Chromobacterium haemolyticum Infection Subsequent to Experiencing a Traumatic Event in a Rice Field: A Case Report and Literature Review
Abstract
The incidence of infections caused by Chromobacterium haemolyticum, phylogenetically related however distinct from Chromobacterium violaceum, has increased since its identification in 2008. Differences in their unique microbiological features have been highlighted, particularly regarding their phenotypic distinctions in the colony pigmentation and hemolysis. This is largely due to C. haemolyticum being misidentified as C. violaceum, using the current automated microbial identification systems. However, clinical aspects and outcomes of C. haemolyticum infections remain unclear as few clinically relevant cases have been reported and considered similar to C. violaceum infections. Consequently, we reported an extremely rare case of C. haemolyticum bacteremia, which was initially diagnosed as a C. violaceum infection, however was later confirmed to be a C. haemolyticum infection, using 16S ribosomal ribonucleic acid (rRNA) sequence analysis. Abscess formation was not observed, and the patient was treated with a short course of antibiotics. Ultimately, his condition resolved, without recurrence during the 1-year follow-up. Clinicians should be aware that if the isolated organism is originally identified as C. violaceum, however is phenotypically mismatched with colony nonpigmentation and beta-hemolysis; the organism may be C. haemolyticum. Mortality, abscess formation, and recurrence rates are lower than those of C. violaceum, and chronic broad-spectrum antibiotic suppression may not be required, potentially avoiding unnecessary antibiotic use and preventing multidrug resistance.
1. Introduction
Chromobacterium haemolyticum is a Gram-negative bacterium primarily found in the water and soil of tropical and subtropical ecosystems [1]. This organism is one of 14 species of the genus Chromobacterium [2, 3]. Initially, upon its identification in clinical samples, the association of C. haemolyticum with human diseases was not well understood. However, it has recently been recognized as a pathogen in extremely rare instances of human infections [4, 5].
Before the discovery of C. haemolyticum, this bacterium was considered the same as Chromobacterium violaceum, another bacterium that has been recognized as a rare but lethal opportunistic pathogen in humans [6]. Since C. haemolyticum was found to be phylogenetically related to, but different from, C. violaceum, the differences between them have been debated. In particular, microbiological differences in colony pigmentation and hemolysis were highlighted. This is because C. haemolyticum may be misidentified as C. violaceum using the current automated microbial identification systems. However, the clinical aspects and outcomes of C. haemolyticum infection are yet to be elucidated because of its rarity, and only seven clinically relevant cases have been reported [4, 5, 7–11], although it is considered similar to C. violaceum infection.
Here, we report an extremely rare case of C. haemolyticum bacteremia. The organism was initially identified as C. violaceum; however, colony pigmentation and hemolysis were inconsistent with the features of C. violaceum and was later identified as C. haemolyticum using 16S ribosomal ribonucleic acid (rRNA) sequence analysis. Unlike C. violaceum infection, the patient was successfully treated with a short course of antibiotics, without abscess formation or recurrence. This is the first report to indicate that, although C. haemolyticum is phylogenetically related to C. violaceum, its impact on humans as an infectious disease may differ completely.
2. Case Presentation
The 80-year-old male patient had a known history of hypertension, diabetes mellitus, dyslipidemia, chronic renal disease, and chronic cardiac failure. He attempted to avoid another car while driving and overturned into a rice field 7 days prior to the current admission. He crawled out of his car; however, he collapsed in the rice field, remaining there for several hours. He was transported to the emergency department, and necrotic wounds were identified on his right hand. Subsequently, these wounds were irrigated and sutured. Two days thereafter, the patient presented with fatigue and a loss of appetite. His symptoms gradually worsened to include lethargy and dyspnea. The patient was subsequently admitted to our hospital.
On physical examination, the patient was lethargic, with a blood pressure of 153/80 mm Hg, pulse of 93 beats/min, respiratory rate of 25 breaths/min, oxygen saturation of 96% on room air, and body temperature of 36.6°C. Exudates from the wounds on the dorsal side of the right hand were observed. Breath sounds of the lower pulmonary lobes had decreased bilaterally. Moreover, he had bilateral lower extremity pitting edema. He had a tachycardia, without systolic murmurs. On palpation, his abdomen was soft, without tenderness.
Laboratory investigations were performed. The complete blood count revealed leukocyte and platelet counts of 9.9 × 109/L and 247 × 109/L, respectively, in addition to C-reactive protein and hemoglobin levels of 8.96 mg/dL and 9.4 g/dL, respectively. Renal functions test revealed blood urea nitrogen and creatinine levels of 23 and 2.01 mg/dL, respectively. Liver function tests revealed levels of alanine and aspartate aminotransferases and albumin of 19, 28 U/L, and 3.2 g/dL, respectively. The B-type natriuretic peptide measured 891.2 pg/mL. The serum glucose was 175 mg/dL, and the hemoglobin A1c measured 8.3%.
Chest radiography revealed bilateral pleural effusions with cardiomegaly. A provisional diagnosis of cellulitis of the right hand, complicated by the exacerbation of cardiac failure, was made. Subsequently, two sets of blood cultures were obtained. Empirical antibiotic piperacillin–tazobactam was administered. Diuretic treatment was initiated for the cardiac failure. Three days postadmission, the blood culture revealed a Gram-negative rod. The organism was identified as C. violaceum by WalkAway 40 SI (Beckman Coulter, Brea, CA, USA). Most beta-lactam antibiotics, including cefepime, ampicillin–sulbactam, and piperacillin–tazobactam, were inactive against this bacterium. However, this bacterium was sensitive to imipenem, meropenem, ciprofloxacin, levofloxacin, minocycline, doxycycline, and trimethoprim–sulfamethoxazole. Based on these susceptibility results, the piperacillin–tazobactam was switched to meropenem. Abdominal and pelvic ultrasonography revealed no foci of infection, such as abscesses. Sputum culture revealed the presence of normal flora.
When the isolated organism was identified as C. violaceum, the colonies grown on the sheep blood agar plates were gray and nonpigmented, exhibiting beta-hemolysis (Figure 1). These findings were inconsistent with the features of C. violaceum. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI Biotyper, MBT Compass library Ver.9.0.0.0, 8468 MSPs, Bruker Daltonics GmbH, Bremen, Germany) was performed; however, the results were inconclusive due to low score values. Homology analysis of the 16S rRNA sequence involving 1458 base pairs of the organism was performed, using the basic local alignment search tool. This demonstrated that the sequence shared the greatest similarities with that of C. haemolyticum MDA0585T at 99.38% and consecutively with that of Chromobacterium rhizoryzae LAM1188T at 99.30%. Finally, we confirmed the organism to be C. haemolyticum because C. rhizoryzae produces tan-colored colonies and is oxidase-negative [12].
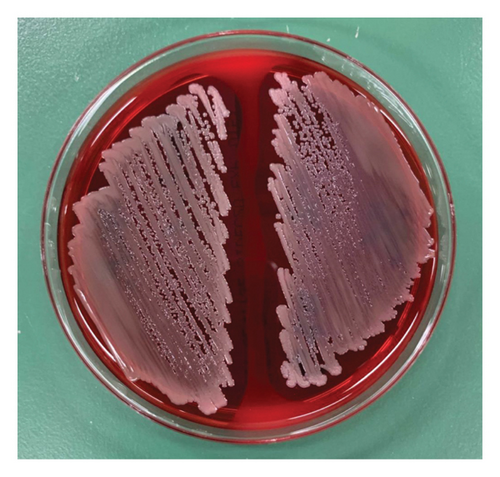
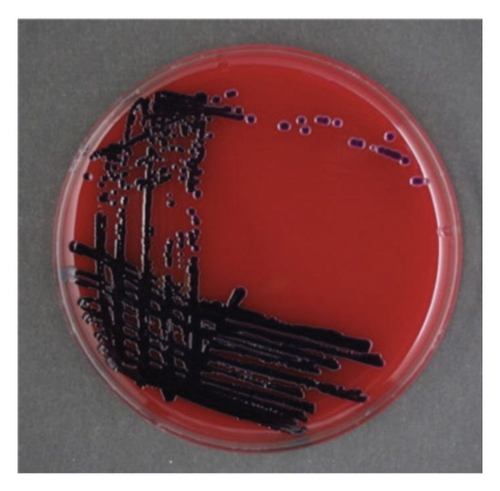
The patient’s general condition improved after antibiotic and diuretic treatment. His cellulitis improved. The repeated blood culture results were negative. On day 11 postadmission, he was discharged home with oral minocycline, to complete a total of 14 days of effective antibiotic treatment. At the 1-year follow-up, the patient was still in good health with no recurrence of infection.
3. Discussion
C. haemolyticum was first identified in 2008 by Han et al. [6], who investigated a nonpigmented species of the genus Chromobacterium in clinical samples. Prior to its discovery, C. haemolyticum was thought to be the same as C. violaceum; however, the presence of this novel strain was suggested by the fact that C. violaceum produces a violet pigment that contrasts with C. haemolyticum. They discovered that C. haemolyticum was phylogenetically closely related to C. violaceum. However, C. haemolyticum has been identified as a different organism due to the lack of colony pigmentation, the ability to hemolyze sheep blood, and differences in several biochemical reactions. The identification of C. haemolyticum is important because current automated microbiological identification systems have misidentified C. haemolyticum as C. violaceum, which occurred in our case.
In previous cases documenting C. haemolyticum, this bacterium was not accurately identified, and the authors have highlighted the principal findings differentiating C. haemolyticum from C. violaceum, which is characterized by colony pigmentation and hemolysis [4, 5, 7–11]. C. violaceum is usually present as violet-pigmented colonies because of the production of violacein, without hemolysis occurring in sheep blood agar cultures. In contrast, C. haemolyticum is nonpigmented and does not produce violacein, with beta-hemolysis occurring in sheep blood agar cultures [6]. Differences exist in many of the biochemical reactions of the species belonging to the genus Chromobacterium; nonetheless, only C. violaceum is available for species identification in this genus, using the mass spectrometry database [9]. Therefore, 16S rRNA sequencing is required for definitive diagnosis of C. haemolyticum infection. If the isolated organism is identified as C. violaceum, however is phenotypically mismatched, clinicians should be aware that the isolated organism may be C. haemolyticum instead [8].
Apart from the microbiological features of C. haemolyticum, the overall clinical picture of C. haemolyticum infection remains unclear owing to its rarity. C. haemolyticum and C. violaceum are the only causative pathogens of human infections belonging to the genus Chromobacterium. However, the clinical similarities and differences between C. haemolyticum and C. violaceum infections have not received much attention and have been considered similar. C. violaceum infection typically occurs after exposure to soil and water [13]. The most prominent feature of this infection is its severity, which rapidly induces acute progression and multiple organ abscesses, resulting in fatal sepsis [13]. Additionally, C. violaceum is resistant to commonly used beta-lactam antibiotics and tends to relapse posttreatment, with a reported relapse rate of 6.6% [14]. Relapses are postulated to occur because of residual suppurative foci [15]. Thus, chronic suppression with oral antibiotics is recommended for 2–3 months to prevent relapse [14]. Due to its severity, rapid progression, tendency to develop abscesses, drug resistance, and high recurrence rate, the mortality rate of C. violaceum infection is reportedly 35%–53% [13].
Compared to C. violaceum infection, C. haemolyticum infection is extremely rare; only eight clinically relevant cases have been reported to date including ours [4, 5, 7–11] (Table 1). The diagnostic presentations include necrotizing fasciitis [4], proctocolitis [5], pneumonia [7, 9], meningitis [10], bacteremia [4, 8–11], and cellulitis in one, one, two, one, six, and two patients, respectively [11], some of which overlapped. These cases have been reported in the United States, Japan, and Thailand [4, 5, 7–11]. Six of the eight patients (75%) had known experiences of aquatic exposure and developed infections, including bacteremia, necrotizing fasciitis, and meningitis [4, 7, 9–11]. The findings of severe C. haemolyticum infections after exposure to contaminated aquatic environments are very similar to those of C. violaceum infections; nonetheless, the outcomes and recurrence rates differ between the two species. Compared with the mortality rate of C. violaceum, only one case (13%) of C. haemolyticum infection resulted in mortality [10]. One patient with alcohol dependence had neck pain due to a cervical fracture secondary to head trauma, which obscured the diagnosis of meningitis, resulting in delayed initiation of antibiotics. All other patients with C. haemolyticum infections survived [4, 5, 7–9, 11], including older adults with multiple comorbidities who developed bacteremia [9]. Remarkably, none of the eight patients (100%) developed abscesses; and four of the eight patients who were successfully cured had not been treated with chronic suppressive antibiotics [5, 7, 11]. Recurrence of infection in the short term has not been reported in these cases. C. haemolyticum infection did not recur in our patient during the long-term 1-year follow-up period (Table 2).
| No. | Age | Sex | Diagnosis | Positive culture | Incident | Presumed source of organism | Comorbidity | Abscess formation | Antibiotics | Effective antibiotics | Outcome | Recurrence | Country | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | M | Necrotizing fasciitis, bacteremia | Blood | Road accident | River | None | No |
|
6 weeks | Survived | None for 6 months | Japan | [4] |
| 2 | 4 | F | Proctocolitis | Stool | — | — | — | No |
|
10 days | Survived | None for 6 weeks | Thailand | [5] |
| 3 | 69 | M | Pneumonia | Sputum | Water aspiration | Runoff water |
|
No |
|
11 days | Survived | Transferred to another hospital | Japan | [7] |
| 4 | 11 | M | Catheter-associated bacteremia | Blood | — | — |
|
No |
|
8 weeks | Survived | None for 3 months | United States of America | [8] |
| 5 | 70s | M | Pneumonia, bacteremia | Sputum, blood | Near-drowning | River |
|
No |
|
11 weeks | Survived | — | Japan | [9] |
| 6 | 73 | M | Meningitis, bacteremia | Spinal fluid, blood | Head trauma | Canal | Hypertension, hyperuricemia, alcoholic hepatitis | No |
|
4 days | Demised | — | Japan | [10] |
| 7 | 50s | M | Cellulitis, bacteremia | Blood | Skin injury | Hot spring | — | No |
|
17 days | Survived | — | United States of America | [11] |
| Our case | 80 | M | Cellulitis, bacteremia | Blood | Skin injury | Rice field |
|
No |
|
2 weeks | Survived | None for 1 year | Japan | — |
| Chromobacterium haemolyticum | Chromobacterium violaceum | |
|---|---|---|
| Microbiological features | ||
| Colony pigmentation | Nonpigmentation | Violet pigmentation |
| Hemolysis | Beta-hemolysis | Nonhemolysis |
| Clinical features | ||
| Incidence | Extremely rare: 7 cases | Rare |
| Environment | Tropical/subtropical ecosystems | Tropical/subtropical ecosystems |
| Source of infection | Contaminated soil and water | Contaminated soil and water |
| Progression | Acute progression with sepsis | Acute progression with sepsis |
| Abscess formation | No | Multiple organ abscesses |
| Recurrence | No | Yes: 6.6% |
| Susceptibility | Resistant to common beta-lactams | Resistant to common beta-lactams |
| Duration of antibiotics | May be cured with short duration | Chronic suppression for 2-3 months is recommended |
| Mortality | May not be poor ≤ 13% | Poor: 35%–53% |
Clinicians should be aware that if an isolated organism is originally identified as C. violaceum, however is phenotypically mismatched with colony non-pigmentation and beta-hemolysis, the organism may potentially be C. haemolyticum. C. haemolyticum infections can develop into severe sepsis, postexposure to contaminated water, similar to C. violaceum. However, mortality, abscess formation, and recurrence rates are lower in this species than in C. violaceum, and chronic broad-spectrum antibiotic suppression may not be required, potentially avoiding unnecessary antibiotic use and preventing multidrug resistance.
Ethics Statement
Written informed consent was obtained from the patient for publication of his deidentified data and images.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We would like to express our sincere gratitude to Dr. Shigemi Hitomi for performing the genetic analyses and identifying the isolated C. haemolyticum.
Open Research
Data Availability Statement
The data used to support the findings of this study are included within the manuscript.




