Magnetic Resonance Spectroscopy Insights Into Late-Life Depression: Advances in Brain Metabolic Profiling
Abstract
Background: Late-life depression (LLD) is a primary depression with onset age greater than 60 years old. The typical clinical manifestations of patients include low mood and impaired cognitive function, which may be related to their neurobiochemical changes. Magnetic resonance spectroscopy (MRS) methods have quantified changes in levels of neurotransmitters and other neurometabolites in patients with LLD, clarifying their neurobiochemical characteristics.
Objective: This systematic review examined the existing evidence regarding MRS in LLD, emphasizing the connection between brain metabolites and clinical symptoms, including depression and cognitive deficits.
Methods: A systematic search was conducted across PubMed, Embase, Web of Science, Cochrane, and gray literature to identify studies relevant to this systematic review. The included studies summarized the alterations in metabolites within the brain regions associated with LLD and explored the correlation between these metabolites and clinical symptoms, aiming to elucidate the neurobiochemical features of LLD.
Results: In patients with LLD, the N-acetylaspartate (NAA) level was significantly reduced in the frontal cortex and was positively correlated with the severity of depressive symptoms. Furthermore, lower NAA/creatine (Cr) levels in the hippocampus were positively linked to reduced cognitive functions. The NAA level was also reduced, while myo-Inositol (mI) and the glutathione (GSH) to Cr ratio were increased in the cingulate cortex. Specifically, a higher GSH/Cr ratio in the anterior cingulate cortex correlated positively with depressive symptom severity. Conversely, the GSH/Cr ratio in the left lingual gyrus negatively correlated with language acquisition proficiency. Similarly, depressive symptom improvement was inversely related to a decrease in the glutamate (Glu)/total Cr (tCr) ratio in the posterior cingulate cortex (PCC) after antidepressant treatment. In contrast, the mI level showed a significant rise in the left temporal lobe and basal ganglia, and a negative correlation was observed between the mI/tCr ratio in the basal ganglia and overall cognitive performance. The choline to Cr ratio was also elevated in both the whole brain and the basal ganglia. Additionally, the levels of potential of hydrogen and potential of magnesium (pMg) were increased in gray matter. Conversely, white matter showed reduced nucleoside triphosphates, beta-nucleoside triphosphates (β-NTP), and pMg. Moreover, β-NTP and inorganic phosphate (Pi) were significantly associated with executive function in white matter.
Conclusion: LLD is associated with neurobiochemical alterations in the frontal lobe, cingulate gyrus, and other areas of the brain, which are strongly linked to depressive symptoms and cognitive impairment. In contrast, the neurobiochemical changes observed in the hippocampus and parietal lobe are distinctive features in cognitive disorders, indicating that the underlying mechanisms of cognitive impairment in these two conditions differ.
1. Introduction
Late-life depression (LLD) is marked by a low rate of recognition, a high rate of recurrence, and a poor response to antidepressant therapies when compared to depression in other age groups. Patients typically display symptoms such as sadness, diminished interest, insomnia, and reduced appetite [1]. Some individuals may experience significant cognitive decline and somatic symptoms [2, 3]. The cognitive decline and neuropsychiatric symptoms linked to LLD contribute to increased rates of dementia, disability, and mortality, significantly impacting patient outcomes. The underlying mechanisms of cognitive impairment associated with LLD remain poorly understood.
Previous research on the pathophysiological mechanisms of LLD has primarily concentrated on alterations in brain structure and neurobiochemical irregularities. Findings indicate that the structural abnormalities associated with LLD are predominantly located within the frontal striatal circuit and periventricular white matter, characterized by region-specific brain atrophy and elevated signals on magnetic resonance imaging (MRI) [4, 5]. A longitudinal study revealed a decrease in hippocampal volume following episodes of depression in older adults [6]. While specific brain regions have shown structural abnormalities, there is an increasing body of evidence suggesting that heightened oxidative stress and mitochondrial dysfunction may contribute to neurobiochemical changes in brain areas linked to cognitive impairment.
Research indicates that glucose metabolism in the brains of individuals with LLD is heightened in both the anterior (right and left superior frontal gyrus) and posterior (anterior cingulate cortex, lower parietal lobe) cortical areas [7]. Conversely, other studies have reported a significant decline in glucose metabolism in the prefrontal, parietal, and temporal cortices in LLD patients [8]. Positron emission tomography (PET) investigations of brain glucose metabolism suggest that antidepressant treatments may lead to alterations in specific brain networks linked to improvements in affective symptoms and cognitive function, with the cognitive network regions overlapping those impacted by Alzheimer’s disease (AD) [9, 10]. Thus, examining changes in metabolites and their correlation with clinical symptoms can enhance our understanding of the underlying pathological mechanisms.
Magnetic resonance spectroscopy (MRS) is a noninvasive technique that can measure the concentrations of excitatory and inhibitory neurotransmitters associated with psychiatric and neurodegenerative disorders, such as N-acetylaspartate (NAA), creatine (Cr), phosphocreatine (PCr), choline (Cho), glutamate (Glu), and glutathione (GSH) [11]. This method serves as a valuable tool for investigating the neurological mechanisms underlying LLD [12]. Research has shown that depressed patients exhibit lower levels of Glu and NAA, along with elevated levels of Glx (Glu/glutamine) in the prefrontal cortex (PFC), which are linked to their emotional symptoms and cognitive abilities [13, 14]. Additionally, reduced Glu levels in the hippocampus have been associated with diminished working memory performance in individuals with LLD [15].
As of now, there has not been a comprehensive literature review that consolidates the neurobiochemical features of LLD and examines its effects on clinical symptoms, nor has there been a comparison with cognitive impairment disorders. Consequently, this study aims to explore the existing literature in databases to elucidate the patterns of brain metabolites in LLD and their association with clinical symptoms, ultimately offering insights for the advancement of more effective differential diagnosis and treatment strategies.
2. Materials and Methods
To ensure the quality of our report, we adhered closely to the PRISMA guidelines for systematic review recommendations [16].
2.1. Search Strategy
Articles were obtained through a systematic search of the online databases PubMed, Embase, Web of Science, and Cochrane using the search terms ([geriatrics OR “late life” OR “older adult” OR elderly OR older OR aged] AND [depression OR “depressive disorder” OR “depressive symptoms” OR “major depressive disorder”]) AND (MRS OR magnetic resonance spectroscopy). All studies published online up to April 8, 2024, were included.
2.2. Selection Criteria and Study Selection
After removing duplicates, the titles and abstracts of all entries were carefully assessed against a strict set of inclusion criteria: (1) the study must be an original research article published in a peer-reviewed journal, excluding conceptual or review articles; (2) single-case studies and case series were not eligible; (3) the use of MRS was a required criterion; (4) the research needed to focus on depression in the context of LLD; (5) animal studies were not allowed; and (6) the original work had to be in English. Any publications that clearly did not meet these criteria were promptly excluded. The final selection of studies for inclusion was based on a comprehensive review of the full manuscripts, with a preference for studies involving confirmed LLD. To assess the quality of the remaining articles, additional exclusion criteria were established to maintain high research standards: (1) all subjects must have been diagnosed with depression at an age younger than 60 years; (2) subjects with comorbid conditions related to depression; (3) meeting abstracts. Any articles that met even one of the exclusion criteria were excluded from consideration.
To thoroughly evaluate and address potential biases in the reviewed articles, we have developed and implemented strict inclusion and exclusion criteria. Each exclusion criterion highlights a possible bias, and by removing any articles that fulfill even one of these criteria, we significantly decrease the chances of encountering biased reporting. Furthermore, the selected articles clearly differentiate between patient and control groups, as demonstrated by statistically significant differences, which enhances the accuracy of in vivo diagnoses. The literature search and selection process were conducted by two independent reviewers. In cases of disagreement, the reviewers discussed their reasoning for including or excluding a specific study. If they could not reach an agreement, a third reviewer was brought in to help with the decision-making process.
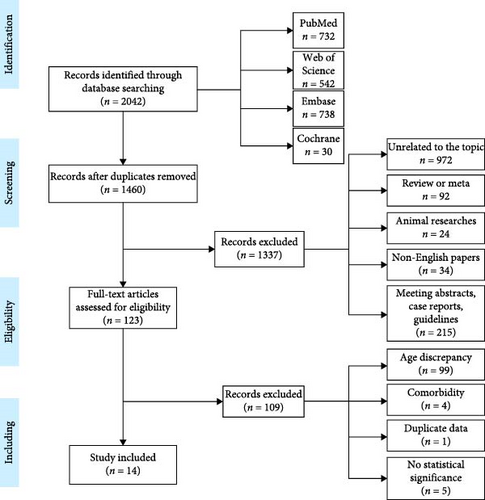
In conclusion, after reviewing the complete texts of 123 studies, 14 publications were selected for inclusion. The article screening process is illustrated in Figure 1.
2.3. Recorded Variables, Data Extraction, and Analysis
Data was collected and extracted by two independent reviewers. For each publication included in the review, the following variables were carefully recorded: the country and institution where the research was conducted, the authors and the year of publication, the study design, details of the experimental group (including the number of participants, their characteristics, gender, and age range), details of the control group (also including the number of participants, their characteristics, gender, and age range), the MRS technique used, the analytical methods applied, the extent of MRS coverage, and the study outcomes. We focused on studies that reported significant differences in brain metabolic levels between the patient group and the healthy control (HC) group, studies that showed a significant correlation between brain metabolic levels in the patient group and depressive symptoms, or studies that indicated a significant correlation between brain metabolic levels in the patient group and cognitive impairment. Results were deemed statistically significant if p < 0.05.
3. Results
Our systematic search resulted in 14 original investigations, which were included for the review, as detailed in Table 1. First, we provided a detailed description of the changes in metabolites in different brain regions. Subsequently, we described changes in brain metabolites associated with LLD depressive symptoms and cognitive impairment.
| References | Numbers of patients/controls | Age of patients (SD)/controls (SD) | Diagnosis | Nucleus | Scanner strength | Equipment | Metabolites reported | Metabolites with significant increase in patients with LLD | Metabolites with significant decrease in patients with LLD | Brain region | Correlation between metabolites and depressive symptoms | Correlation between metabolites and cognitive impairment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [17] | 20 EOD, 27 LOD | 62.7 (6.7), 65.6 (5.4) |
|
1H | 1.5T | MRS | NAA/Cr | — |
|
Frontal WM | — | — |
| [18] | 27/19 | 72.6 (4.7)/71.2 (7.2) | LLD | 1H | 3T | MRS | NAA/Cr, Cho/Cr, mI/Cr |
|
|
The left frontal WM, left periventricular WM, and left basal ganglia of the brain | — | The MMSE score correlated with the mI/tCr ratio (r = −0.50, p = 0.02) in the basal ganglia |
| [19] | 75LLD (patients were classified into those with and without self-awareness based on MDIS scores) | 69.5 (6.7) | LLD | 1H | 3T | MRS | NAA/tCr | — |
|
Left frontal lobe | Total scores of MDIS correlated with NAA/tCr (rho = 0.31; p = 0.006) in the left frontal lobe | — |
| [20] | 14/12 | 72.1 (5.1)/72.7 (4.6) | LLD | 1H | 3T | MRS | NAA, Cho, Cr, mI |
|
|
Whole brain | — | — |
| [21] | 7/10 | 63–76/65–75 | LLD | 1H | 1.5T | MRS | Cho/Cr, NAA/Cr |
|
— | Whole brain | — | — |
| [22] | 20/18 | 69.95 (8.18)/72.39 (5.79) | LLD | 1H | 1.5T | MRS | NAA, Cho, Cr, mI |
|
— | Prefrontal lobe | — | — |
| References | Numbers of patients/controls | Age of patients (SD)/controls (SD) | Diagnosis | Nucleus | Scanner strength | Equipment | Metabolites reported | Metabolites with significant increase in patients with LLD | Metabolites with significant decrease in patients with LLD | Brain region | Correlation between metabolites and depressive symptoms | Correlation between metabolites and cognitive function |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [23] | 25 LLD with MCI, 18 MCI/19 | 64.7 (3.1), 64.2 (3.4)/63.9 (3.3) | LLD with MCI OR MCI | 1H | 1.5T | MRS | mI, mI/Cr | — | — | Frontal lobe, PCG, occipital lobe | — | Frontal lobe mI (p = 0.007) and mI/Cr ratio (p = 0.031) significantly higher in MCI than HCs. Occipital and PCG mI/Cr ratios (p = 0.033) also higher in MCI |
| [24] | 58/12 | 72.1 (3.9)/61.5 (4.9) | DEP | 1H | 3T | 3D MRS | GSH/Cr |
|
— | ACC | The elevated GSH/Cr ratio was associated with greater depressive symptoms (r = 0.28, p = 0.038) | The elevated GSH/Cr ratio was correlated with the RAVLT-encoding (r = −0.28, p = 0.040) |
| [25] | 9/9 | 70 (7)/67 (7) | LLD | 1H | 7T | MRS | GABA, GSH, NAAG, NAA, and mI | — |
|
ACC and PCC |
|
— |
| [26] | 13/10 | 70.7 (9.5)/73.6 (6.3) | LLD | 31P | 4T | 3D MRS | β-NTP, NTP, pMg, pH |
|
|
Whole brain | — | — |
| [27] | 10/8 | 68.300 (9.499)/74.125 (5.249) | LLD | 31P | 4T | 3D MRS |
|
— |
|
Whole brain | — | Altered levels of β-NTP (b1 = 0.037; t135 = 2.57; p = 0.022) and Pi (b1 = −0.067; t106 = −3.51; p = 0.001) in white matter were associated with executive function in LLD group only |
| [29] | 51 (one group with/without drug therapy) | 72.4 (4.1) | DEP | 1H | 3T | MRS | GSH/Cr |
|
— | Thalamus | The increase of GSH/Cr was, in turn, associated with a worsening of depressive symptoms (r = 0.43; p = 0.043) | — |
| [15] | 35/21 | 63.57 (7.55)/65.48 (7.00) | LLD | 1H | 3T | MRS | NAA/Cr, Glu/Cr | — | — | Hippocampus | — | Lower levels of NAA/Cr were related to poorer verbal learning (r = 0.44, p = 0.008) and memory retention (r = 0.41, p = 0.018) in the depressed subjects |
| [28] | 3/33 | 81.24 (5.43) | LLD | — | — | MRS | NAA/Cr | — | — | Hippocampal | — | Hippocampal NAA/Cr measures were associated with performance on the FCSRT-IR (LH: rs = 0.35, p = 0.035; TH: rs = 0.43, p = 0.010) |
- Abbreviations: β-NTP, beta-nucleosidel triphosphates; NAA, N-acetyl aspartate; NAAG, N-acetylaspartylglutamate; PE, phosphoetanolamine; PEtn, phosphoethanolamine; PME, phosphomonesters; pMg, potential of magnesium; ACC, anterior cingulate cortex; ALCAR, acetyl-L-carnitine; ATP, adenosine triphosphate; BDI, Beck depression inventory; Cho, choline; Cr, creatine; DEP, “At-risk” of depression; EOD, early-onset depression; GABA, γ-aminobutyric acid; Glx, glutamate/glutamine; GM, gray matter; GPCho, glycerophosphocholine; GPEtn, glycerophosphoethanolamine; GSH, glutathione; HDRS, Hamilton depression rating scale total score, 17-item version; LH, left hippocampal; L-MTL, the left medial temporal lobe; LOD, late-onset depression; MDIS, the mood disorders insight scale; mI, myo-Inositol; MMSE, mini-mental state examination; MT, magnetization transfer; NTP, nucleoside triphosphates; PCC, posterior cingulate cortex; PCG, precentral gyrus; PCho, phosphocholine; PCr, phosphocreatine; PFC, prefrontal cortex; pH, potential of hydrogen; Pi, inorganic phosphate; RAVLT1–5, Rey verbal learning test, measure of total learning over trials 1-5, expressed as a z score; RAVLT7/5: Rey auditory verbal learning test, percent retention ([trial 7/trial 5) × 100%]), measure of delayed recall, expressed as a z score; RH, right hippocampal; SD, standard deviation; tCr, total creatine (creatine plus phosphocreatine); TH, total hippocampal; WM, white matter.
3.1. Changes of Metabolites in Various Brain Regions of LLD
3.1.1. PFC
Seven studies have reported abnormalities in frontal brain metabolites in LLD. Current MRS research on the frontal lobe primarily examines NAA, Cr, Cho, and myo-Inositol (mI). Murata et al. [17] and Chen et al. [18, 19] discovered that the NAA/Cr ratio in the left PFC is lower in individuals with LLD. Venkatraman et al. [20] reported reduced levels of NAA, Cr, and Cho in the bilateral PFC of LLD patients compared to a control group, noting that Cr levels increased following antidepressant treatment. However, some studies have yielded conflicting results; for instance, Kumar et al. [22] found elevated Cr and Cho levels in the frontal white matter of LLD patients, while metabolites in the gray matter showed no significant differences between patients and HC [21, 22]. Other researches have confirmed metabolic changes in the dorsolateral PFC (DLPFC) in LLD, indicating higher mI content and increased Cho/Cr and mI/Cr ratios in DLPFC white matter compared to controls [19, 22]. Conversely, Aldossary et al. [23] found no significant differences in mI, Cr, or the mI/Cr ratio in the frontal lobes of LLD patients with when compared to HC. Overall, while LLD patients exhibit decreased NAA and NAA/Cr ratios in the frontal lobe cortex, there remains controversy regarding changes in metabolites such as Cr, Cho, and mI.
3.1.2. Cingulated Gyrus
Two studies have identified abnormalities in brain metabolites within the cingulate gyrus in individuals with LLD. Currently, research has primarily concentrated on metabolites such as GSH and NAA. Duffy et al. [24, 29] found that the GSH/Cr ratio in the anterior cingulate cortex (ACC) of the LLD group was significantly elevated compared to HC. Meanwhile, Smith reported a decrease in the NAA/total Cr (tCr) ratio in the posterior cingulate cortex (PCC) of the LLD group. Furthermore, while initial comparisons did not reveal any significant differences in the ratios of γ-aminobutyric acid (GABA) to tCr and Glu to tCr between LLD patients and HC, subsequent analysis postcitalopram intervention demonstrated a reduction in these metabolic ratios within the LLD cohort [25].
3.1.3. Whole Brain
Three studies examined alterations in brain metabolites. Forester and Harper et al. discovered that levels of NTP (nucleoside triphosphates), beta-nucleoside triphosphates (β-NTP), and magnesium in white matter were decreased in patients with LLD, while the levels of magnesium and pH in gray matter were increased in LLD, which indicating a potential presence of neuronal mitochondrial dysfunction in these individuals [26, 27]. Additionally, Charles et al. [21] reported that the overall brain Cho/Cr ratio in LLD patients was significantly elevated compared to the control group.
3.1.4. Basal Ganglion
One study examined changes in metabolites within the basal ganglia. Chen et al. [19] discovered that the ratios of Cho/Cr and mI/Cr in the left basal ganglia of patients with LLD were elevated.
3.1.5. Hippocampus
Two studies investigated midbrain metabolites in the hippocampus of patients with LLD. Jayaweera et al. [15] and Ezzati et al. [28] observed that, in comparison to the control group, the ratios of NAA/Cr, Glu/Cr, and Cr in the hippocampus of LLD patients exhibited a decreasing trend; however, these differences were not statistically significant.
3.1.6. Temporal Lobe
A study focused on the metabolites in the temporal lobe of patients with LLD. Venkatram et al. [20] discovered that, in comparison to the control group, levels of NAA and mI in the left medial temporal lobe were significantly elevated in LLD patients.
3.2. Study on Correlation Between Brain Metabolites and Depressive Symptoms
Chen et al. [19] discovered that a reduced NAA/Cr ratio in the left PFC of individuals with LLD was positively linked to depressive symptoms. Additionally, a lower NAA/Cr ratio in the left-DLPFC was positively associated with self-awareness [19]. In a related study, Duffy et al. [24] indicated that an elevated GSH/Cr ratio in the ACC was positively correlated with severe depressive symptoms. Smith et al. [25] proposed that the alleviation of depressive symptoms following citalopram treatment was negatively correlated with the decrease of Glu/tCr in the PCC and positively correlated with the increase of GSH/tCr in the PCC. In contrast, Duffy et al. [29] observed that the placebo group exhibited greater changes in GSH/Cr levels in the thalamus compared to those taking omega-3 fatty acids, and this increase in GSH/Cr was positively associated with a worsening of depressive symptoms.
3.3. Study on the Correlation Between Brain Metabolites and Cognitive Impairment
Chen et al. [18] discovered a negative correlation between basal ganglia mI/tCr levels and overall cognitive function. In addition, Aldossary et al. [23] observed that patients with mild cognitive impairment (MCI) exhibited significantly higher mI and mI/Cr ratios in the frontal lobe, occipital lobe, and posterior cingulate gyrus compared to healthy individuals; however, no diffenreces were found between patients with depression and cognitive impairment and those with MCI. Duffy et al. [24] reported that in individuals with LLD, GSH/Cr ratios in the cingulate gyrus were inversely related to language learning abilities. Jayaweera et al. [15] found that lower NAA/Cr levels in the hippocampus were positively associated with deficits in verbal learning and memory. Similarly, Ezzati et al. [28] found a correlation between hippocampal NAA/Cr values and verbal memory performance. Lastly, Harper et al. [27] have demonstrated that the Stroop task, a well-established measure of executive function, is capable of predicting reductions in β-NTP and is negative with inorganic phosphate (Pi) within the white matter of individuals with LLD.
4. Discussion
In this review, we have compiled the latest clinical research on MRS in patients with LLD, emphasizing the connection between brain metabolites and depressive symptoms, cognitive deficits, and contrasting these with metabolic irregularities observed in cognitive impairment disorders. Our analysis indicates that most studies report a reduction in the NAA/Cr ratio in the frontal lobe cortex among LLD patients, along with a correlation between NAA/Cr levels in depressive symptoms [17–19]. However, findings regarding changes in metabolites such as Cr, mI, and Cho are inconsistent [20–22]. LLD patients also demonstrated reduced NAA levels in the cingulate gyrus [25] and increased Cho/Cr and mI/Cr ratios in the left basal ganglia [19, 30]. The changes in NAA/Cr in the frontal and cingulate gyrus show some overlap between LLD and MCI patients [23, 31, 32]. Contrary to what was observed in individuals with cognitive disorders, LLD patients did not exhibit metabolic irregularities within the hippocampus and parietal lobe. The metabolic changes associated with LLD are closely linked to its depressive symptoms and cognitive impairment but do not align with the brain regions exhibiting metabolic abnormalities in cognitive disorders.
4.1. Brain Metabolites Associated With LLD
4.1.1. Neuronal Viability Marker: Changes in NAA and Its Significance
NAA is a crucial metabolite predominantly found in neurons and serves as a marker for neuronal health and integrity. In the context of LLD, studies have shown that NAA levels are significantly altered. For instance, research indicates that elderly individuals suffering from depression exhibit reduced NAA concentrations in the PFC, which correlates with depressive symptoms and cognitive decline. This reduction suggests a potential neurodegenerative process or impaired neuronal function associated with depression in older adults. The significance of these findings lies in the potential for NAA to serve as a biomarker for diagnosing LLD and monitoring treatment responses. Elevated NAA levels posttreatment may reflect improved neuronal health and recovery from depressive episodes, highlighting its utility in both clinical and research settings as a neurochemical marker of mood disorders in the elderly [33, 34].
4.1.2. Energy Metabolites in Brain Neurons: The Role of PCr, NTP, ATP, Pi, Magnesium, and pH
The metabolism of PCr, NTP (represented by ATP), and Pi are critical in understanding the energetic state of cells, particularly in the context of LLD. In geriatric depression patients, studies utilizing dynamic phosphorus MRS (31P-MRS) have revealed significant alterations in the levels of PCr, NTP, β-NTP, ATP, and Pi. These metabolites are essential for energy production and management within neurons. Given that the β-NTP peak is predominantly derived from ATP [35], so in the field of phosphorus spectroscopy, particularly when examining 31P-MRS data, the β-NTP peak is predominantly indicative of the ATP concentrations [27]. The reduced NTP and β-NTP, especially ATP levels, indicate a compromised energy metabolism, which can contribute to the symptoms of depression, such as fatigue and cognitive dysfunction. Cr kinase (CK), a cytoplasmic isoform, predominantly facilitates the reverse reaction, transforming PCr back into Cr, thereby producing ATP [36]. This process serves not merely as an energy buffer but also as a metabolic regulator. By catalyzing the regeneration of ATP from PCr, CK plays a crucial role in stabilizing intracellular ATP levels and maintaining a high ATP to adenosine diphosphate (ADP) ratio, both of which are vital for the efficient execution of cellular functions. This mechanism is essential in preventing a precipitous decline in ATP levels during periods of heightened energy demand, thereby ensuring the continuity of cellular energy supply [37]. Therefore, the relationship between PCr and ATP dynamics suggests that disturbances in energy homeostasis may play a fundamental role in the pathophysiology of LLD. Enhancing energy production through therapeutic interventions aimed at restoring ATP levels could potentially alleviate depressive symptoms and improve overall brain function in older adults [38, 39]. Pi in this reaction acts as a metabolic signal, aiding in the clearance of ADP and hydrogen ions (H+), which are byproducts of ATP hydrolysis, involving in cellular energy metabolic processes. It was observed increase trend in the white matter of LLD patients, which is negatively correlated with executive function. This correlation suggests that elevated Pi may serve as a metabolic indicator of neurobiological changes associated with executive dysfunction in LLD. The increased Pi could reflect heightened neuronal activity, energy metabolism demands, or potential mitochondrial dysfunction, which are all implicated in the pathophysiology of cognitive impairment in LLD [27, 40]. Magnesium plays a significant regulatory role in cellular pH, cell volume, and intracellular cation concentrations, which may indirectly influence the CK enzyme activity and the equilibrium between ATP and PCr in brain neurons [41]. Consequently, aberrant magnesium levels and increased pH could result in dysregulated energy metabolism within neuronal cells of the brain, offering potential avenues for the development of future antidepressant therapies in individuals with LLD.
4.1.3. Representatives of Glial Activity: Cho and mI
Cho and mI have emerged as an important metabolite in the study of LLD. Both Cho and mI exhibit higher concentrations within glial cells compared to their levels in neurons accessible by MRS. This differential concentration can be indicative of glial cell activity to a certain extent [42]. Cho is associated with cell membrane metabolism and myelination [43] and is a precursor for the neurotransmitter acetylcholine, which is vital for cognitive functions and mood regulation. Research has indicated that Cho levels are often altered in individuals with depression, and these changes may be linked to cholinergic dysfunction. The cholinergic system interacts with other neurotransmitter systems, including noradrenergic, serotonergic, dopaminergic, GABAergic, glutamatergic, and cannabinoid systems, to modulate mood [44]. The elevated mI levels observed in LLD are significant, as mI serves as a glial marker, is integral to the lipid composition of biomembranes, and is crucial for the phosphatidylinositol second messenger system, which is linked to the pathophysiology of LLD, and changes in brain mI concentrations are also associated with various neuropsychiatric disorders [45, 46]. Increased concentrations of mI and Cho are frequently indicative of glial cell activation, which is typically linked to neuroinflammatory processes. This is consistent with the inflammation hypothesis [47, 48]. Furthermore, astrocytes, a type of glial cell, are crucial for maintaining the neuronal environment, which undergo significant changes at the transcriptomic and functional levels in both healthy aging brains and in the context of neurodegenerative diseases [49]. The parallel increase of mI and Cho levels were observed consistently in two studies in Table 1, which suggests a potential mechanism in which astrocytic function becomes dysregulated in response to pathological stimuli. Collectively, Cho and mI are significant biomarkers and therapeutic targets in the context of LLD, with its levels in the brain being associated with the disease’s pathophysiology and its potential use in the treatment being an area of active research [45].
4.2. Metabolic Changes Between LLD and Major Depression Disorder (MDD) in Other Age Groups
The changes in brain metabolites of depression in other age groups and LLD have similarities but also differences.
4.2.1. PFC, Cingulated Gyrus, and Parietal Lobe
Both LLD and MDD in adults exhibited reduced levels of NAA in the frontal lobe, indicating dysfunction of brain neurons and alterations in energy metabolism. In terms of other metabolites in frontal, Kumar et al. [22] and Binesh et al. [50] noted that the LLD patients had higher levels of mI than the controls, although the differences were not statistically significant. Wyckoff et al. [51] found a correlation between the content of macromolecular proteins in the white matter of left-DLPFC and the mI and NAA in LLD, suggesting that metabolites and protein metabolism may be related.
4.2.2. Cingulate Cortex and Parietal Lobe
In the ACC, depression in different age groups also shows significantly reduced levels of NAA and NAA/Cr, but there was no consistent trend at Cho and other metabolite levels [52–54]. LLD has metabolite changes in both the anterior and posterior cingulate gyrus. For the patients with MDD, the MRS studies on the cingulate gyrus mainly focused on the ACC, and the conclusions are inconsistent. Both He et al. [53] and Wade-Bohleber’s [55] studies proposed that the levels of Glu, Gln (glutamine), and mI in ACC in the MDD group were significantly higher than those in the HC, while the concentration of NAA was decreased, and the concentration of Gln was correlated with the severity of depressive symptoms. Benson et al. [56] found that patients with MDD had 15% lower Glu/Cr in ACC compared to the HC, and the Glu/Cr ratio was negatively correlated with the degree of lack of pleasure. However, some studies on the altered concentration of ACC metabolites have also suggested that there was no significant difference in ACC metabolites between the MDD group and the control group [57, 58]. Additionally, abnormalities in glutamatergic function are observed in the DLPFC and ACC, such as decreased levels of Glu, Glx, GABA, Glu/Cr, and GABA/tCr ratios [14, 56, 59, 60]. Treatment-resistant depression patients also demonstrated significantly decreased levels of Glu and Glx in the left DLPFC, as well as reduced levels of NAA, Cho, and NAA/Cr ratio in the left ACC, which improved after rTMS treatment [61]. It suggests that the cingulate gyrus may alter the distribution of metabolites in the brain through its connections to the prefrontal and limbic projections, thereby regulating negative stimulation and self-attention and thus playing an important role in emotional regulation and cognitive function.
However, in the parietal lobe, MDD in adults also shows reduced levels of NAA, Cr, and Cho [62], and no studies have found similar changes in LLD.
4.2.3. Basal Ganglion, Hippocampus, and Thalamus
Furthermore, a study found significantly elevated levels of Cho in the left hippocampus in MDD [63], different with LLD results. Haroon et al. [64] found that compared with the control group, Glu increased in the left basal ganglia of MDD patients, which is related to anhedonia and psychomotor inhibition, suggesting that glial cell dysfunction was associated with depressive symptoms.
These studies suggest that LLD and MDD in adults share similar alterations in metabolite levels in regions such as the frontal lobe and cingulate gyrus. However, MDD in adults also shows reduced levels of NAA, Cr, and Cho in the parietal lobe [62]; changes in metabolites in the parietal are not found in LLD. Future research could further investigate whether neuro-metabolic alterations resembling those observed in adults with MDD exist in brain regions such as the parietal lobe and the occipital lobe in patients with LLD to clarify the similarities and differences of pathological mechanisms between LLD and MDD.
4.3. Metabolic Changes Between LLD and Cognitive Disorders
LLD is a risk factor for MCI [65] and for AD. Between LLD and cognitive impairment, the study found that metabolites such as NAA/Cr had similar trends in the frontal lobe and cingulate gyrus. A meta-analysis showed that patients with cognitive impairment had significantly reduced frontal lobe NAA and Cr concentrations, along with elevated levels of myocardial infarction in various brain regions, particularly the hippocampus, and while GSH levels were significantly lower in MCI patients, no changes were detected in AD patients [46]. A meta-study by Liu et al. [66] found that the ratio of NAA/Cr in the hippocampus and the concentration of NAA in PCC decreased dramatically during the pathologic progression from MCI to AD. The concentration of mI in PCC increased significantly in patients with AD and MCI compared to HC. NAA and mI levels are associated with cognitive decline.
In contrast to patients with LLD, those with cognitive impairment demonstrated altered metabolite levels within the hippocampus and parietal lobes. The study by Valatkevičienė et al. [32] identified a moderate to strong positive correlation between NAA/Cr, NAA/mI, and FA of the white matter tracts in the hippocampus and dorsal PCC in MCI patients. This suggests that the biochemical integrity of the hippocampus and cingulate cortex is linked to the microstructural characteristics of the ipsilateral white matter tract originating from the hippocampus [32]. Studies on MRS of MCI showed that mI was higher and NAA was lower [67] in the parietal cortex than in the control group, and mI level could predict the progression of MCI to AD with high specificity [68]. However, no similar changes in parietal metabolites were found in LLD. The pathology of AD is characterized by the deposition of amyloid-beta (Aβ) in the brain [69, 70]. The reason for the high risk of cognitive impairment in LLD patients may be the existence of AD pathology. Current studies mostly involve Aβ protein, but the results are controversial at present. Some studies have found that the level of Aβ in LLD is increased [71]. Mackin et al. [72] reported that Aβ levels were reduced in LLD patients. A meta-analysis showed significant differences in Aβ metabolism in LLD, the same changes as in patients with AD [73].
Some recent researches on cognitive impairment combined PET molecular imaging and assisted MRS measurement to explore the relationship between brain metabolites and Aβ protein and found that NAA and Aβ were negatively correlated in AD and MCI patients [74, 75]. Some studies have also found a positive correlation between mI and Aβ in the cingulate cortex in AD [76], while Sheikh-Bahaei et al. [74] did not find a correlation between LLD and MCI. Additionally, a positive correlation was suggested between total Cho (tCho) and Aβ deposition in a univariate model specific to PCC in LLD patients, excluding the influence of sex. Furthermore, only in the ACC of HC lower levels of GABA were associated with higher Aβ protein [77]. More studies are likely to be conducted in the future to explore the relationship between LLD metabolites and Aβ proteins and to reveal its possible pathophysiological mechanism.
There are also some shortcomings in previous studies, such as small sample size, retrospective study, failure to fully consider the possible impact of drug use on the results of MRS, and technical limitations. Studies in the future can be explored from the following aspects: first, the sample size should be expanded to study more representative samples to further verify and improve the existing research results. Second, more longitudinal studies are needed to determine the underlying susceptibility to LLD and how the MRS changes before and after the onset of the disease and before and after treatment. Third, future research could build on existing research to explore the differences between LLD patients with and without cognitive impairment to help identify patients at risk for cognitive impairment and reduce associated harm. Fourth, with the development of imaging technology, multimodal magnetic resonance studies can be carried out to analyze the same sample by integrating multiple MRI techniques to mutually verify and supplement the results of various MRI techniques and provide more comprehensive clinical clues and imaging evidence. MRS technology has great application potential in the research of LLD and provides new ideas and methods in promoting the diagnosis, treatment, and prevention of LLD. It can be used as an effective auxiliary diagnostic tool to help doctors more accurately diagnose LLD. At the same time, by monitoring changes in brain metabolic activity, it can provide more references for developing personalized treatment programs.
5. Conclusions
LLD is associated with neurobiochemical alterations in the frontal lobe, cingulate gyrus, and other areas of the brain, which are strongly linked to depressive symptoms and cognitive impairment. In contrast, the neurobiochemical changes observed in the hippocampus and parietal lobe are distinctive features in cognitive disorders, indicating that the underlying mechanisms of cognitive impairment in these two conditions differ.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Sha Liu, Yong Xu, and Yao Gao created the study’s concept and design. Qian Wu and Wu-Yu Mao wrote the initial manuscript and carried out the analyses. Wu-Yu Mao, Yao Gao, Qian Wu, and Zhong-Ping Wen contributed to the revision of the manuscript. Bo Chen and Ji-Hui Zhang contributed to the data analysis. Each author accepted the submitted version of the paper and made contributions to it. Qian Wu, Wu-Yu Mao, and Yao Gao contributed equally to this work and are share first authorship.
Funding
This work was supported by the National Natural Science Foundation of China (82271546 and 82301725), National Key Research and Development Program of China (2023YFC2506201), Key Project of Science and Technology Innovation 2030 of China (2021ZD0201805), China Postdoctoral Science Foundation (2023M732155), Shanxi Science and Technology Innovation Talent Team (202304051001049), Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20240041), Fundamental Research Program of Shanxi Province (202203021212028), research project supported by Shanxi Scholarship Council of China (YC2034), Shanxi Medical University School-Level Doctoral Initiation Fund Project (XD2102), Youth Project of First Hospital of Shanxi Medical University (YQ2203), and Doctor Fund Project of Shanxi Medical University in Shanxi Province (SD2216).
Acknowledgments
We would like to thank Dr. Sha Liu and her lab for the assistance and support it has provided us with. This project is supported by the above-mentioned funds.
Open Research
Data Availability Statement
The template data collection forms and data extracted from included studies can be requested to the corresponding author upon justified request for academic purposes only.




