A Physical Exercise Intervention, Delivered Through In-Person or Home-Based Programs, in Patients Living With Cancer and Bone Metastases: A Single-Arm, Feasibility Study
Abstract
Evidence of the feasibility of physical exercise for patients with bone metastases is still scarce. This study assessed the feasibility of in-person or home-based exercise programs and explored their efficacy in patients with bone lesions. Twenty-one patients with bone metastases were invited to participate in an exercise intervention consisting of 12 weeks of in-person or home-based aerobic and resistance training twice a week. The primary study endpoint was the feasibility of the program, including recruitment, completion, attendance, adherence, tolerance rates, and safety. Secondary outcomes were physical fitness and patient-reported parameters. To analyze data descriptive statistics, Student’s t test and Wilcoxon signed-rank test were applied. The recruitment rate was 84%, and only one patient withdrew (5%). The median program attendance was 93%, and adherence was 85%, with the home-based program showing a higher adherence rate compared to the in-person delivery. Tolerability was 98%, and 13 mild (Grade 1 or 2) adverse events were registered. At postintervention, an increase in functional capacity was observed, while no significant changes in muscle strength, flexibility, or anthropometric parameters were detected. Enhancements in quality of life domains, including physical, role, and emotional functioning, as well as reductions in fatigue and appetite loss, were also reported. Our exercise intervention, whether delivered in person or through a home-based program, was highly feasible and safe.
Trial Registration: ClinicalTrials.gov identifier: NCT04226508
1. Introduction
Despite the advances in novel therapeutic options, bone metastasis is still a fearful complication for patients with cancer, associated with shortened survival and poorer quality of life [1]. For patients with metastatic disease, the incidence of bone metastases is around 54% for breast cancer, 89% for prostate cancer, 37% for lung adenocarcinoma, and 39% for renal carcinoma, with the spine, pelvis, ribs, humeri, and femora as the most involved lesion sites [2, 3]. Bone metastases may expose patients to a higher risk of skeletal-related events, including spinal cord compression, pathological fractures, hypercalcemia, and pain exacerbation, requiring locoregional or systemic bone-targeting therapies to prevent progression and ameliorate symptoms [4, 5]. Furthermore, patients with metastatic cancer often experience a broad spectrum of symptoms and treatment-related side effects, such as fatigue, gastrointestinal problems, depression, sleep disturbance, and pain [6].
Over the past decade, physical activity and exercise have been shown to confer various benefits to patients with cancer by improving physical fitness parameters (e.g., cardiorespiratory fitness, muscle strength, body composition, and flexibility) and alleviating several cancer-related sequelae, such as pain, fatigue, peripheral neuropathy, and anemia, with some preliminary evidence available for bone health in patients with bone lesions [1, 7, 8]. In addition, there is epidemiological evidence of a positive association between physical activity and survival in patients with cancer [9], even in those with an advanced stage of disease [10, 11]. The American College of Sports Medicine (ACSM) guidelines for patients with cancer, including those with an advanced stage of disease, recommend moderate-intensity aerobic activity at least three times per week for 30 min per session combined with resistance training twice weekly [12]. Furthermore, the International Bone Metastases Exercise Working Group introduced additional recommendations for clinicians and exercise professionals to ensure the safe management of exercise in patients with bone lesions [13].
However, despite these recommendations, some additional considerations should be made. Whereas a considerable amount of data comes from patients with an early disease stage, research-derived knowledge of physical exercise and bone metastases is still scarce. Most of the available evidence comes from studies including a mixed cancer population (e.g., with/without bone metastases) [1, 14] or conducted in patients with prostate cancer [15, 16]. Exercise safety in patients with bone metastases also warrants further consideration. Although patients living with bone lesions are interested in participating in exercise [17], about 40%–65% of clinicians working in cancer settings are still worried about the potential increased risk of fracture for these patients and believe that exercise should be avoided in this population [18, 19]; however, this is not an option given that exercise is essential for preventing other chronic diseases, supporting the patient through their cancer treatment, and maintaining physical function and quality of life [20]. From the exercise prescription perspective, a fully supervised program coordinated by dedicated exercise specialists probably remains the best option to minimize risk in this setting [13]. However, distance from appropriate exercise facilities and qualified professionals is one of the main barriers reported by patients, which may discourage their participation [21, 22]. In this sense, it is crucial to make exercise accessible to maximize the number of individuals benefiting without neglecting optimal supervision and adaptation. With these premises, the aim of the present study was to assess the feasibility of a 12-week exercise intervention delivered through two methods: in-person or home-based, based on patients’ preferences. Secondary endpoints were to evaluate the effectiveness of the exercise in improving physical fitness components and patient-reported outcomes. Exploratory aims were to investigate if patients may experience a differential benefit from an exercise intervention according to the type of bone lesions (i.e., osteolytic or osteoblastic bone metastases) and the type of treatment (i.e., hormone therapy or other treatments).
2. Materials and Methods
2.1. Study Design
To investigate the feasibility of a 12-week tailored physical exercise program in patients with cancer metastasized to their bones, a single-arm trial was carried out at the Verona Hospital. The current study followed the Good Clinical Practice principles, ensuring that all procedures adhered to the Helsinki and Oviedo Declarations. Approval for the project was granted by the Verona University Ethics Committee for Clinical Trials (Prot. N. 33320), and protocol registration was made.. The study is reported following the CONSORT Statement: extension for randomized pilot and feasibility trials [23].
2.2. Eligibility Criteria and Procedures
Between January 2021 and January 2024, patients diagnosed with solid cancer with bone metastasis were recruited by oncologists and dietitians at the Oncology Unit of the Verona Hospital to join a tailored physical exercise program. Patients’ inclusion criteria were (i) adult age (≥ 18 years old), (ii) a diagnosis of solid cancer, (iii) presence of one or multiple osteolytic or osteoblastic bone lesions, (iv) performance status ≤ 2 (evaluated with the Eastern Cooperative Oncology Group [ECOG] scale), (v) medical clearance to exercise, and (vi) signed the written informed consent for participation. Exclusion criteria were (i) major surgery ≤ 2 months at enrollment and (ii) scheduled surgery within the 3 months of exercise intervention. Potentially eligible patients were contacted by the study staff to organize a first appointment at the Sport Science facilities of the University of Verona. In this meeting, the research staff confirmed eligibility, described in detail the study procedure and intervention, and invited patients to sign written informed consent for study participation. Thereafter, patients performed the baseline assessments.
2.3. Exercise Program
The 12-week tailored physical exercise program was created in accordance with the guidelines of the ACSM for patients with cancer [12] and the recommendations for patients with bone metastases [13]. The exercise program was individualized based on patients’ needs (preferences/barriers), physical/functional assessments, symptoms, and medical/treatment history (Figure 1). Additionally, the following exercise adaptations specific to bone disease were applied in designing the program: (i) starting with moderate loads or bodyweight exercises and gradually increasing intensity throughout the program, (ii) selecting exercises that might stimulate bone anabolism (e.g., those which produce a mechanical stimulus), (iii) avoiding exercise placing rapid or high load movements that stress the area of bone lesions, (iv) stopping or modifying exercise when experiencing pain, particularly at the site of metastasis, during the sessions, (v) instructing patients on proper exercise technique and posture alignment, and (vi) continuously checking the patient’s adaptation to exercise and adjusting when necessary, throughout the sessions.
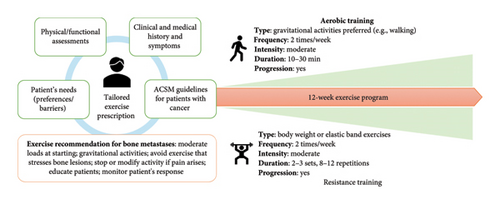
The exercise program was composed of two weekly sessions of aerobic and resistance training, which could be carried out consecutively or separately based on patients’ preferences. Aerobic training was predominantly walking activity, a ground-based activity that may promote osteogenesis [24]; however, cycling was offered if necessary (e.g., in patients at risk of falls). The duration of aerobic activity started at 10–15 min and gradually increased to 25–30 min, whereas intensity was maintained at a moderate level using the 10-point Borg Rating of Perceived Exertion Scale (i.e., 3–5 RPE). Resistance training was prescribed as 2-3 sets of 8–12 repetitions at moderate intensity (i.e., 3–5 RPE), with the volume progressively adjusted throughout the weeks based on individual capabilities. Resistance activities comprised 5 body weight or elastic band exercises (i.e., lunges, row, push press, calf raises, and abdominal exercises), adapting each exercise based on bone metastatic site and focusing on the correct technique. For instance, in patients with osteolytic bone metastases of the spine, abdominal exercises were entirely avoided to mitigate the risk of vertebral compression or fractures, while in other patients exercises consisted of planks and isometric abdominal muscle activation, which are safer alternatives. An additional balance exercise was added to each session with the aim of reducing the risk of patient falls. Each training session included a warm-up (5 min of dynamic stretching) at the beginning of the session and ended with a cool-down (5 min of static stretching).
The exercise sessions were delivered by two methods, based on patients’ preference (i) in-person, i.e., fully supervised sessions led by exercise specialists at the facilities of the Department of Sport Science, or (ii) home-based, consisting of a tailored exercise plan designed for patients to complete on their own. The home-based program was provided in paper format and detailed the type, frequency, intensity, and duration of activities, and patients were provided with a diary for recording and checking adherence and compliance, as well as elastic bands for resistance exercises. Scheduled in-person meetings (at 2, 4, and 6 weeks) at the facilities were organized to hand the exercise program, instruct patients on proper exercise techniques, self-monitor intensity, and train them on self-monitoring intensity. Weekly phone calls by the research staff provided additional support and monitored patients’ progress. While participants had the flexibility to exercise where and how they preferred, all opted to perform resistance training at home and engage in outdoor walking for aerobic activity. Participants could not change the training modality during the study.
2.4. Outcomes
2.4.1. Primary Outcomes
Feasibility was the primary endpoint of the study, comprising a series of evaluations monitored by the research staff during the program: (i) recruitment rate, i.e., the ratio of enrolled patients to those eligible; (ii) completion rate, i.e., the number of participants who completed compared with withdrawals; (iii) program attendance, calculated as the number of attended sessions out of the 24 scheduled sessions and categorized as permanent discontinuation (i.e., loss to follow-up), missed sessions (one or two consecutive), or interruption (three or more consecutive); (iv) program adherence, evaluated as the ratio of the completed volume versus the programmed dosage; adherence was also described by dose modifications, i.e., any session requiring an increase or a decrease in the exercise dosage or early session interruption when the planned session terminated before reaching the target intensity or duration; (v) tolerance, measured by comparing the perceived exercise intensity by RPE versus the prescribed RPE; for the in-person program, RPE was gathered both during and after the aerobic sessions and after each set of strength exercises. If the perceived intensity was higher or lower than prescribed, the trainer adjusted the workload accordingly to ensure alignment with the target intensity. Adjustments were documented as either a dose increase or reduction, with the corresponding RPE perceived initially noted to track the mismatch between prescribed and perceived intensity. In the home-based program, patients recorded their perceived RPE for both aerobic and strength exercises after each session in the log diary. These data were reviewed during weekly phone consultations to adjust the exercise load for the subsequent week, ensuring the intended moderate intensity was maintained; and (vi) safety, evaluated by recording the adverse events derived from exercise intervention according to the Common Terminology Criteria for Adverse Events, Version 5.0 [25]. Adverse events were checked by the exercise specialists and reviewed by an independent oncologist. For the in-person program, exercise feasibility was monitored by the exercise specialists, while for the home-based program, patients were instructed to complete the diary which was then collected and analyzed by the researchers. Based on prior studies, the program was considered feasible if no severe or life-threatening adverse events occurred and three of the following conditions were achieved: recruitment rate > 50% [26], completion rate > 80% [27], median attendance > 80% [27], median adherence > 75% [28], and median tolerance > 70% [29].
2.4.2. Secondary Outcomes
Secondary outcomes included the changes from baseline to postexercise intervention in physical fitness parameters and patient-reported outcomes. All the assessments were conducted at the Department of Sports Science by trained exercise specialists. To ensure safety, blood pressure and saturation were measured before the evaluations. Physical fitness included functional capacity, muscle strength, flexibility, and anthropometric measures. Functional capacity was evaluated using the “Six-minute walk test,” performed following the procedures of the American Thoracic Society guidelines [30]. Muscle strength was measured with the handgrip strength test and the isometric leg press or isometric leg extension [31, 32]. Isometric leg extension was used to replace leg press for patients affected by bone metastasis at the spine, pelvis, or proximal femur to avoid any potential skeletal-related risk derived from compression [13]. Lower limb flexibility was measured using the chair sit and reach test, while upper arm flexibility was assessed with the back scratch test, both following standardized procedures [33]. Height, weight, waist, and hip circumferences were measured in accordance with the World Health Organization protocols [34], and body mass index and waist-to-hip ratio were subsequently obtained.
Patient-reported measures included quality of life and amount of regular physical exercise. Quality of life was evaluated with the Italian Version of the European Organization for Research and Treatment of Cancer Quality of Life and Core Questionnaire (EORTC QLQ C-30) [35], which comprises 30 items categorized into functional scales (physical, emotional, role, cognitive, and social), symptom scales (fatigue, pain, nausea, and vomiting), and a global health index. Additional single items were used to assess symptoms commonly reported by cancer patients, such as dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea. Physical activity levels were assessed with Godin’s Shepard Leisure Time Exercise Questionnaire, in which patients had to indicate the weekly duration and frequencies of physical activity performed at strenuous, moderate, and mild intensities [36]. Sociodemographic data, including age, gender, education, marital status, employment, and financial resources, were collected during the assessments. Clinical and treatment data were obtained from medical charts.
2.5. Statistical Analysis
Descriptive statistics, such as mean, standard deviation, median, interquartile range (IQR), and percentages, were used to present baseline and feasibility outcomes. The normality assumption for secondary endpoints was assessed using the Shapiro–Wilk test, and pre–post intervention changes were analyzed using either Student’s t test or the Wilcoxon signed-rank test as appropriate. Data analysis was conducted using SigmaStat V. 4.0 (Systat Software Inc.), all tests were two-tailed, and a p value < 0.05 was the criterion for statistical significance.
Given the nature of this feasibility study, a formal sample size calculation was not conducted. However, based on prior literature and considering the number of patients being treated at the Oncology Unit, a sample comprising 21 patients was considered suitable for assessing feasibility and examining the impact of the exercise intervention on secondary outcomes.
3. Results
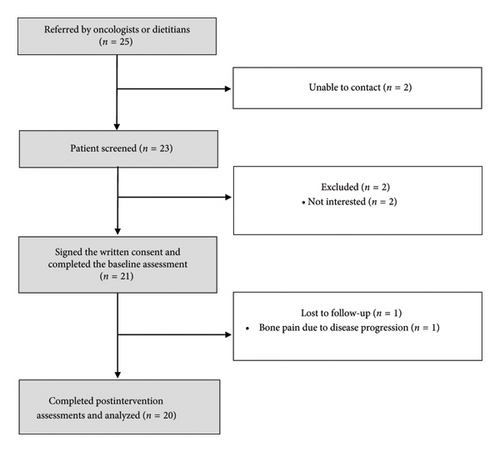
Twenty-five potentially eligible patients were referred (Figure 2), of which 4 were excluded due to inability to contact (n = 2) and lack of interest (n = 2). A total of 21 patients underwent the baseline assessments (Table 1). Most patients were female (61.9%), mean age of 58.7 ± 10.6 years, and 52.4% had a high school degree. The most common cancer types were breast (28.6%), lung (28.6%), and prostate (19.0%), with a median time since diagnosis of 15 months (IQR: 8.0–52.0 months). The thoracic (90.5%) and lumbar (80.9%) spine were the most frequent sites of bone metastases, and 57% of patients presented osteoblastic lesions. Almost all the patients (90.5%) were concomitantly treated with bone-targeted agents. Regarding the exercise program, 48% of patients opted for the home-based program and 52% for the in-person program.
| Characteristics | Participants (n = 21) |
|---|---|
| Age, mean (SD) | 58.7 (10.6) |
| Male, n (%) | 8 (38.1) |
| Female, n (%) | 13 (61.9) |
| Education, n (%) | |
| Secondary | 4 (19.0) |
| High school degree | 11 (52.4) |
| Undergraduate degree | 6 (28.6) |
| Marital status, n (%) | |
| Married | 21 (100) |
| Employment, n (%) | |
| Full-time employed | 7 (33.3) |
| Part-time employed | 2 (9.5) |
| Retired | 7 (33.3) |
| Sick leave | 5 (23.8) |
| Family income, n (%) | |
| Inadequate | 1 (4.8) |
| Barely adequate | 2 (9.5) |
| Adequate | 14 (66.7) |
| More than adequate | 4 (19.0) |
| Tumor site, n (%) | |
| Breast | 6 (28.6) |
| Lung | 6 (28.6) |
| Pancreas | 3 (14.3) |
| Prostate | 4 (19.0) |
| Thymus | 1 (4.8) |
| Liver | 1 (4.8) |
| Metastases sites, n (%) | |
| Bone | 21 (100) |
| Lymph nodes | 10 (47.6) |
| Liver | 8 (38.1) |
| Other | 6 (28.6) |
| Bone metastases sites, n (%) | |
| Cervical spine | 9 (47.6) |
| Thoracic spine | 19 (90.5) |
| Lumbar spine | 17 (80.9) |
| Sacral spine | 14 (66.7) |
| Proximal humerus | 3 (14.3) |
| Ribs | 9 (42.9) |
| Pelvis | 10 (47.6) |
| Proximal femur | 5 (23.8) |
| Type of bone metastases, n (%) | |
| Osteolytic | 11 (52.4) |
| Osteoblastic | 10 (47.6) |
| Metastatic involvement, n (%) | |
| Single organ | 2 (9.5) |
| Multiorgan | 19 (90.5) |
| Prior pathological fractures, n (%) | |
| Yes | 3 (14.3) |
| No | 18 (85.7) |
| Months since diagnosis, median (IQR) | 15.0 (8.0–52.0) |
| Type of treatment, n (%) | |
| Surgery | 8 (38.1) |
| Chemotherapy | 8 (38.1) |
| Radiotherapy | 4 (19.0) |
| Immunotherapy | 4 (19.0) |
| Target therapy | 7 (33.3) |
| Hormone therapy | 10 (47.6) |
| Current anticancer treatment status, n (%) | |
| Ongoing | 21 (100.0) |
| Treatment for bone metastases, n (%) | |
| Yes | 19 (90.5) |
| No | 2 (9.5) |
| Type of treatment for bone metastases, n (%) | |
| Zoledronic acid | 10 (47.6) |
| Denosumab | 9 (42.9) |
| Palliative radiotherapy | 10 (47.6) |
| Exercise program methods, n (%) | |
| In-person training program | 11 (52.4) |
| Home-based program | 10 (47.6) |
3.1. Feasibility
Feasibility outcomes are presented in Table 2. The study’s recruitment rate was 84%, and the completion rate was 95%, with only one patient who dropped out, in this case due to disease progression.
| Variable | Total cohort | Home-based program | In-person program | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Aerobic training | Resistance training | Overall | Aerobic training | Resistance training | Overall | Aerobic training | Resistance training | |
| Lost to follow-up, n (%) | 1 (5) | — | — | 1 (9) | — | — | 0 (0) | — | — |
| Attendance, median (IQR) | 93% (78%–98%) | 94% (82%–96%) | 96% (78%–100%) | 98% (90%–100%) | 96% (83%–100%) | 100% (96%–100%) | 93% (78%–96%) | 94% (83%–96%) | 92% (73%–96%) |
| Treatment interruption, n (%) | 5 (25) | — | — | 1 (11) | — | — | 4 (36) | — | — |
| Missed session, n (%) | 149 (16) | 70 (7) | 79 (8) | 33 (7) | 16 (7) | 17 (7) | 116 (24) | 54 (23) | 62 (26) |
| Adherence, median (IQR) | 85% (74%–96%) | 88% (82%–96%) | 83% (65%–96%) | 90% (75%–100%) | 96% (83%–100%) | 96% (58%–100%) | 89% (78%–94%) | 83% (79%–86%) | 81% (67%–85%) |
| Dose modification, n (%) | 3 (15) | 0 (0) | 3 (15) | 3 (27) | 0 (0) | 3 (27) | 0 (0) | 0 (0) | 0 (0) |
| Early session termination, n (%) | 0 (0) | — | — | 0 (0) | — | — | 0 (0) | — | — |
| Tolerability, % | 98 | 98 | 98 | 100 | 100 | 100 | 95 | 95 | 95 |
| Perceived intensity, median (IQR) | 4 (4–4 RPE) | 4 (4–4 RPE) | 4 (4–4 RPE) | 4 (3–4 RPE) | 4 (3–4 RPE) | 4 (3–4 RPE) | 4 (4–5 RPE) | 4 (4–5 RPE) | 4 (4–5 RPE) |
- Note: Definition: lost to follow-up, number of patients who did not complete the study; adherence, number of completed planned exercise dosage compared to the total programmed; attendance, number of attended sessions compared to the total; treatment interruption, number of patients who missed ≥ 3 continuous sessions; missed session, number of sessions not attended by the patients; dose modification, number of patients that required ≥ 10% of sessions dose escalation/reduction; early session termination, number of sessions interrupted before the planned intensity/duration; tolerability, number of sessions performed at the planned intensity; perceived intensity, median RPE perceived during the aerobic and resistance sessions.
The total cohort reported a median attendance of 93% (IQR: 78%–98%); attendance to the in-person program was 93% (IQR: 78%–96%), whereas patients who participated in the home-based program had a slightly higher attendance rate to the exercise sessions (98% [IQR: 90%–100%]). Both aerobic and resistance training result in more than 90% attendance rates. Overall, 149 sessions (out of 960 scheduled) were missed, 53% of them were resistance training sessions, and 78% occurred in the in-person program. Non–health-related reasons (25.5%) and lower limb thrombosis (24.2%) were the main reasons for missed sessions (Table S2; Supporting Information). Five out of 20 patients (25%) interrupted the treatment due to surgical procedures (n = 1), hospital appointments (n = 1), personal reasons (n = 1), shoulder pain (n = 1), and anticancer treatment–related adverse events (n = 1).
The total cohort median adherence was 85% (IQR: 74%–96%), with 781 sessions performed at the prescribed dosage and minimal differences between aerobic and resistance session adherence (88% vs. 83%). Regarding the training methods, patients in the in-person program showed lower median adherence for both aerobic (96% IQR: 83%–100% vs. 83% IQR: 79%–86%) and resistance (96% IQR: 58%–100% vs. 81% IQR: 67%–85%) training compared to the home-based program. Three patients in the home-based program required a dose modification of > 10% for the resistance training due to muscle pain (n = 2) and lymphedema exacerbation (n = 1). Tolerability was high, above 90%, for the two training methods. Individual attendance and adherence to the exercise program are reported in Figure S1 (Supporting Information).
No severe or life-threatening adverse (Grade 3 or 4) events occurred throughout the study. Table 3 reports the adverse events experienced during the training sessions. Grade 1 dizziness was reported in one session, whereas 12 other adverse events of Grade 2 requiring a program adjustment were registered, 10 of them in the home-based program. Additionally, during the training period, no falls were observed among the study participants.
| Variable | Total cohort | In-person program | Home-based program |
|---|---|---|---|
| Adverse events, n (%) | 13 (100) | 3 (23.1) | 10 (76.9) |
| Dizziness | 1 (7.7) | 1 (33.3) | 0 (0.0) |
| Tachycardia | 2 (15.4) | 2 (66.7) | 0 (0.0) |
| Leg muscle pain | 4 (30.8) | 0 (0.0) | 4 (40.0) |
| Back pain | 1 (7.7) | 0 (0.0) | 1 (10.0) |
| Neck/shoulder muscle pain | 5 (38.5) | 0 (0.0) | 5 (50.0) |
3.2. Physical Fitness and Patient-Reported Outcomes
Changes in physical fitness after 3 months of the exercise programs are presented in Table 4. A significant increase was observed in functional capacity (480.3 ± 75.9 vs. 514.2 ± 68.7 m; p < 0.001), whereas no statistically relevant improvements were detected for upper or lower limb muscle strength or flexibility, as well as for anthropometric measures.
| Variables | Baseline, mean (SD) | Postintervention, mean (SD) | p value |
|---|---|---|---|
| Anthropometric measures | |||
| Body weight (kg) ∗ | 73.20 (63.95–79.50) | 71.00 (63.70–83.50) | 0.674 |
| Body mass index (kg/m2) | 25.97 (4.29) | 26.10 (3.99) | 0.670 |
| Waist (cm) | 90.91 (13.84) | 89.70 (12.45) | 0.304 |
| Hip (cm) | 103.00 (9.28) | 101.84 (8.04) | 0.227 |
| Waist-to-hip ratio (cm) | 0.88 (0.09) | 0.88 (0.08) | 0.902 |
| Chair sit and reach (cm) | |||
| Right leg | −8.70 (11.09) | −6.07 (14.02) | 0.229 |
| Left leg | −10.32 (12.54) | −6.28 (13.42) | 0.070 |
| Back scratch (cm) | |||
| Right arm ∗ | 0.00 (−6.00–2.75) | 1.50 (−9.25–3.75) | 0.784 |
| Left arm ∗ | −10.25 (−15.50–−4.25) | −11.00 (−16.25–−0.50) | 0.541 |
| Handgrip (kg) | |||
| Right arm ∗ | 28.50 (22.50–33.25) | 28.00 (24.00–32.50) | 0.284 |
| Left arm | 27.30 (6.99) | 28.40 (5.09) | 0.111 |
| Leg press (kg) | 111.08 (37.45) | 91.90 (43.44) | 0.492 |
| Leg extension (kg) | 27.49 (19.46) | 44.95 (34.11) | 0.238 |
| Six-minute walking test (m) | 480.27 (75.95) | 514.20 (68.67) | < 0.001 |
- ∗Data presented as median and interquartile range.
Patient-reported outcomes before and after intervention are described in Table 5. At postintervention, significant enhancements in multiple domains of quality of life, such as physical functioning (76.3 ± 17.1 vs. 84.3 ± 12.5 points; p < 0.012), role functioning (66.7 [IQR: 41.7–83.3] vs. 100.0 [IQR: 75.0–100.0] points; p < 0.011), and emotional functioning (67.5 ± 16.2 vs. 79.2 ± 12.5 points; p < 0.018), were evident. In addition, a significant reduction in symptoms, including fatigue (43.9 ± 17.5 vs. 33.3 ± 18.7 points; p < 0.026) and appetite loss (0.0 [IQR: 0.0–33.3] vs. 0.0 [IQR: 0.0–0.0] points; p < 0.031), was also found. No significant change in habitual physical activity at any intensity was noted.
| Variables | Baseline, median (IQR) | Postintervention, median (IQR) | p value |
|---|---|---|---|
| EORTC QLQ-C30 | |||
| Physical functioning ∗ | 76.33 (17.09) | 84.33 (12.48) | 0.012 |
| Role functioning | 66.67 (41.67–83.33) | 100.00 (75.00–100.00) | 0.011 |
| Emotional functioning ∗ | 67.50 (16.20) | 79.17 (12.54) | 0.018 |
| Cognitive functioning | 83.33 (75.00–100.00) | 83.33 (83.33–100.00) | 1.000 |
| Social functioning | 66.67 (58.33–83.33) | 75.00 (66.67–83.33) | 0.110 |
| Global health status ∗ | 59.58 (16.51) | 65.42 (17.37) | 0.135 |
| Fatigue ∗ | 43.89 (17.47) | 33.33 (18.73) | 0.026 |
| Nausea/vomiting | 0.00 (0.00–16.67) | 0.00 (0.00–8.33) | 0.563 |
| Pain | 33.33 (16.67–41.67) | 16.67 (8.33–33.33) | 0.067 |
| Dyspnea | 33.33 (0.00–33.33) | 0.00 (0.00–33.33) | 0.492 |
| Insomnia | 0.00 (0.00–50.00) | 33.33 (0.00–33.33) | 0.496 |
| Appetite loss | 0.00 (0.00–33.33) | 0.00 (0.00–0.00) | 0.031 |
| Constipation | 0.00 (0.00–16.67) | 0.00 (0.00–0.00) | 0.563 |
| Diarrhea | 0.00 (0.00–33.33) | 0.00 (0.00–33.33) | 0.438 |
| Financial problems | 0.00 (0.00–33.33) | 0.00 (0.00–0.00) | 0.375 |
| Physical activity level (min/week) | |||
| Vigorous | 0.00 (0.00–0.00) | 0.00 (0.00–15.00) | 0.313 |
| Moderate | 0.00 (0.00–180.00) | 120.00 (60.00–120.00) | 0.093 |
| Light ∗ | 163.25 (197.70) | 243.25 (281.47) | 0.225 |
| Total | 232.50 (0.00–480.00) | 300.00 (127.50–615.00) | 0.241 |
- ∗Data presented as mean and standard deviation.
3.3. Exploratory Analysis According to Exercise Delivery Methods
Regarding the delivery methods (Table S1; Supporting Information), at baseline, the two groups were balanced for the sociodemographic (with the exception of education), medical, and physical fitness, as well as patient-reported outcomes. An increment in the “Six-minute walk test” was observed in both home-based (+45.08 m, p < 0.001) and in-person (+37.80 m, p = 0.004) training groups, while no changes in the other physical fitness variables were found (Table S3; Supporting Information). For patient-reported outcomes, a significant enhancement in emotional functioning was detected from the in-person program (+16.66 points, p = 0.020) (Table S4; Supporting Information).
3.4. Exploratory Analysis According to the Type of Bone Metastases
Patients with osteoblastic and osteolytic lesions (Table S5; Supporting Information) did not display differences in baseline sociodemographic, physical fitness, or patient-reported outcomes, except for gender, cancer type, left-arm strength (higher in the group with osteoblastic lesions), pain level (higher in the group with osteolytic lesions), and the total amount of physical activity (higher in the group with osteoblastic lesions). Regarding feasibility, the group of patients with osteolytic lesions reported a lower number of missed sessions with respect to those with osteoblastic metastases (49 vs. 100 missed sessions), which resulted in a higher attendance rate (96% vs. 79%). Similar findings were observed for adherence (Table S6; Supporting Information). Whereas a significant increment in functional capacity was detected in both groups (+41.78 m, p = 0.018 osteoblastic lesions groups; +42.61 m, p < 0.001 osteolytic lesions group), patients with osteolytic bone lesions also reported improvements in left-leg flexibility (+5.2 cm, p = 0.041) and left-arm strength (+2.1 kg; p < 0.001)(Table S8; Supporting Information). Concerning patient-reported outcomes, patients suffering from osteolytic bone metastases exhibited a significant reduction in fatigue (−16.16 points, p = 0.024) and pain (−19.69 points, p = 0.040) levels and increments in physical functioning (+10.91 points, p = 0.020) and in the amount of moderate-intensity physical activity (+65.45 min/week, p = 0.007). Even if positive trends were observed in the patients with osteoblastic lesions, these did not reach statistical significance (Table S9; Supporting Information).
3.5. Exploratory Analysis According to the Hormone Therapy Treatment
Grouped by the type of therapy received (Table S10; Supporting Information), the two groups were balanced at baseline. Whereas both groups exhibited significant improvements in functional capacity (+42.67 m, p = 0.002, hormone-therapy group; +83.76 m, p = 0.001, nonhormone therapy group), patients undergoing hormone therapy reported a decrease in waist circumference (−3.58 cm, p = 0.026) and enhancements in left-leg flexibility (+6.48 cm, p = 0.034) and physical functioning (+10.66 points p = 0.033). Patients undergoing other anticancer treatments had an increase in left-arm strength (+1.65 kg, p = 0.007) (Tables S11 and S12; Supporting Information).
4. Discussion
This is one of the first studies that have deeply analyzed the feasibility of in-person or home-based physical exercise programs in patients diagnosed with solid cancers and living with bone metastases. The physical exercise intervention, with an overall recruitment rate of 84%, attendance of 93%, adherence of 85%, tolerability of 98%, dropout rate of 5%, and the absence of severe adverse events, was feasible for patients with bone lesions. Our findings are higher compared to those found in the systematic review of Weller and colleagues, who reported a mean of 64% recruitment rate, 79% attendance at the exercise sessions, and a 26% dropout rate in studies including only patients with bone lesions [14] and also to those presented in a recent investigation of patients with bone metastatic prostate cancer which described a 55% of recruitment rate and 89% of attendance [37]. The exact reasons for the discrepancies could be related to the proposed physical exercise program. In our study, we requested participants to perform aerobic and resistance training twice a week, whereas, in the other investigations, the program prescription was more challenging in terms of frequency (3–5 days per week), thus requiring more commitment by the patients [14, 37]. In addition, offering different exercise settings for participant choice (i.e., in-person or home-based) may reduce the negative impact of some common barriers (e.g., distance from facilities or lack of time) related to exercise practice that usually discourage patients from participation and may negatively impact attendance.
In line with prior research [1, 14], our study confirms the safety profile of physical exercise through the absence of severe side effects, particularly those skeletal-related, such as pathological fractures. This finding is particularly crucial in the context of bone metastatic cancer, where a traditional dogma relating physical exercise with a higher risk of skeletal-related adverse events persists [19]. In addition, our study offers valuable insight into the safety of different physical exercise methods. Indeed, traditionally, home-based programs are considered unsupervised settings, which are not generally advised and, therefore, tested (almost all the available studies were conducted in a supervised manner) in patients with bone metastases [13]. However, we have developed a home-based prescription that addresses some important issues of supervision and adaptation by including a tailored program based on patient condition, scheduled meetings in which patients were educated on the correct exercise practice, self-monitoring the intensity, recognition of the “red flags” associated with high-risk related to exercise, and weekly phone calls to supervise the program status and remotely offer support to patients.
Concerning physical fitness, a significant increase in functional capacity was observed, while no improvements in strength, flexibility, and anthropometric parameters were detected. Previous research into patients with bone metastases currently shows mixed results; for instance, a randomized controlled trial including 57 patients with bone metastatic prostate cancer found that 3-month fully supervised aerobic and resistance training performed thrice a week was able to increase lower-limb strength but failed to obtain significant benefits for body composition and functional capacity [15]. Conversely, a single-arm investigation on 20 men with prostate cancer and bone metastases implementing resistance training twice a week for 12 weeks resulted in improvements in strength, functional capacity, and lean mass [16]. Whereas prior research reported that a physical exercise program did not significantly impact quality of life [1], in our study, enhancements in functioning scales such as physical, role, and emotional, as well as in some symptoms, especially fatigue and appetite loss, were noted. This may suggest a potential effect of exercise on quality of life, even if the design of our study limits the possibility of drawing definitive conclusions. In this sense, future randomized controlled trials are necessary to demonstrate the real efficacy of physical exercise in the context of bone metastases on physical fitness and quality of life parameters.
Although, to our knowledge, no research has yet been reported on the different impacts of physical exercise according to the type of bone metastases, our exploratory analysis suggests that patients with osteolytic lesions may experience an increased magnitude of benefit from physical exercise. Indeed, whereas a significant increase in functional capacity was observed in both groups, patients with osteolytic lesions also obtained improvements in upper-limb strength, physical functioning, fatigue, and pain levels. While these results could be partially due to the imbalance between the two groups in the baseline values, especially for strength and pain level, and at the differential efficacy of bone-targeted agents among the bone metastatic types [38], this does give rise to some interesting speculation. Compared to those with osteoblastic bone metastases, patients with lytic lesions may experience more pain and have a more impaired quality of life, probably given the predominance of bone resorption versus deposition, which results in bone destruction [39]. In this sense, physical exercise might help to mitigate a more compromised situation from the physical and quality of life point of view, but also, as suggested by the preliminary results of Rief and colleagues, might have a synergistic role in accelerating bone formation at the metastatic site [40]. Although these observations are purely speculative, they might also pave the way for future investigations to understand if physical exercise may have a different impact with respect to the type of bone lesions.
The heterogeneity of the study population, lack of follow-up, absence of assessments dedicated to bone density evaluation, and lack of history of falls before enrollment constitute some limitations of the present study. Among the strengths, this is the first study on patients with bone metastases that provides a comprehensive collection of feasibility data as well as offers two different types of exercise settings based on patients’ preferences without neglecting their supervision and adaptation.
5. Conclusion
Overall, a 12-week in-person or home-based physical exercise program is feasible and safe in patients affected by metastatic bone cancer and seems effective in increasing functional capacity, enhancing quality of life, and ameliorating disease and treatment-related symptoms. Future studies implementing a randomized controlled design could confirm these preliminary results.
Ethics Statement
Approval for the project was granted by the Verona University Ethics Committee for Clinical Trials (Prot. N. 33320).
Consent
All patients signed the written informed consent prior to study participation.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Anita Borsati: conceptualization, formal analysis, methodology, investigation, resources, data curation, writing – original draft, writing – editing, and visualization.
Christian Ciurnelli: investigation, formal analysis, data curation, visualization, and writing – review and editing.
Lorenzo Belluomini: resources, writing – review and editing, and visualization.
Linda Toniolo: resources, writing – review and editing, and visualization.
Ilaria Trestini: resources, writing – review and editing, and visualization.
Daniela Tregnago: resources, writing – review and editing, and visualization.
Marco Sposito: resources, writing – review and editing, and visualization.
Jessica Insolda: resources, writing – review and editing, and visualization.
Francesca Zacchi: resources, writing – review and editing, and visualization.
Elena Fiorio: resources, writing – review and editing, and visualization.
Francesco Bertoldo: resources, writing – review and editing, and visualization.
Federico Schena: resources, writing – review and editing and visualization.
Michele Milella: writing – review and editing, visualization, supervision, and project administration.
Sara Pilotto: conceptualization, methodology, investigation, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision, and project administration.
Alice Avancini: conceptualization, methodology, investigation, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision, and project administration.
Sara Pilotto and Alice Avancini share the co-last authorship.
Funding
This work was supported by the extraordinary fund of the University of Verona for open-access publication.
Acknowledgments
The authors thank the participants in this study. This work was supported by the extraordinary fund of the University of Verona for open-access publication.
Supporting Information
Figure S1: Individual exercise program attendance and adherence; Table S1: Patients’ characteristics at baseline according to exercise program methods; Table S2: Reasons for missed sessions according to exercise program methods; Table S3: Patients’ changes in physical fitness after intervention according to exercise program methods; Table S4: Patients’ changes in quality of life and exercise levels after intervention according to exercise program methods; Table S5: Patients’ characteristics at baseline according to the type of bone metastases; Table S6: Feasibility outcomes of the entire cohort and according to the type of bone metastasis; Table S7: Reasons for missed sessions according to the type of bone metastases; Table S8: Patients’ changes in physical fitness after intervention according to the type of bone metastases; Table S9: Patients’ changes in quality of life and exercise levels after intervention according to the type of bone metastases; Table S10: Patients’ characteristics at baseline according to hormone-therapy treatment; Table S11: Patients’ changes in physical fitness after intervention according to hormone-therapy treatment; Table S12: Patients’ changes in quality of life and exercise levels after intervention according to hormone-therapy treatment.
Open Research
Data Availability Statement
The data underlying this article are available in the article and in its online supporting information.




