Efficacy and Safety of Tofacitinib in Patients With Refractory Prurigo Nodularis: A 16-Week, Single-Center, Prospective, Observational Pilot Study
Abstract
Background: The current management for prurigo nodularis (PN) is challenging. Tofacitinib may emerge as a promising treatment for PN.
Aim: This study aimed to assess the efficacy and safety of tofacitinib in treating refractory PN.
Design and methods: A 16-week prospective observational pilot study was conducted in patients with moderate-to-severe refractory PN. The enrolled patients received oral tofacitinib. The primary endpoints included improvement in pruritus and quality of life, as measured by investigator’s global assessment (IGA), prurigo activity score (PAS), visual analog scale (VAS), numeric rating scale (NRS), verbal rating scale (VRS), Dermatology Life Quality Index (DLQI), and Itchy Specific Quality of Life (Itchy QoL) at week 12. Secondary endpoints were the proportion of patients with a ≥ 4-point reduction in Worst Itch-Numeric Rating Scale (WI-NRS) from baseline at week 12 and week 16. Safety assessments were conducted through week 16. Besides, historical controls, mixed-effects model (MMRM), and post-hoc analyses were performed to evaluate the efficacy of tofacitinib.
Results: Twenty-four PN patients included demonstrated clinical improvement in terms of skin lesions, pruritus, and quality of life. IGA, PAS items, VAS, NRS, VRS, DLQI, and Itchy QoL significantly improved from the baseline to 12 weeks of tofacitinib treatment (p < 0.05 for all). However, the response to tofacitinib became less pronounced at week 16, with reduced improvement in skin lesions, itch, and quality of life compared to that at week 12. The percentage of patients experiencing a ≥ 4-point WI-NRS reduction decreased from 75% at week 12% to 66.67% at week 16. The results of historical controls, MMRM model, and post hoc analyses supported the clinical efficacy of tofacitinib in PN, with baseline NRS24h,worst appearing to be a potentially clinical feature impacting treatment efficacy. No severe adverse events were reported up until the end of the study period.
Conclusions: Tofacitinib demonstrates effectiveness in reducing itch and skin infiltration in patients with moderate-to-severe PN, whereas its long-term efficacy requires further observation.
Trial Registration: ClinicalTrials.gov identifier: NCT06201715
1. Introduction
Prurigo nodularis (PN), also known as chronic nodular prurigo, is a dermatological disorder characterized by intense pruritus and inflamed excoriated nodules localized to the extensor surfaces of the extremities [1]. PN dramatically impairs patients’ quality of life, leading to an increased burden on mental health and social economy [2]. While PN can manifest at any age, it is more prevalent in females and older individuals. The pathogenesis of PN remains largely unknown, but it has been hypothesized to be a neurodermatitis [3]. Immune reaction and neuropeptides play a crucial role in cutaneous inflammation, with altered neural circuitry contributing to pruritus associated with PN [4]. Additionally, PN patients often coexist with systemic, dermatologic, and neuropsychiatric conditions [5]. Despite the development of emerging treatments for PN, their clinical application is limited due to low efficacy or a high frequency of side effects [6–10].
Tofacitinib, a non-selective Janus Kinase (JAK) inhibitor, presents as a promising therapeutic option for PN. Previous cases have demonstrated its beneficial effects on both skin lesions and itch improvement in patients with PN; JAK inhibitors are also likely to exert itch-specific effects [11, 12]. JAK inhibitors provide anti-inflammatory effects through the disruption of the JAK-signal transducer and activator of transcription (STAT) signaling pathways used by multiple cytokine receptors, including those for IL-4, IL-13, and IL-31. Selective targeting of JAK1 and JAK3 allows for the concurrent inhibition of multiple cytokine pathways. Moreover, it is suggested that JAK inhibitors also have specific effects on itch sensation [13]. Herein, we conducted a single-center pilot study to evaluate the effectiveness and tolerability of tofacitinib in treating PN patients during an observation period of 16 weeks.
2. Methods
2.1. Study Design
Our study was a prospective, single-center, open-label, observational pilot study that receive approval from the Ethics Committee of Zhejiang University School of Medicine Second Affiliated Hospital (IRB20231186) and was conducted in compliance with the Declaration of Helsinki.
2.2. Patients
Between September 2022 and February 2023, patients were evaluated for eligibility based on the following inclusion criteria: (1) age ≥ 18 years old; (2) diagnosed with PN with a duration exceeding 6 months; (3) presence of at least 10 pruritic nodules; (4) a worst itch-numeric rating scale (WI-NRS) score ≥ 7 one week prior to the study; (5) a history of more than 2 weeks of ineffective topical glucocorticoid treatment or antihistamine therapy; and (6) signed informed consent and willingness to cooperate with the follow-up as well as adherence to the study protocol. Exclusion criteria included the current use of biologic, systemic glucocorticoid, or immunosuppressive agents; previous use of JAK inhibitors; pregnancy or lactation status; abnormal findings in complete blood count, liver functions, and kidney function tests for patients; presence of any infection or inflammation; active tumors or an increased risk of tumor complications; systemic comorbidities that could interfere with or complicate study assessments. Additionally, individuals experiencing active atopic dermatitis were not included in this study. The fundamental characteristics and clinical data of the patients were documented.
2.3. Treatment
During a 16-week observational study, all patients were subjected to an assessment of the efficacy and safety of tofacitinib. They were administered a 5-mg tofacitinib tablet twice daily. Patients who had been using a stable regimen of low-to-moderate potency topical corticosteroid and topical calcineurin inhibitors prior to screening were permitted to continue their usage throughout the duration of the study.
2.4. Outcomes
A comprehensive medical history and physical examination were conducted for all participants. The efficacy and safety of the treatments were evaluated by the investigator and subjects. A blinded investigator assessed the clinical efficacy using the investigator’s global assessment (IGA) and prurigo activity score (PAS). The IGA of PN severity was evaluated on an 11-point scale ranging from −5 (obvious aggravation) to 5 (marked improvement) points. The PAS is a seven-item questionnaire evaluating the type, distribution, and size of pruriginous lesions, representative body area, exact number of lesions, activity in terms of percentage of pruriginous lesions with excoriations/crusts on top, and the percentage of healed pruriginous lesions. The PAS has demonstrated to be useful in objectively measuring PN patients over time [14]. Patients assessed their degree of efficacy using visual analog scale (VAS), numeric rating scale (NRS), verbal rating scale (VRS), Dermatology Life Quality Index (DLQI), and itchy specific quality of life (Itchy QoL) [15]. The VAS is a visual scale for itch on a horizontal 10-cm line, with “no itch” marked at the left end and “worst imaginable itch” marked at the right end. We defined the average pruritus over the past 24 h as VAS24h,average and both worst and average pruritus over the past 4 weeks as VAS4w,worst and VAS4w,average, respectively. The NRS is a similar tool for measuring itch ranging from 0 (no itch) to 10 (unbearable itch). Similarly, we defined the average and worst pruritus of the past 24 h using NRS as NRS24h,average and NRS24h,worst, respectively. The VRS is a five-point questionnaire indicating varying levels of itching intensities as follows: 0 = none; 1 = mild; 2 = moderate; 3 = severe; 4 = very severe. The VRS24h,average, VRS24h,worst, and VRSevening represented 24-h average, worst, and average evening pruritus by VRS. The DLQI is a questionnaire with scores ranging from 0 to 30, where a total score of 30 points indicates the worst possible quality of life due to pruritus and a change of the score of ≥ 4 points is considered to be clinically important. The quality of life for patients with PN was evaluated using Itchy QoL, which includes 22 pruritus-specific items such as symptoms, functional limitations, and emotions.
The primary outcomes include the mean changes from baseline in IGA, PAS, VAS24h,average, VAS4w,worst, VAS4w,average, NRS24h,average, NRS24h,worst, VRS24h,average, VRS24h,worst, VRSevening, DLQI, and Itchy QoL at week 12. Secondary outcomes included the proportion of patients experiencing reduction in WI-NRS by ≥ 4 points from baseline at week 12 and week 16. To comprehensively evaluate the treatment effect over time, a mixed-effects model (MMRM) was employed on our study. The MMRM was implemented using the “nlme” and “emmeans” packages in R. The model includes the following fixed effects: time (as a categorical variable with levels at each visit week), baseline NRS24h, worst, disease duration, atopic history, age, sex, and body mass index (BMI). The random effects included a random intercept for each subject to account for individual variability. An unstructured covariance matrix was used to model within-subject correlations. The analysis focused on the four efficacy outcomes: 0% or 1%–25% activity of pruriginous lesions with excoriations/crusts (corresponds to IGA PN-A score of 0 or 1), NRS24h,worst, VAS4w,average, and VRSevening. The significance of the treatment effect over time was evaluated through likelihood ratio tests and analysis of variance (ANOVA) for fixed effects. Estimated marginal means (EMMs) were calculated, and contrasts were performed to compare each follow-up time point with the baseline. For post hoc analyses, we first examined interaction effects of baseline NRS24h,worst, BMI, and age in NRS24h,worst and VAS4w,average following the identification of differences from the MMRM model. Additionally, post hoc analyses with the Bonferroni correction were performed for multiple clinical demographics including baseline NRS24h,worst, disease duration, atopic history, age, sex, and BMI also being conducted.
Since our study is an observational study without placebo control, we included a comparison with historical placebo groups from PRIME and PRIME2 trials. Historical controls are an alternative to randomization to a placebo in these settings, providing a comparison that is helpful in understanding the clinical effect of an intervention [16]. LIBERTY-PN PRIME and PRIME2 were two similarly designed phase 3 trials of dupilumab in adults with moderate-to-severe PN [17], which shared comparable eligibility criteria and several end points with our study. The detailed study designs and baseline patient characteristic of these trails have previously been studied.
2.5. Statistical Analysis
All statistical analyses were conducted using SPSS for Mac software (version 26.0), GraphPad Prism 9 (version 9.2.0), and RStudio (version 2024.12.1). Descriptive data were reported as mean values with standard deviation (SD). A paired samples t-test was employed to compare before and after treatment with tofacitinib. In cases where the assumption of the paired t-test was not met, the Wilcoxon matched-pairs singed rank test was utilized to determine subjects’ assessment of the effectiveness and satisfaction score for different treatments. Given the relatively small sample size of our study and the uncertainty regarding variance homogeneity, we employed Welch’s t-test and Fisher’s exact test to compare our findings with historical placebo data from the PRIME and PRIME2 trials. Welch’s t-test was chosen to accommodate potential unequal variances between groups, while Fisher’s exact test was applied for categorical variables to ensure robustness in statistical comparisons. A p value < 0.05 was considered statistically significant, and all probability values were two-tailed.
3. Results
3.1. Patient Characteristics
In total, 27 patients were initially screened, with 24 patients completing the study. Three patients withdrew because of COVID-19, while none of the remaining 24 patients discontinued treatment due to adverse events. The mean age of patients was 58.88 ± 15.17 years, ranging from 31 to 89 years. The mean period of PN was 5.92 ± 7.42 years. Elevated IgE and elevated eosinophil were observed in 16.67% (4/24) and 8.33% (2/24) of patients, respectively, with atopy reported in 29.17% of patients. All patients had previously received topical agents, and a quarter (6/24) had previously been treated with more than two systemic medications. At baseline, the mean values for VAS24h,average and VAS4w,worst were recorded as being at a level of severity corresponding to scores of 6.04 ± 2.22 and 7.71 ± 2.44, respectively. The mean baseline average itch NRS24h,worst score was 7.58 ± 2.6. Assessed by a 0-to-4 VRS score, the mean baseline VRSevening score for night-time itch was 2.75 ± 1.15. The mean baseline DLQI and Itchy QoL scores for quality of life were 21.63 ± 4.44 and 67.00 ± 14.46, respectively. The demographic and disease characteristics of the patients at baseline are presented in Table 1.
| Characteristics | (N = 24) |
|---|---|
| Age (years), mean ± SD | 58.88 ± 15.17 |
| Male sex, n (%) | 18 (75%) |
| BMI (kg/m2), mean ± SD | 23.65 ± 3.95 |
| Disease duration (years), mean ± SD | 5.92 ± 7.42 |
| Disease onset (years), mean ± SD | 52.96 ± 14.72 |
| Family history of atopy | 3 (13%) |
| Comorbidities, n (%) | |
| Hypertension | 8 (33%) |
| Diabetes | 7 (29%) |
| Depression | 3 (13%) |
| Presence of atopy | 7 (29%) |
| Others | 7 (29%) |
| Personal habits, n (%) | |
| Drinking | 4 (17%) |
| Smoking | 7 (29%) |
| Prior treatment, n (%) | |
| Topical medication | 24 (100%) |
| Systemic medication (≤ 2 agents) | 11 (46%) |
| Systemic medication (> 2 agents) | 6 (25%) |
| Baseline disease severity | |
| Estimated no. of nodules on body, n (%) | |
| > 100 | 10 (42%) |
| 50–100 | 11 (46%) |
| < 50 | 3 (13%) |
| Elevated IgE (> 200 IU/mL), n (%) | 4 (17%) |
| Elevated eosinophil (> 1∗10^9/L), n (%) | 2 (8%) |
| Mean VAS24h,average (0–10 scale), mean ± SD | 6.04 ± 2.17 |
| Mean VAS4w,worst (0–10 scale), mean ± SD | 7.71 ± 2.39 |
| Mean NRS24h,worst (0–10 scale), mean ± SD | 7.58 ± 2.60 |
| Mean VRSevening (0–4 scale), mean ± SD | 2.71 ± 1.21 |
| Mean DLQI (0–30 scale), mean ± SD | 21.63 ± 4.44 |
| Mean itchy QoL (0–110 scale), mean ± SD | 67.00 ± 14.46 |
- Abbreviations: DLQI, Dermatology Life Quality Index; IgE, immunoglobulin E; NRS, numeric rating scale; QoL, quality of life; SD, standard deviation; VAS, visual analog scale; VRS, verbal rating scale.
3.2. Assessment of Efficacy
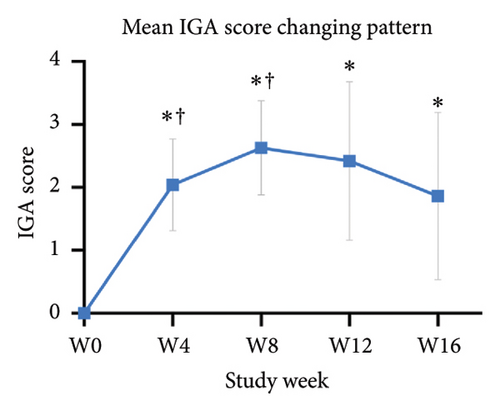
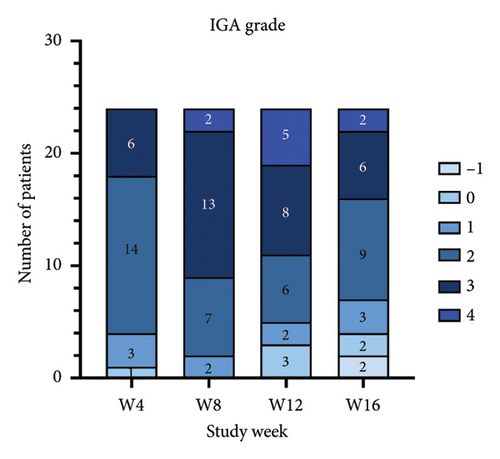
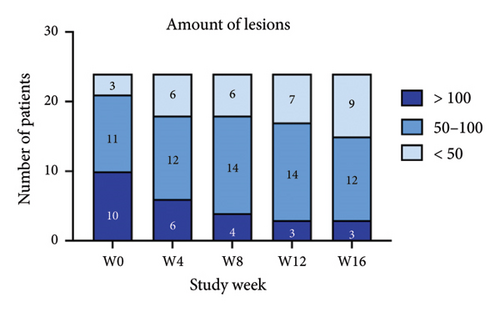
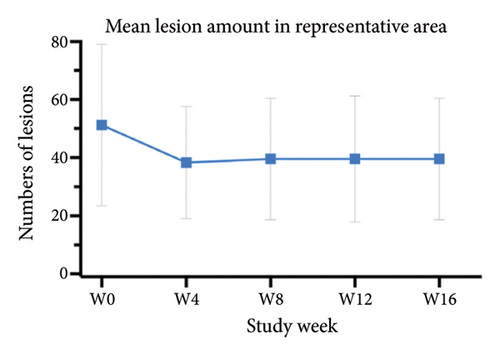
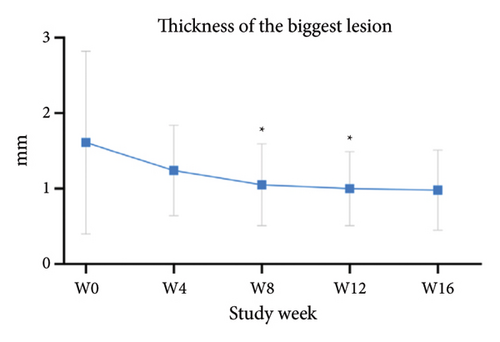
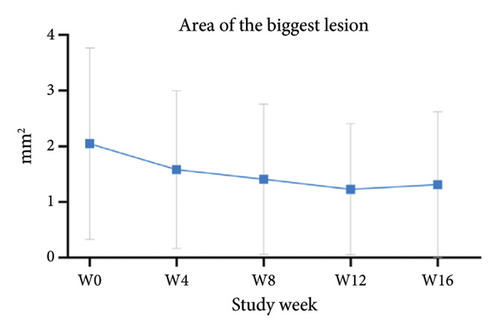
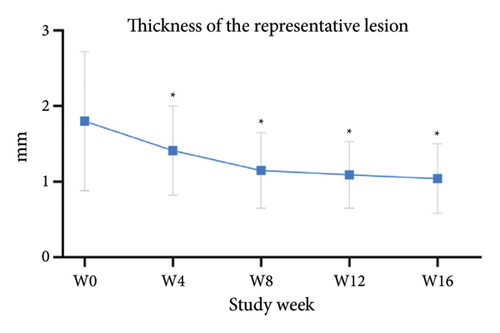
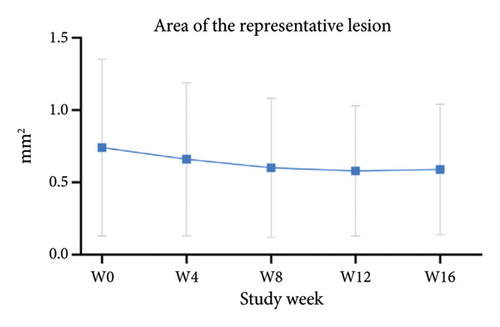
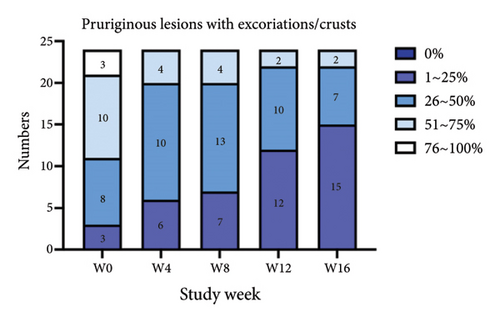
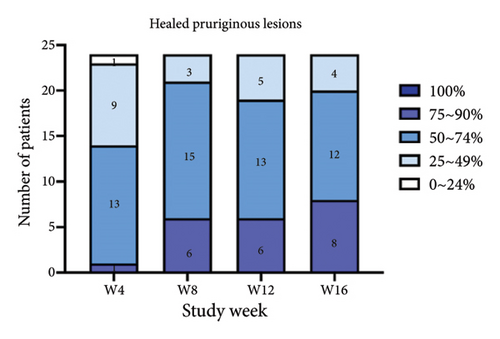
For the primary efficacy outcomes assessed by the investigator, the mean IGA score at week 12 was 2.41, indicating a significant improvement compared to baseline (Figure 1(a); p < 0.05). Nineteen out of 24 patients (79.16%) achieved at least a two-point improvement in the IGA score, with 5 patients (20.83%) treated with tofacitinib achieving a ≥ 4-point improvement in the IGA score (Figure 1(b)). From week 4 to week 8, there was a progressive increase in the values of the IGA score, increasing from 2.04 ± 0.73 to 2.63 ± 0.75 (p < 0.05). However, at week 12 and week 16, the mean value of IGA scores decreased to 2.42 ± 1.26 and 1.89 ± 1.33, respectively, although not statistically different (p = 0.054). Similarly, the proportion of tofacitinib recipients who achieved a ≥ 2-point improvement in the IGA score decreased by week 16 (70.8%, 17/24) compared to week 12. Twenty patients maintained clinical efficacy after receiving continuous treatment for 16 weeks. Figure 2 illustrates the changes in PAS items following tofacitinib treatment. At week 12, only 3 out of 24 (12.5%) patients with more than 100 prurigo lesions and most patients (14/24, 58.33%) have lesions between 50 and 100 (Figure 2(a)). The number of lesions in the representative area decreased from a mean of 51.25 ± 27.89 at baseline to a mean of 39.58 ± 21.69 at week 12 (Figure 2(b); p = 0.79). As for the biggest pruriginous lesion, the highest elevation decreased from 1.61 ± 1.21 mm at baseline to 1.00 ± 0.49 mm at week 12 (Figure 2(c); p < 0.05), and the area changed from 2.05 ± 1.72 to 1.23 ± 1.18 mm2 (Figure 2(d); p = 0.76). Regarding the representative lesion, the highest elevation changed from 1.80 ± 0.92 to 1.09 ± 0.44 mm (Figure 2(e); p < 0.05), and the area changed from 0.74 ± 0.61 to 0.58 ± 0.45 mm (Figure 2(f); p = 0.30). As for the items reflecting PN activity, 22 out of 24 (91.67%) patients achieved at least a two-level response of active scratching and 20 out of 24 (83.33%) patients achieved at least a two-stage healing of PN at week 12 (Figures 2(g) and 2(h)).
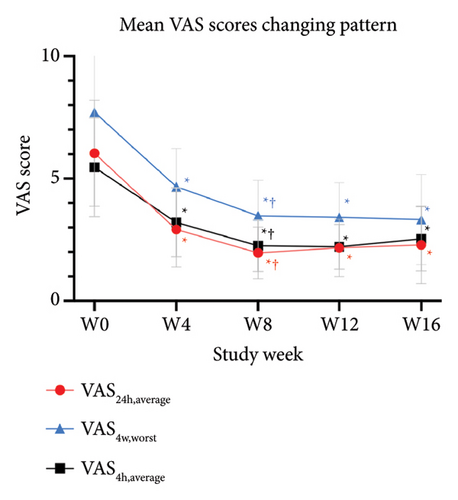
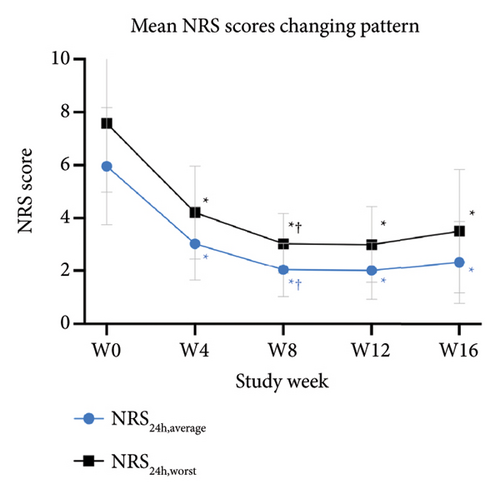
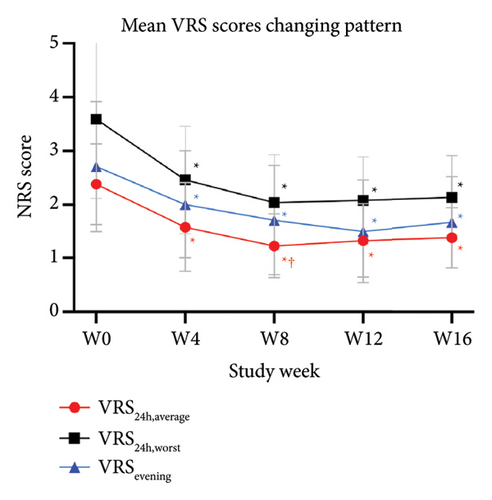
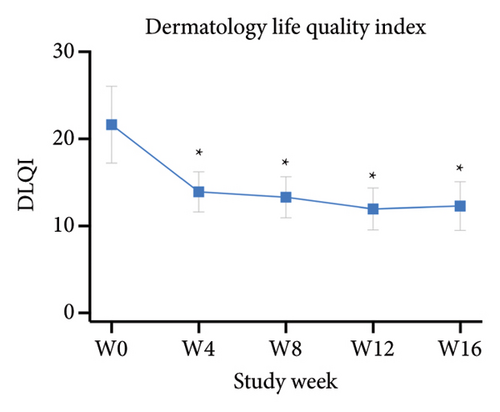
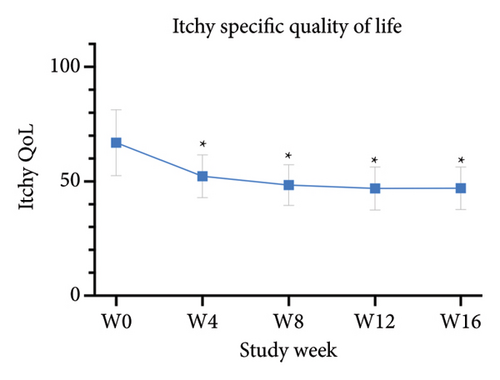
The primary outcomes assessed by patients showed significant improvement in mean scores of VAS24h,average, VAS4w,worst, and VAS4w,average from the baseline to 12 weeks after tofacitinib treatment (p < 0.05 for all; Figure 3(a)). The mean values of VAS24h,average, VAS4w,worst, and VAS4w,average at baseline were 6.04 ± 2.17, 5.46 ± 2.06, and 7.71 ± 2.22, respectively. By week 12, the mean values of VAS24h,average, VAS4w,worst, and VAS4w,average scores had decreased to 2.17 ± 1.18, 2.21 ± 0.92, and 3.42 ± 1.42, respectively. Consistent with the trend in VAS scores, itching severity assessed by NRS24h,average, NRS24h,worst, VRS24h,average, VRS24h,worst, and VRSevening at week 12 also showed significant improvement from baseline. The NRS24h,average and NRS24h,worst decreased from 5.96 ± 2.21 and 7.58 ± 2.55 at baseline to 2 ± 1.08 and 3 ± 1.44 at week 12 (p < 0.05 for all; Figure 3(b)). Similarly, the VRS24h,average changed from 2.38 ± 0.75 to 1.33 ± 0.69, the VRS24h,worst changed from 3.58 ± 1.47 to 2.04 ± 0.84, and VRSevening changed from 2.71 ± 1.21 to 1.5 ± 0.96 (p < 0.05 for all; Figure 3(c)). Furthermore, the DLQI and Itchy QoL scores evaluated at week 12 also showed significant improvement. The mean DLQI reduced from 21.63 ± 4.44 at baseline to 11.96 ± 2.42 (p < 0.05; Figure 4(a)), and the mean Itchy QoL scores reduced from 67 ± 14.46 at baseline to 46.96 ± 9.38 at week 12 (p < 0.05; Figure 4(b)).
For the secondary endpoints related to itch, 18/24 (75%) and 16/24 (66.67%) patients achieved a reduction of ≥ 4 points in WI-NRS with tofacitinib treatment at week 12 and week 16, respectively (Figure S1). The proportion of patients achieving a ≥ 4-point reduction in WI-NRS with tofacitinib treatment was higher at week 12 than that at week 16 (p > 0.05), although the difference was not statistically significant.
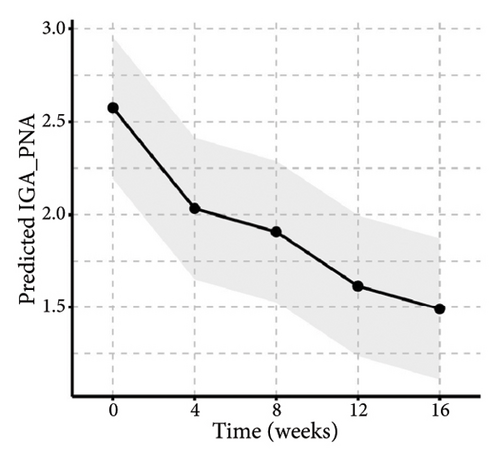
Similarly, the results from the MMRM model indicated significant improvements in skin lesions and itch severity throughout the 16-week treatment period (Figure 5 and Table S1), with clinically meaningful changes observed at each follow-up assessment. By week 16, the proportion of patients achieving 0% or 1%–25% activity of pruriginous lesions with excoriations/crusts (IGA PN-A) had significantly increased over time (F(4, 92) = 14.57, p < 0.01). Furthermore, there were highly significant reductions in NRS24h,worst (F(4, 92) = 47.75, p < 0.01), VAS4w,average (F(4, 92) = 38.20, p < 0.01), and VRSevening (F(4, 92) = 11.33, p < 0.01). Notably, we found that age and baseline NRS4h,worst score may impact the tofacitinib efficacy in the items of NRS24h,worst and VAS4w,average. Additionally, BMI may be another variance for the result of VAS4w,average. The results of interaction effects (TableS1) revealed that the baseline NRS24h,worst showed a significant interaction with time in both NRS24h,worst (p < 0.01) and VAS4w,average (P = 0.03), indicating slower improvement in high baseline subgroups. Age and BMI had no significant interactions. Additionally, post hoc analyses also indicated that pruritus scores demonstrated a significant reduction from week 4 onwards, with sustained efficacy through week 16 following tofacitinib treatment (Table S2). The most pronounced improvement occurred between weeks 8 and 12, after which scores stabilized. Notably, while the treatment effect showed a slight reduction at week 16 compared to the earlier time point of week 12, this difference did not reach statistical significance (Table S2), potentially due to a therapeutic plateau or limited sample size. All analyses were adjusted for baseline scores, disease duration, atopic history, sex, age, and BMI and are averaged over the levels of atopic history and sex, reflecting the average treatment effect across the entire population.

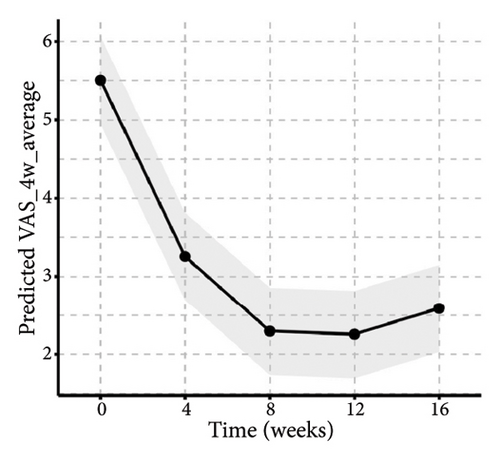
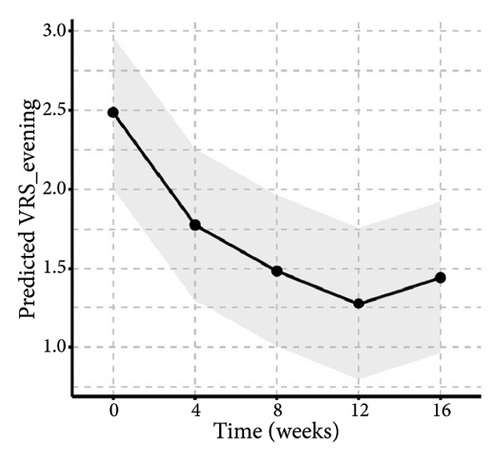
When compared with historical placebo groups from the PRIME and PRIME2 trials (Table 2), the baseline NRS score was generally well-balanced among different groups (p > 0.05), indicating similar baseline pruritus characteristics. However, patients in our study were older, had fewer female, and exhibited higher baseline DLQI score than those in placebo groups. At week 12, significantly more tofacitinib-treated patients achieved the ≥ 4-point reduction in WI-NRS compared to placebo-treated patients in both trials: 18/24 (75%) versus 12/76 (15.8%) in PRIME (95% confidence interval (CI), 4.71–57.89; p < 0.01); and 18/24 (75%) versus 18/82 (22%) in PRIME2 (95% CI, 3.35–36.89; p < 0.01). The IGA for PN Activity (IGA PN-A) in PRIME and PRIME2 is a clinician-reported outcome used to assesses disease activity based on the percentage of pruriginous lesions with excoriations or scars. An IGA PN-A score 0 or 1 corresponds to 0% or 1–25% of activity, respectively. At week 12, the proportion of patients achieving the IGA PN-A score of 0 or 1 was significantly higher in the tofacitinib group (12/24, 50%) compared to that in placebo (PRIME: 11/76, 14.5%; PRIME2: 16/82, 19.2%; both p < 0.01). Regarding the least-squares (LS) mean change in DLQI at week 12, no significant differences were observed between our study and placebos from PRIME and PRIME2.
| Our study, N = 24 | Placebo from PRIME, N = 76 | Difference versus placebo (%), (95% CI) | p | Placebo from PRIME2, N = 82 | Difference versus placebo (%), (95% CI) | p | |
|---|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 58.88 (15.17) | 51.1 (15.8) | — | < 0.05 | 46.7 (15.2) | — | < 0.01 |
| Female sex, n (%) | 6 (25) | 48 (63.2) | — | < 0.01 | 51 (62.2) | — | < 0.01 |
| NRS24h,worst or WI-NRS at baseline, mean (SD) | 7.58 (2.60) | 8.3 (1.1) | — | 0.2 | 8.5 (1.0) | — | 0.1 |
| DLQI at baseline, mean (SD) | 21.63 (4.44) | 15.7 (7.3) | — | < 0.01 | 18.2 (7.0) | — | < 0.01 |
| WI-NRS by ≥ 4 points from baseline at week 12, n (%) | 18 (75) | 12 (15.8) | 15.36 (4.71, 57.89) | < 0.01 | 18 (22) | 10.36 (3.35, 36.89) | < 0.01 |
| IGA PN-A score 0 or 1 at week 12, n (%) | 12 (50) | 11 (14.5) | 5.77 (1.87, 18.54) | < 0.01 | 16 (19.5) | 4.06 (1.39, 12.07) | < 0.01 |
| LS mean change in DLQI at week 12, mean (SE) | −9.67 (4.76) | −5.7 (0.9) | −3.97 (−13.97, 6.03) | 0.42 | −7.1 (1.1) | −2.57 (−12.63, 7.49) | 0.60 |
- Note: WI-NRS from PRIME and PRIME2 is a patient-reported outcome comprising one item, the worst itch over the past 24 h, rated from 0 (“no itch”) to 10 (“worst imaginable itch”). This corresponds to the NRS24h,worst used in our study. The IGA PN-A in PRIME and PRIME2 is a clinician-reported outcome assessing disease activity based on the percentage of pruriginous lesions with excoriations or crusts. An IGA PN-A score of 0 or 1 corresponds to 0% or 1%–25% activity, respectively, in terms of the percentage of pruriginous lesions with excoriations/crusts.
- Abbreviations: DLQI, Dermatology Life Quality Index; IGA PN-A, investigator’s global assessment for PN activity; LS, least squares; NRS, numeric rating scale; SD, standard deviation; VRS, verbal rating scale; WI-NRS, worst itch-NRS.
3.3. Assessment of Safety
In our study, tofacitinib exhibited favorable tolerability, demonstrating an overall safety profile consistent with its established characteristics. Throughout the duration of the study, no serious adverse effects were reported. Three adverse events were documented during treatment, including one case of transient elevation of liver enzymes and two cases of gastrointestinal symptoms. None of the adverse events led to drug withdrawal.
4. Discussion
Patients with PN often experience severe itching that does not respond well to traditional treatments, posing as significant challenge in managing this condition. In our single-center, prospective, observational pilot study, we present compelling evidence that tofacitinib may offer a promising therapeutic approach for PN. Our findings demonstrate that tofacitinib effectively improved PN lesions and pruritus symptoms, leading to an enhancement in disease-related quality of life.
The pathogenesis of PN remains unknown, while the JAK-STAT signaling pathway plays a pivotal role in the pathophysiology and clinical manifestation of PN. The JAK-STAT signaling cascade is essential for transducing signals from numerous pro-inflammatory and inflammatory cytokines, which have been implicated in immune pathogenesis across various inflammatory conditions [18]. There are four known types of JAK members: JAK1, JAK2, JAK3, and TYK2. JAKs belong to the group of the cytoplasmic tyrosine kinases, which are activated upon the simulation of cellular receptors by their specific factors. When activated, they phosphorylate intracellular receptors, leading to the transportation of STAT factors into the nucleus where they modulate the expression of specific genes [13, 19, 20]. Pruritus mediated by IL-31 is potentiated by JAK-STAT signaling, and inhibition of this pathway may alleviate itch. In PN lesions, it was reported that STAT1, STAT3, and STAT6 were upregulated as well as associated cytokines of Th2, Th17, and Th22 [21–23]. It was noteworthy that neuronal sensitization to itch and the development of an itch-scratch cycle also contribute to the pathogenesis of PN. In the mouse model of atopic dermatitis-like disease, the topical application of a JAK inhibitor reduced levels of IL-22 and IL-31 while increasing the epidermal nerve fiber density. Furthermore, the neuron-specific genetic deletion of JAK1 resulted in a markedly reduced pruritus independent of skin inflammation levels [24]. Therefore, the utilization of JAK inhibitors may prove effective in delaying progression in PN [25].
Tofacitinib, an oral pan-JAK inhibitor, exhibits predominant selectivity against JAK1 and JAK3. JAK1 and JAK3 are responsible for mediating the signaling of the γc family of cytokines, including IL-4, IL-13, and IL-31 [24]. Blocking JAK1 and JAK3 may attenuate the transcription of itch-associated cytokines such as IL-4 and IL-3 [13, 26]. Previous studies have indicated a favorable response to tofacitinib in patients with PN [12, 27]. A case report demonstrated successful reduction of tofacitinib in lesion number, as well as improvements in DLQI and NRS in a PN patient who did not respond to conventional treatments. Another case report illustrated that oral tofacitinib led to a 50% decrease in skin lesions and complete alleviation of itch in a patient with refractory PN [28]. Furthermore, a case series involving nine patients from a single center provided additional evidence supporting the efficacy and safety of tofacitinib by demonstrating a rapid and complete resolution of itch in PN patients. Subjective patient-reported itch showed complete remission within 7 days, with sustained efficacy over 9 months. DLQI scores were decreased from 18 at baseline to 0 at month 6 [11]. Despite lacking approval from the United States Food and Drug Administration for PN treatment, JAK inhibitors hold great promise for the management of PN.
Our study was a 16-week observational pilot study aimed at evaluating the efficacy and safety of tofacitinib in the management refractory PN. Despite the previous use of topical and, in over 70% of patients, systemic treatments, the enrolled patients had a high disease burden at baseline, with severe itch and skin lesions significantly impacting their quality of life. In one analysis between patients with PN and their matched controls, an increased association with comorbidities was found in patients with PN, including mental health disorders, systemic illness, HIV infection, and dermatologic conditions [5]. In consistent with previous observations, our study found that 87.5% (21/24) of patients had dermatologic, systemic, neurologic, or other comorbidities. Furthermore, approximately 30% of patients (7/24) had an atopic background, whereas three patients had a family history of atopy. Notably, all primary and secondary endpoints addressing itch and the number of skin lesions were successfully achieved in our study. After 12 weeks of treatment, 79.17% of patients demonstrated satisfactory improvement in IGA score, with 5 out of 24 patients achieving significant remission. Notable enhancement in skin lesions and itch was observed as early as 4 weeks, leading to improvement in IGA, PAS, VRS, NRS, VRS, DLQI, and Itchy QoL, with a progressive improvement at week 8. The results from MMRM model and post hoc analyses indicated significant improvements in symptoms over the 16-week treatment period, with notable changes observed at each follow-up time point. Notably, our study suggested that baseline NRS24h,worst appears to be a significant clinical feature impacting treatment efficacy. In addition, compared with historical placebo controls, our study demonstrated a higher proportion of patients achieving ≥ 4-point reductions in WI-NRS and IGA PN-A scores.
While historical placebo comparisons have inherent limitations due to potential confounding factors and differences in study design, these findings suggest clinical meaningfulness and support the potential efficacy of tofacitinib in treating refractory PN.
However, we noted that response to tofacitinib became less evident after 12 weeks, with a reducing improvement in the IGA score, lesion number and area, VAS, NRS, VRS, DLQI, and Itchy QoL. Furthermore, the results at week 16 were not superior to those at week 12 and week 8, indicating a declining response to tofacitinib treatment. At week 16, the number of patients achieving a two-point improvement in IGA scores decreased from 18 to 16. Additionally, the values of VAS, NRS, VRS, WI-NRS DLQI, and itchy QoL slightly increased compared to those at week 12, though the difference was out of significance. One possible reason is that patients may develop tolerance or resistance to tofacitinib over time, leading to a reduced therapeutic effect. Another possible explanation for this condition is the immune imbalance, as the potential molecular mechanism of PN remains unclear [29]. The over-suppression of the JAK-STAT signaling may lead to an excessive expression of other cytokines. Patient compliance and adherence to the treatment regimen could also be a factor, as non-adherence can lead to suboptimal drug levels and reduced efficacy. Previous phase 2 trial of nemolizumab presented a similar response pattern in patients with moderate to severe PN. A maximal reduction of the weekly peak pruritus score was observed at week 12 (change, −61.9%). However, the reduction in the pruritus score becomes less evident at week 16 (change, −60.4%) and at week 18 (change, −57.9%) [30]. Another study conducted in Singapore comparing dupilumab and oral JAK inhibitors in the treatment of PN also found that oral JAK-inhibitors have a faster first response than dupilumab, while disease flare seemed more common in the JAK inhibitors group, although both groups achieved similar response targets at weeks 12–16 [31]. Therefore, caution should be exercised in considering the clinical use of tofacitinib for a prolonged period in the treatment of PN, as its long-term efficacy and mechanism still require further observation and exploration.
Our study demonstrated the safety of tofacitinib, with no reports of serious adverse events. The majority of patients (21/24) tolerated the treatment well, and all expressed their intention to continue using tofacitinib. However, several limitations warrant careful consideration. First, the relatively small sample size (n = 24) may have restricted statistical power to detect minor treatment effects or rare adverse events. Replication in larger cohorts is essential to validate these findings. Second, the absence of a placebo control group limited our ability to distinguish treatment-specific effects from natural disease variability or placebo responses, particularly after multiple comparison adjustments. Third, the follow-up duration was limited to 16 weeks and should be extended to obtain longer-term results. Finally, further investigation into the effects of tofacitinib on STAT and JAK signaling pathways and cytokine expression in PN is warranted, particularly through experimental data from cellular and animal models. Additionally, exploratory analyses should be interpreted cautiously as they only suggest potential associations rather than definitive evidence. Due to the absence of a prospective sample size calculation and the single-arm, open-label design, the generalizability of these findings remains constrained. Future investigations should employ multicenter randomized controlled trials with larger sample sizes and standardized follow-up protocols to validate the long-term efficacy and durability of treatment outcomes.
In summary, our study demonstrated that tofacitinib effectively improved the lesion and pruritus of PN patients without serious adverse events. This observational study represents the largest evaluation to date of the safety and efficacy of tofacitinib in treating refractory PN. However, it is important to note that this study is limited by the absence of a placebo control. Therefore, further confirmation through forthcoming placebo-controlled randomized prospective studies is necessary. Additionally, larger and longer-term trials are required to determine the durability and safety of tofacitinib in the treatment of PN.
Ethics Statement
The study was approved by the Human Ethics Committee of the Zhejiang University School of Medicine Second Affiliated Hospital (20231186) and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects before enrollment. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
R.D. and X.-Y.M. contributed to the conception and design of study, analysis, and interpretation of data. C.-C.C., Y.L., and I.-J.H. were involved in the acquisition of data and implementing of the research. R.D. and C.-C.C. wrote the manuscript. X.-Y.M. gave the final approval of the version to be published. All authors read and approved the final manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (NSFC) (nos. 82103754, 81930089, 82230104, and 81630082).
Supporting Information
Figure S1. Proportion of PN patients with WI-NRS score reduction by ≥ 4 at week 12 and at week 16. PN, prurigo nodularis; WI-NRS, Worst Itch-Numeric Rating Scale.
Table S1. Marginal ANOVA results for fixed effect in the MMRM model and interaction effect results.
Open Research
Data Availability Statement
The data that support the findings of this study are not publicly available due to the privacy of research participants but are available from the corresponding author upon reasonable request.




