult patients was the SF-36, designed as a multipurpose health survey measurement, and the EORTC QLQ-C30, which is designed for adult cancer patients. Both questionnaires assessed multiple domains with several items and are validated and translated in many different languages. The EORTC Quality of Life group is currently working on developing questionnaires for children aged 8–14 years, creating a tool for children younger than 8 years, and validating the EORTC QLQ-C30 for the age group of 12–17 years.
For pediatric patients, the QoL data are mostly collected by using the PedsQl. For pediatric patients treated for cancer, the PedsQl-specific Cancer Module is available and is frequently used to assess HR-QoL during and after osteosarcoma treatment. Recently, a study in patients treated for osteosarcoma demonstrated that physical functioning scores were in sufficient concordance between the PedsQl in pediatric patients and the EORTC QLQ-C30 in adult patients [96]. Accordingly, to other review articles that looked at the most frequently used PROMs for HR-QoL in patients with bone sarcoma [82, 90, 92, 93], we found that most of the used questionnaires are developed for the generic or cancer-generic population and hardly any specific questionnaires tailored to (bone) sarcoma patients are used. In a review conducted by den Hollander et al. [90], patients with sarcoma reported lower HR-QoL compared to the general population, and considerable differences in HR-QoL were found between the different sarcoma locations.
After literature search, only one bone sarcoma-specific questionnaire was found. The Bt-Dux has been designed to assess the HR-QoL in patients who have undergone treatment for a malignant bone tumor in the lower extremity and gather information regarding cosmetic, social, emotional, and functional aspects. In a study conducted by Bekkering et al. [66], it was considered a practical and valid instrument. However, this questionnaire has only been validated and translated in four languages, making it unsuitable for use in an international cohort. Although not found in the literature search, the Sarcoma Assessment Measure (SAM) for adult patients has been developed recently [97, 98]. The SAM is based on the experiences of patients with soft tissue sarcoma, bone sarcoma, and gastrointestinal stromal tumors and comprises 22 items reflecting physical, emotional, social, and financial well-being and sexuality. Currently, this questionnaire has been validated only in English and will be adapted for use in pediatric patients; however, it cannot be used in all participating countries for a trial at this time.
To examine the diverse domains and subdomains of HR-QoL to identify gaps in their coverage, the conceptual HR-QoL model designed by Anthony et al. [13] was used. From the different aspects evaluated in the selected articles, “general health” was the least assessed major domain, whereas “psychological health” was the most extensively evaluated domain. The subdomains, “physical function” and “symptoms,” as well as “social function,” were the most frequently examined aspects. As shown in our overview, the subdomains self-esteem, body image, and behavior were least frequently evaluated. Also, in the study by Holzer et al. [60], it was found that body image was significantly lower compared with healthy controls, whereas self-esteem was not affected. Most questionnaires focus on the four primary dimensions of HR-QoL (physical, functional, emotional, and social); however, aspects such as economic and vocational aspects, spirituality, sexuality, communication, and satisfaction with treatment are underexposed [99]. Recently, based on a literature review, Rothmund et al. [100] suggest updating of the model of Anthony et al. [13] and to assign a distinct domain concerning “financial issues” and also making a differentiation between “general emotional distress” and “treatment burden,” which covers involvement in shared decision-making and the social relation and communication with healthcare providers within the social domain. The subdomain “body image” was suggested to relocate into the subdomain “self-esteem” understanding it as one identifying concept of self-esteem [101]. The abovementioned aspects are indeed crucial in bone sarcoma patients. Within the FOSTER consortium, patient advocates utilized a questionnaire to collect information on the various aspects that influence QoL. Aspects such as economic health, getting information, communication, prognosis, involvement in decision-making, management of uncertainty, and of short- and long-term effects are mentioned as important but are frequently not included in the different PROMs. Aspects such as financial well-being and sexuality are now included in the Sarcoma Assessment Measurement [97].
In previous sarcoma trials in pediatric and adult population, there is in general an underutilization of PROMs evaluation [9, 102]. Moreover, the content and validity of the current questionnaires have a moderate level of evidence and comprehensiveness should be improved [103]. Based on the results of the literature search, a final selection of PROMS was made within the “Quality of Life” subgroup of the FOSTER consortium by using the Delphi method (Table 3). For adult patients (> 18 years), the EORTC-QLO-C30 was recommended because it is frequently used in selected literature and is validated and available in multiple languages. The advantage of the EORTC-QLQ-C30 over the SF36 is that it is cancer-specific and has been used in previous international sarcoma trials. The PedsQl general and PedsQl-cancer-specific are recommended for use in pediatric patients (< 18 years), and these are also frequently used in selected literature, validated, available in multiple languages and, also used in previous international sarcoma trials. The EORTC-QLQ-C30 does not include items concerning body image and self-esteem, however during and after the treatment of bone sarcoma, especially the subdomain “body image” is relevant. While the PedsQl-cancer-specific module asks for some specific items concerning the perceived physical appearance, the Body Image Scale (BIS) could be added for adult patients. The BIS is developed to evaluate body image in adult cancer patients. This 10-item questionnaire has a good structural validity and reliability and is especially developed for the use in clinical trials [104]. After the Delphi round, it was decided to implement the BIS for patients above the age of 16 years.
| Pediatric patients < 18 years | Adult patients > 18 years | ||||
|---|---|---|---|---|---|
| Pediatric Quality of Life Inventory (PedsQl)-generic | European Organization for the Research and Treatment of Cancer-Core Module (EORTC-QLQ-C30) | ||||
| PedsQl-cancer-specific | |||||
| Body Image Scale (BIS) (> 16 years) | BIS | ||||
|
|
||||
Although none of selected articles showed the use of the Patient-Reported Outcomes Measurement Information System (PROMIS) to assess HR-QoL, this system could be used to effectively measure other relevant symptoms such as anxiety, pain, and fatigue which hold particular significance following treatment in osteosarcoma patients. PROMIS is a comprehensive and flexible system for assessing the various aspects of adult and pediatric patient’s HR-QoL and has been translated into multiple languages. Specific item banks that measure a specific health concept could be used to create custom questionnaires. PROMIS has not been used in international sarcoma trials so far; however, it is validated and seems to have greater precision than most conventional measurements [105]. One of the features of PROMIS is its ability to use computer-adaptive testing (CAT). Instead of administering a fixed set of questions, CAT selects questions based on a person’s responses and stops when a reliable measurement is achieved [106–108]; however, for use in international studies where QoL is a secondary outcome, the fixed Short Forms seem to be more useful.
In conclusion, to comprehensively address as many relevant aspects as possible for osteosarcoma sarcoma patients in the upcoming trial within FOSTER, we recommend the use of the EORTC-QLQ-C30 for adult patients; for patients up to 18 years, we recommend the PedsQl general and PedsQl-cancer-specific, and the BIS for patients > 16 years. To measure additional important aspects of fatigue, pain, and anxiety, for adult patients PROMIS-29 and for pediatric patients PROMIS-25 could be used.
Separate recommendations will be provided for assessing functional outcomes. The development of new bone sarcoma-specific questionnaires, or the validation and translation of existing bone sarcoma-specific questionnaires like the SAM or the Bt-Dux, along with the utilization of new digital possibilities, holds great value for upcoming bone sarcoma trials.
Last, site of the primary tumor matters. It would be important to prospectively validate qualitative research interviews to identify predominant osteosarcoma symptoms and impacts, site-specific (i.e., upper or lower limb or trunk), as for other tumors (71).
This review has some limitations. It was restricted to full publications in English, which may have led to the omission of relevant studies in other languages or formats. The included studies varied widely in their aims, designs, and populations, making comparisons and generalizations challenging. Additionally, the selection criteria for the included studies might have introduced bias. These limitations underscore the need for more standardized, comprehensive, and longitudinal research in this field.
5. Implications for Cancer Survivors
This review offers an overview of the opportunities and shortcomings of existing questionnaires measuring different domains of HR-QoL for patients with osteosarcoma during and after their treatment. PROMS are use in different contexts. In clinical trials, PROMS can provide important information about the influence of (new) treatment or agents regarding on risks and benefits from the patient’s perspective, such as in the upcoming CaBOs trial, which compare Cabozantinib as maintenance therapy with placebo within the FOSTER consortium. The PROs collected during clinical trials could offer insights into the benefits and risks of the treatment arm. However, selected PROMS could also be used at an individual patient level. They can facilitate shared decision-making, monitor symptoms, and screen for long-term effects in survivors after osteosarcoma treatment. These recommendations for PROMS could lead to more comprehensive information about the treatment and can be expanded by exploring new possibilities, such as incorporating PROMIS. This final selection could hopefully also be used in other bone sarcoma treatments (such as the upcoming international Ewing protocols), enabling the comparison of treatments.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding has been used in the preparation of this manuscript. FOSTER Consortium was supported by “Enfants Cancers Sante (ECS)” and the “Societe Française de Lutte Contre les Cancers et les Leucemies de l’Enfant et de l’Adolescent (SFCE)”.
Acknowledgments
We would like to thank Ivan Sola from the Iberoamerican Cochrane Center for his help in constructing the bibliographic search.
Supporting Information
PRISMA 2020 Checklist.
Open Research
Data Availability Statement
The data supporting this systematic review are from previously reported studies and datasets, which have been cited in the text. A review protocol was not prepared.




