Isotretinoin and Thyroid Dysfunction: A Call for Routine Monitoring
Abstract
Isotretinoin is a widely prescribed medication for severe acne and other dermatological conditions. While effective in managing acne, some of its systemic effects were widely discussed. However, its impact particularly on thyroid function remains underexplored. This narrative review highlights current evidence on the relationship between isotretinoin use and thyroid dysfunction, evaluating the need for routine thyroid function testing to help clinicians assess the risk of thyroid dysfunction in their patients. We searched PubMed, Scopus, and Google Scholar from inception to February 2025. Interpretation was guided by a systematic approach emphasizing study relevance, methodological quality, and recency. Inclusion criteria focused on peer-reviewed research addressing isotretinoin’s impact on thyroid function. Study designs, sample sizes, and risk of bias were critically assessed to maintain objectivity and reliability in synthesizing current evidence. Studies consistently report alterations in thyroid hormone levels during isotretinoin therapy, including elevated thyroid-stimulating hormone (TSH) and decreased free triiodothyronine (FT3) and free thyroxine (FT4) levels. Studies suggest that these changes may be mediated through mechanisms involving thyroid cell apoptosis, immunomodulatory effects, or central regulatory disruptions. Females and individuals undergoing prolonged isotretinoin therapy appear to be at higher risk. These findings highlight the importance of routine thyroid function monitoring in patients on isotretinoin, particularly those with a predisposition to autoimmune disorders or prolonged treatment courses. Further research with larger sample sizes and rigorous methodologies is needed to comprehend the underlying mechanisms and refine clinical guidelines. This review emphasizes on the need for a multidisciplinary approach involving dermatologists and endocrinologists to ensure optimal patient care and safety.
1. Introduction
Isotretinoin is a widely used dermatological drug. Often prescribed for common dermatological conditions such as acne vulgaris, the prevalence of its use varies depending on the parent disease across different regions. Nearly 2 in every 1000 women use isotretinoin in Germany, whereas a higher prevalence at 58% is noted in Saudi Arabia [1, 2]. The usage of isotretinoin is approved for severe acne, but it has also been used for acne rosacea, or to treat scars [3]. Despite its status as a “prescription drug,” the growing knowledge of the effects of the drug has prompted widespread over-the-counter use, with a lack of awareness of the side effects carried along with it [4].
Isotretinoin is a nonsteroidal antiacne medication. Despite this, it is associated with considerable adverse effects, the most well-known being its teratogenic potential [5]. Despite this, its use in women of childbearing age is still high [1]. Additionally, certain adverse events have been noted in the liver and the pancreas [6]. Therefore, its oral administration has been highlighted for its side effects, and concerns about the risk–benefit ratio for the drug have been raised.
There is growing evidence that isotretinoin has certain effects on the pituitary–thyroid axis. Studies have shown an elevation of thyroid-stimulating hormone (TSH) levels and reduced thyroid levels following chronic use of the drug [7, 8]. The need for routine thyroid function tests (TFTs) while on chronic use of the drug has been emerging to allow for early identification of adverse events. However, there is still a lack of clarity on the systemic effects of isotretinoin and its mechanism of action on the thyroid axis.
This review aims to summarize current findings on the relationship between isotretinoin use and thyroid functioning and to assess whether routine TFTs should be considered when prescribing the drug. Additionally, it highlights potential thyroid-related adverse effects of isotretinoin to assist clinicians in assessing the risk of thyroid dysfunction in patients prescribed isotretinoin.
2. Methods
A detailed outline was developed prior to conducting the literature review to ensure a systematic and focused approach. This outline defined the key research questions, topics to be covered, and the sequence of steps for searching, screening, selecting, and analyzing studies. The outline helped structure the review to comprehensively address the relationship between isotretinoin use and thyroid dysfunction, guiding inclusion/exclusion criteria, data extraction, and synthesis methods.
2.1. Eligibility Criteria
For this narrative review, we included studies that investigated the relationship between isotretinoin use and thyroid dysfunction, encompassing clinical trials, observational studies, case reports, and relevant reviews published in English. We excluded articles that did not address thyroid outcomes, nonhuman studies, conference abstracts without full text, and publications not in English. This approach ensured a focused and relevant selection of literature while maintaining a broad perspective on the topic.
2.2. Literature Search and Study Selection
We conducted a comprehensive literature search using PubMed, Scopus, and Google Scholar from inception to February 2025. No publication date limits were imposed during the literature search to ensure the inclusion of relevant foundational concepts and definitions from earlier studies. However, the majority of included references are recent, reflecting the most recent clinical and research developments related to isotretinoin and thyroid function. References of selected articles were also manually screened to identify additional relevant studies.
Studies were selected based on methodological quality, relevance, and credibility. Peer-reviewed articles directly addressing isotretinoin and thyroid function were prioritized. We evaluated study design, sample size, and risk of bias, excluding sources with insufficient detail, unclear methods, or from gray literature.
3. Results
Thyroid hormones form one of the key components of the delicate endocrine’s system, primarily involved in regulating basal metabolism and cellular functions across various organs. Triiodothyronine (T3) and thyroxine (T4) influence many physiological processes, including early fetal neurogenesis in utero, to playing an essential role in puberty, to finally maintaining cellular metabolism; the myriad functions of T3 and T4 manifest in every stage of growth and development [9–11]. Thyroid disorders, henceforth, lead to widespread complications, often worsening both the physiological and mental status of an individual [12]. Due to the complexity of interactions at various steps, the synthesis, release, and action of these hormones are influenced by both internal and external milieu at every step.
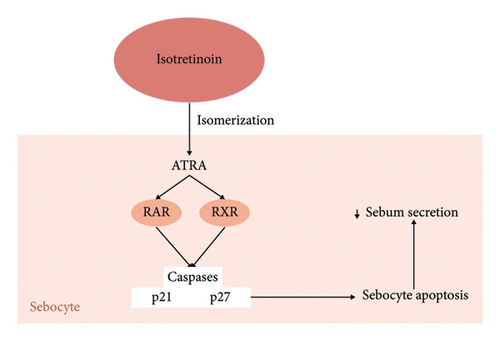
Isotretinoin, a systemic retinoid, isomerizes to all-trans retinoic acid when entering any cell (Figure 1). Here, it acts over retinoid-acid receptors, stimulating consequent genes and leading to the formation of proteins which finally culminate in activating caspases and proteins p21 and p27, commonly involved in cell cycle arrest and apoptosis [13, 14] (Figure 1). Studies have shown a p53-mediated apoptosis of sebocytes, thus decreasing baseline sebum production [15] (Figure 1). Other peripheral actions include its effect on the testosterone pathway, decreasing the formation of androgen receptors, which are shown to facilitate sebum secretion (Figure 1). Thus, the action of isotretinoin aims to suppress internal stimuli for sebum production and secretion [16].
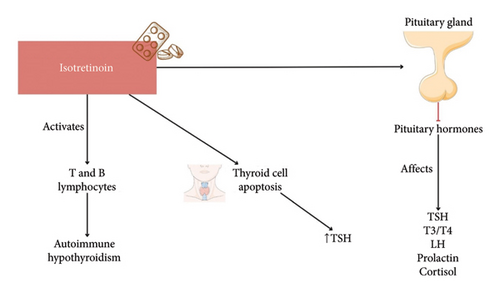
Isotretinoin is known for causing multiple side effects, one of the most prominently known being its high potential as a teratogen. This results mainly due to certain “peripheral” apoptosis apart from sebaceous cells like that of neural crest cells [14]. In all, isotretinoin is regarded as a safe drug to use except in those planning for pregnancy. However, recent evidence suggests an important role of isotretinoin in conversely affecting the pituitary–thyroid axis (Figure 2). A study noted an increase in TSH levels following 4 months of treatment, whereas free triiodothyronine (FT3), free thyroxine (FT4), and thyroid volumes decreased significantly [17]. One of the postulated mechanisms of this interaction is its effect on pituitary hormones directly. A suppression of pituitary hormone levels can also happen, resulting in effects not only in T3/T4 but also in other downstream hormones like luteinizing hormone (LH), prolactin, and cortisol among others [18] (Figure 2). Another proposed mechanism is the action of bexarotene, a metabolite of isotretinoin, that stimulates the peripheral metabolism of thyroid hormones [19]. However, certain other studies suggest a suppression of TSH levels by bexarotene; therefore, its mechanism of action is still debatable [20, 21].
In a prospective study conducted to investigate the effect of isotretinoin on TFTs, 66 patients (14 males and 52 females) were given isotretinoin twice daily with meal for 4 months, while 38 patients including 8 males and 30 females did not receive any treatment. Fasting blood samples were taken before and after 4-month treatment. The levels of TSH, FT3, FT4, antithyroglobulin, and antithyroid peroxidase antibodies (TPO-Abs) were measured. The level of TSH had a significant increase in the isotretinoin group between the initial and later values, whereas no statistical significance was observed in the control group. Furthermore, with FT3, FT4, and TPO-Abs, thyroid volume significantly decreased, whereas no statistical significance was observed in the control group (Table 1) [17].
| Study | Type of study | Sample size/demographics | Isotretinoin duration and frequency | Main outcomes | Statistical significance |
|---|---|---|---|---|---|
| Uyar et al. [17] | Case-control | 66 treated (14M/52F), 38 control (8M/30F) | 4 months | ↑ TSH, ↓ FT3, FT4 in treatment group | Significant in treatment group only |
| Yıldırım et al. [22] | Prospective study | 51 patients (22M/29F), mean age 20 | 0.5–1 mg/kg/day until 120 mg/kg total for 6 months | ↑ TSH, ↓ FT3 & FT4 at 3rd and 6th month | Statistically significant; no clinical symptoms reported |
| Salem Hareedy et al. [7] | Prospective study | 47 patients (33M/14F), mean age 21 | 0.5 mg/kg/day for 6 months | ↑ TSH, ↓ FT3 and FT4 | Significant (one-way and two-way ANOVA, Sidak’s test) |
| Masood and Hakeem [23] | Case report | 25-year-old female | 20 mg/day for 6 months | TSH rose to 78.97 mIU/mL, normalized 12 weeks post-drug | Clinically significant |
| Schöps et al. [24] | Case report | 36-year-old female | 40 mg/day | ↑ TSH, ↓ FT3/FT4, signs of subclinical hypothyroidism | Not reported |
| Schöps et al. [24] | Case report | 26-year-old female | 40 mg/day for 9 months | Diagnosed with Graves’ disease 2.5 months post-treatment | Temporal association; causality unclear |
- Note: F: female; M: male; FT3: free triiodothyronine; FT4: free thyroxine. ↑ = increased, ↓ = decreased.
- Abbreviation: TSH, thyroid-stimulating hormone.
Another study aimed to observe variations in serum TFTs of acne vulgaris patients treated with systemic isotretinoin. 51 patients (22 males and 29 females) with a mean age of 20 years were given 0.5–1 mg/kg/day isotretinoin until 120 mg/kg systemic isotretinoin was reached. For 6 months, dermatological examination took place once a month and FT3, FT4, and TSH levels were measured at baseline, 3rd, and 6th month of the treatment. Mean serum TSH levels showed a statistically significant increase following isotretinoin therapy. Mean serum FT3 and mean serum FT4 showed a significant decrease in 3rd and 6th months. No weight gain, menstrual irregularity, hair loss, or mood changes were reported (Table 1) [22].
According to another study, 47 patients (33 males and 14 females) with a mean age of 21 years underwent 0.5 mg/kg oral isotretinoin treatment for severe acne. The patients were suffering from severe acne for 13 months. Levels of TSH, FT4, and FT3 were measured at baseline, 3rd, and 6th month after treatment. A significant decrease was observed in FT4 and FT3 after 3 and 6 months compared to baseline. This can be further elaborated with a more significant reduction after 6th month compared to the 3rd month. One-way ANOVA and Sidak’s multiple comparison tests were done (ANOVA compares the averages of three or more groups to check for significant differences; Sidak’s test follows ANOVA to identify which groups differ while minimizing errors) which clarified that gender does not correlate with levels of FT4 and FT3 during isotretinoin treatment. This was further confirmed by a two-way ANOVA test which showed that the duration of isotretinoin treatment plays a significant role in decreasing FT3 and FT4 levels but not gender. In addition to this, the effect of isotretinoin on patients who received it in the past but were not able to complete the treatment course or used isotretinoin intermittently was compared with patients who took isotretinoin for the first time through one-way ANOVA and Sidak’s multiple comparison test. Both showed a significant decrease in FT4 and FT3 after 3 and 6 months as compared to baseline. For further confirmation, a two-way ANOVA test was done which showed significant decrease in FT4 affected by duration of treatment and past treatment. In comparison, FT3 was only affected by the duration of treatment. Moreover, there was a significant increase seen in the level of TSH during the treatment of isotretinoin. Through repeated measures of ANOVA and Sidak’s multiple comparison test, females showed a significant increase in TSH as compared to males with no significant difference. Further confirmation showed the level of TSH was affected by the duration of treatment and gender but not by previous isotretinoin treatment (Table 1) [7].
A 25-year-old female presented at the Agha Khan University Hospital, Pakistan, with a 3 kg-weight gain, dull mood, and menorrhagia for the previous 3–4 months. Drug history reported using 20 mg isotretinoin for 6 months to cure her acne. However, at the time of her presentation, she had stopped using it for 6 weeks. 2 weeks after stopping isotretinoin, her TST showed a serum TSH of 78.97 mIU/mL (normal range 0.4–4.2). The test was repeated after stopping isotretinoin for 6 weeks, in which her TSH was 15.03 mIU/mL. During her follow-up visit, approximately after 12 weeks, her TSH level was 3.33 mIU/mL, which means it came back to normal (Table 1) [23].
In a case reported in Denmark, 36-year-old previously healthy female started developing acne right after stopping oral contraceptives and was given 40 mg isotretinoin daily for acne. Her gynecologist evaluated her for polycystic ovary syndrome, for which she got a serum thyroid profile test done. In this result, her TSH level started increasing, FT4 and FT3 started decreasing, and symptoms of subclinical hypothyroidism were seen (Table 1) [24].
A 26-year-old female, previously healthy, was diagnosed with Graves’ disease. She had been on a daily dose of 40 mg of isotretinoin for 9 months, with treatment completed two and a half months before her symptoms began. Notably, her medical records indicated normal TSH levels 8 months before starting isotretinoin (Table 1) [24].
A trend that is worth observing is the elevation of TSH with the dosage of isotretinoin, and simultaneously the levels of FT3 and FT4 have been decreasing. However, as soon as the acne treatment with isotretinoin was stopped, the TSH level began to return to normal. Furthermore, females in between 20 and 30 years old were more likely to be affected than males. Certain limitations can be observed. Most studies had an overall small sample size and females had a larger sample size compared to males which affects the reliability of the result. In addition to this, most of the studies were conducted in Asia, Europe, and Africa which shows a lack of diversity. Studies need to be conducted on American and Australian people to have more reliable results. More randomized controlled trials should be conducted to avoid the risk of bias in the findings.
4. Discussion
Isotretinoin-induced hypothyroidism was first documented in 2012 in a case report of a 19-year-old male treated with isotretinoin for severe acne [25]. Retinoids primarily function through two key nuclear hormone receptors: retinoic acid receptors (RARs) and retinoid X receptors (RXRs), which also modulate the activity of steroid and thyroid hormones, establishing a connection with hypothyroidism [26] (Figure 1).
Isotretinoin use has been linked to a reduction in both FT3 and FT4 levels, with a concomitant elevation in TSH levels [7, 17, 22] (Figure 2). Similar findings were noted after 3–6 months of isotretinoin use. In contrast, a study observed a decrease in both FT3 and TSH levels with isotretinoin use [27] (Figure 2). Interestingly, another found that females had a more significant increase in TSH compared to males [7]. Studies have also indicated that adult women with acne are at a significantly higher relative risk for elevated antithyroglobulin antibody levels, suggesting thyroid autoimmunity [28] (Figure 2).
Yıldırım et al. also noted a substantial effect of therapy duration, rather than dose, suggesting that prolonged isotretinoin use may be a critical factor in thyroid dysfunction. This observation was supported by another research, which found that patients with a history of intermittent isotretinoin therapy had significantly lower FT4 levels compared to those receiving isotretinoin for the first time, highlighting the potential cumulative effects of repeated treatments [22].
One possible explanation for the increase in TSH levels during isotretinoin therapy is a reduction in thyroid volume [7] (Figure 2). Isotretinoin may induce apoptosis (programmed cell death) in thyroid cells, leading to a decrease in thyroid tissue and a subsequent elevation in TSH as a feedback response (Figure 2). This theory is supported by studies documenting significant changes in thyroid hormone levels, possibly reflecting such cellular changes. However, a study reported a decrease in T3 and T4 levels without a significant change in TSH, suggesting a potential central mechanism involving the pituitary gland, which contradicts this hypothesis [29].
Additionally, isotretinoin’s immunomodulatory effects are a concern. The drug can activate immune cells such as T and B lymphocytes, potentially triggering autoimmune responses, including autoimmune hypothyroidism (Figure 2). This raises concerns about the potential for isotretinoin to induce thyroid autoimmunity, particularly in patients with a predisposition to autoimmune conditions [30] (Figure 2).
Given these findings, clinicians should remain vigilant when prescribing isotretinoin, particularly for long-term use. Regular monitoring of thyroid function, including TSH and FT4 levels, is recommended for high-risk patients undergoing isotretinoin therapy, with increased attention given to those with a history of autoimmune diseases. It is also important to consider other drugs with similar structures to retinoids, such as bexarotene, an antineoplastic agent that has been shown to cause central hypothyroidism, potentially leading to misleading TFTs [31]. This suggests that isotretinoin may have similar thyroid-related effects, reinforcing the need for careful monitoring in susceptible individuals.
There is a pressing need to monitor thyroid-related symptoms when using isotretinoin for acne treatment. Therefore, fostering collaboration between endocrinologists and dermatologists is crucial to ensure comprehensive patient care. Larger studies and clinical trials are needed to clarify the potential link between isotretinoin and thyroid dysfunction and to understand the underlying mechanism. Such research would provide clearer insights and improve screening and treatment protocols.
5. Conclusion and Perspectives
In conclusion, although isotretinoin remains a preferred treatment for severe acne, its potential impact on thyroid function should not be neglected. Studies have shown significant alterations in thyroid hormone levels, including increased TSH and decreased FT3 and FT4, especially in females and those undergoing isotretinoin therapy for longer durations. While the risk of thyroid dysfunction is relatively rare, regular monitoring of thyroid function during isotretinoin therapy is crucial, particularly for high-risk patients. Future research with larger sample sizes and better study designs is needed to fully understand the mechanisms behind isotretinoin-induced thyroid changes and optimize patient care.
Nomenclature
-
- FT3
-
- Free triiodothyronine
-
- FT4
-
- Free thyroxine
-
- LH
-
- Luteinizing hormone
-
- RARs
-
- Retinoic acid receptors
-
- RXRs
-
- Retinoid X receptors
-
- T3
-
- Triiodothyronine
-
- T4
-
- Thyroxine
-
- TFTs
-
- Thyroid function tests
-
- TPO-Abs
-
- Antithyroid peroxidase antibodies
-
- TSH
-
- Thyroid-stimulating hormone
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Zoha Iftikhar: conceptualization, data curation, methodology, project administration, and writing – review and editing.
Laura Ghanem: methodology, visualization, and writing – review and editing.
Maheen Sheraz: writing – original draft.
Mansoor Ahmed: writing – original draft.
Shree Rath: writing – original draft.
Mustafa Husain: writing – review and editing and supervision.
All listed authors have made significant contributions to the manuscript and agree to be accountable for the research presented.
Maheen Sheraz, Mansoor Ahmed, and Shree Rath contributed equally to the work.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
All data were obtained from studies retrieved from PubMed (https://pubmed.ncbi.nlm.nih.gov) and Embase (https://www.embase.com). All supporting data from the included studies are publicly available and are cited within the manuscript.




