Malaria Cytoskeletal Proteins Require Alveolin–Alveolin Interactions for Differential Localization
Recruitment and Organization of Alveolin Proteins
Abstract
The alveolins are a family of intermediate filament-like proteins that form cytoskeletal structures in both free-living and parasitic members of the alveolate kingdom. Despite their important functions, the alveolins’ biochemical properties and organizing principles are still poorly understood. Here, we characterize four alveolins of Plasmodium falciparum, the deadliest malaria parasite, to understand how alveolin domains mediate protein–protein interactions and highly specific recruitment to substructures of the cytoskeleton. Unexpectedly, we uncover variable dependence on alveolin domains for each substructure rather than an overarching mechanism. While PfIMC1e requires 1f to be sequentially recruited to the basal complex, PfIMC1c and PfIMC1g do not require interactions with each other to localize properly to the inner membrane complex. Moreover, alveolin domains are not interchangeable—they contain unique signatures for specialized localization. Finally, we identify a region outside the alveolin domain of PfIMC1e that is important for basal complex recruitment. These results provide direct evidence that alveolin domains mediate both alveolin–alveolin interactions and compartment-specific localization.
1. Introduction
Plasmodium parasites, the causative agents of malaria, have specialized cytoskeletal structures that are essential to support the parasite’s complex lifecycle. One of these structures is the inner membrane complex (IMC), which serves a cytoskeleton-like function in conjunction with the meshwork of proteins that lies attached to its cytoplasmic face, the subpellicular network (SPN) [1]. These highly divergent structures are a distinguishing feature of alveolate cytoskeletal systems and absent from metazoans, including mammals [2–4]. The SPN is a series of filaments that are composed of a family of proteins called alveolins [1, 5, 6]. Studies in both Plasmodium and its relative Toxoplasma have demonstrated that alveolin knockouts lead to defects in cell shape, motility, and infectivity in addition to compromised tensile strength [3, 7–11]. Thus, the SPN filaments are thought to provide mechanical stability, impacting many of the parasite’s critical processes including motility, invasion, and cell division.
All alveolins contain at least one valine- and proline-rich repeat domains (called the alveolin or IMCp domain) with a consensus heptad repeat sequence of EKIVEVP [12]. The regions outside this domain vary greatly in size and do not have conserved features other than predicted posttranslational modification sites [12, 13]. There are 13 alveolins (IMC1a–m) currently annotated in the Plasmodium genome. Despite their “IMC” nomenclature, Plasmodium alveolins have distinct spatial organizational patterns, localizing to either the IMC or the basal complex (BC) [8, 10, 11, 14–17]. The BC is a multiprotein structure that is believed to guide the formation of parasite membranes during cell division, another key component of the cytoskeleton, and likely mediates abscission during cytokinesis [18]. It remains unclear how alveolins achieve these specific spatial patterns or whether alveolins in different compartments hold different biological functions.
The alveolin domain has also been shown to be necessary and largely sufficient for trafficking, often hypothesized to mediate protein–protein interactions for filament formation [19]. Deleting the alveolin domain of PbIMC1h caused loss of protein staining at the IMC in the rodent malaria parasite Plasmodium berghei, and similar observations have been made in the free-living ciliate Tetrahymena [20, 21]. In the parasitic relative Toxoplasma, the alveolin domains of TgIMC3, 6, and 8 on their own showed compartment-specific localization [19, 22]. To achieve wild-type localization, however, TgIMC8 required N- and/or C-terminal sequences, implying important functions also lie outside the alveolin domain [19]. Adding to the complexity, many nonalveolin binding partners have been proposed to participate in the recruitment and stabilization of the alveolin network at the IMC. The most notable examples are the GAPMs in Plasmodium and ILP1 in Toxoplasma, neither of which contains alveolin domains but was demonstrated to bind alveolin proteins [7, 23, 24].
Overall, much remains unknown about the mechanisms behind alveolin function, filament formation, and recruitment to specific cell compartments. Studies thus far have suggested a great diversity in individual alveolin function, localization, and timing of expression during the parasite lifecycle. This diversity indicates that new alveolins in new parasites and lifecycle stages must be individually interrogated. It also motivates us to dissect the primary sequence of these proteins in more detail to identify the molecular signatures of these phenotypes. In this study, we examine the role of the alveolin domain in facilitating interactions between alveolin proteins. We also identify a region outside the alveolin domain, the noncanonical repeat (NCR) domain, that is required for localization. Of the 13 annotated alveolins in the genome, Plasmodium falciparum only expresses four during the asexual blood stage, two that localize to the IMC, and two that localize to the BC. We take advantage of this minimal number to assess the contribution of the alveolin domain to filament formation and compartment-specific recruitment.
2. Results and Discussion
In order to test the determinants of localization for Plasmodium proteins, we generated in trans expression constructs containing either selected domains of interest or full-length sequence of PfIMC1g, an IMC alveolin, and PfIMC1e, a BC alveolin (Figure 1; Supporting Information 2: Table S1) [8]. These constructs were all tagged with either single V5 or smV5 tags [25] and expressed from the Pfbleb locus, a schizont-stage BC protein that is dispensable for asexual development [26]. The in trans constructs replace the coding region of PfBLEB and are expressed from its endogenous promoter, largely matching the level and timing of expression for the alveolins. To mitigate potential toxicity from protein overexpression, we inserted these constructs in an inversion-based Cre-inducible system. Briefly, the ORF of our in trans constructs was inverted relative to the Pfbleb promoter and flanked by two pairs of heterospecific lox sites (loxP511 and loxN) oriented towards each other (see Supporting Information 1: Figure S1A for diagram). These parasite lines also contained a dimerizable Cre (DiCre) recombinase which becomes active upon the addition of rapamycin (RAPA). In theory, this would enable inducible expression of our constructs by irreversibly flipping the ORF in frame of the Pfbleb promoter. However, we found that leakiness in the system led to flipping even without RAPA, resulting in comparable expression levels between uninduced and induced conditions (Supporting Information 1: Figure S1B). Fortunately, these constructs showed no toxicity despite the trans-expression, allowing us to evaluate our constructs in trans. We harvested late (46–48 h postinfection, hpi) schizonts expressing these constructs and used immunofluorescence assays (IFAs) against V5 to visualize the localization of our tagged constructs and against PfGAP45 to visualize the IMC of parasites [27].

2.1. The Alveolin Domain Is Not Always Necessary and Sufficient for Recruitment to the IMC
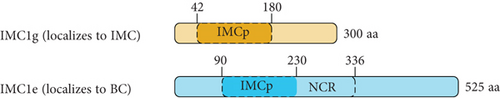
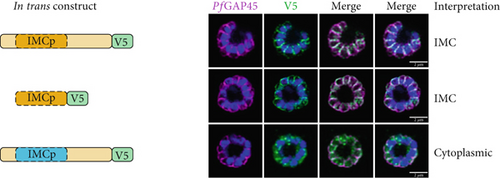
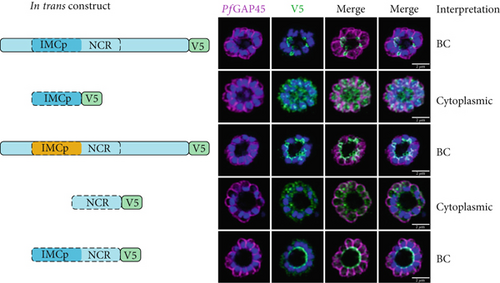
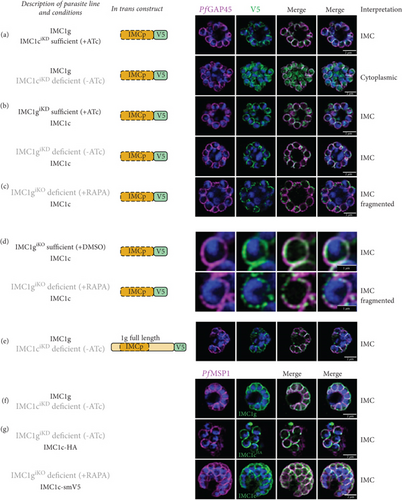
We first tested whether the alveolin domain of PfIMC1g was sufficient for its recruitment to the IMC. The alveolin domain was identified using InterPro and consisted of amino acids 42–180 of the protein (Figure 1a) [28]. Constructs expressing the alveolin domain of PfIMC1g on its own were properly localized, akin to the full-length protein (Figure 1b). We conducted a similar analysis with PfIMC1e. The PfIMC1e alveolin domain, again identified using InterPro, consisted of amino acids 90–230 (Figure 1a). While full-length PfIMC1e localized to the BC, the alveolin domain on its own showed cytoplasmic staining (Figure 1c). To ensure that the mislocalized constructs are stably expressed, we performed western blots (Supporting Information 1: Figure S1A–D). Untagged 3D7-DiCre parasites showed no staining when probed with anti-V5 antibody, as expected (Supporting Information 1: Figure S1C). The IMC1g alveolin domain (Supporting Information 1: Figure S1D) and the IMC1e alveolin domain (Supporting Information 1: Figure S1E) both exhibited robust expression at the expected molecular weight despite only the former localizing properly by IFA. Thus, unlike the alveolin domain of PfIMC1g, the alveolin domain of PfIMC1e was not sufficient for recruitment to the BC (or the IMC).
Next, we tested the necessity of these alveolin domains for recruitment to the IMC and BC. Given the size of the alveolin domain, simple deletions may cause protein misfolding or instability, so we instead created hybrid constructs that replaced the alveolin domain with a different sequence of similar length and amino acid composition.
For PfIMC1g, we replaced amino acids 42–180 with the PfIMC1e alveolin domain, which is similar in length and amino acid composition to the PfIMC1g domain but does not have IMC-targeting properties. This construct was unable to localize to the IMC despite preserving the PfIMC1g N- and C-termini (Figure 1b). Western blots revealed that this hybrid protein is expressed at the expected size, though the intensity is weak, suggesting low abundance (Supporting Information 1: Figure S1F). While we cannot formally exclude the possibility that this construct is unstable, the alveolin domain of PfIMC1g appears to be both necessary and sufficient for IMC recruitment.
For PfIMC1e, we replaced amino acids 90–230 with the PfIMC1g alveolin domain, which does have IMC-targeting properties. Surprisingly, this construct primarily localized to the BC with some minor staining at the IMC (Figure 1c). Thus, the primary targeting sequence that recruits PfIMC1e to the BC must lie outside of its alveolin domain.
It is not obvious what marks the difference between the alveolin domains of PfIMC1g and PfIMC1e. Both domains are the same length (~140 aa) and consist of 13 valine- and proline-rich repeats with the characteristic consensus heptad of the alveolins (EKIVEVP) (Figure 1d). However, the position of this heptad within the 12 amino acid repeats is not consistent between PfIMC1g and PfIMC1e. The heptad begins at Position 3 in PfIMC1g and Position 1 in PfIMC1e (Supporting Information 1: Figure S2). It is unclear to what extent this positioning has any biological relevance.
2.2. PfIMC1e Contains an Additional Repeat Domain With BC-Targeting Properties
We further analyzed the PfIMC1e sequence, which revealed an additional stretch of valine- and proline-rich repeats not identified as an alveolin domain by InterPro [29, 30]. This region spans amino acids 230–336 and contains an additional nine repeats but does not maintain the canonical alveolin consensus sequence (Figure 1d; Supporting Information 1: Figure S2). We have termed this region the non-canonical repeat (NCR) region (Figure 1a).
We tested whether the PfIMC1e NCR region could localize to the BC on its own or when coupled with the canonical alveolin domain identified by InterPro. On its own, the NCR region was not able to localize to the BC, despite robust expression by western blot (Figure 1c; Supporting Information 1: Figure S1G). However, when the NCR was coupled with the alveolin domain, the resulting construct was localized to the BC (Figure 1c). So, while neither the alveolin domain nor the NCR region of PfIMC1e is, on their own, sufficient for BC localization, together they form a minimal domain for localization.
2.3. PfIMC1e and PfIMC1f Are Dispensable and Sequentially Recruited to the BC
We previously showed that PfIMC1f was dispensable in the asexual blood stages by direct knockout [8]. Since we failed to directly knock out PfIMC1e, here, we employed an inducible knockout approach to assess its essentiality. This line used loxP sites and DiCre to excise the PfIMC1e coding sequence upon RAPA addition [8, 31], generating the PfIMC1eiKO line.
Parasite replication, as measured by flow cytometry, was unaffected in PfIMC1e-deficient (+RAPA) conditions when compared to its wild-type (+DMSO) control (Figure 2a). As expected, wild-type (+DMSO) parasites showed PfIMC1e staining at the BC, while PfIMC1e-deficient (+RAPA) parasites showed no staining of PfIMC1e at the BC (Figure 2b). These parasites also lacked any obvious morphological abnormalities.

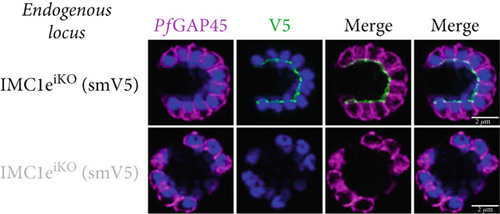
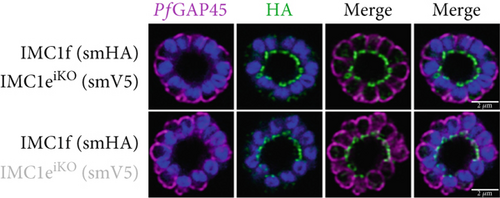
Since PfIMC1e and PfIMC1f share a BC localization and are individually dispensable, we assessed whether there was redundancy between the two proteins. We generated a line where PfIMC1f is directly knocked out and where PfIMC1e can be conditionally knocked out. Once more, we saw no difference in parasite replication when both proteins were absent (Figure 2a).
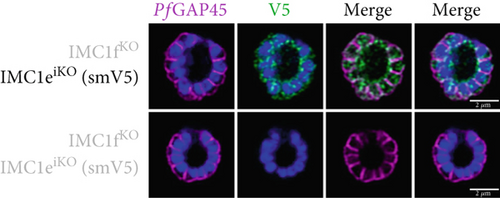
To assess whether PfIMC1e and PfIMC1f showed dependence on each other for recruitment, we performed IFAs on our knockout parasites to visualize the localization of one protein in the absence of the other. When PfIMC1eiKO is knocked out, the localization of endogenous PfIMC1f, visualized via an epitope tag on the endogenous protein, remains at the BC (Figure 2c). Moreover, western blots show that PfIMC1f levels remain comparable when PfIMC1eiKO is knocked out as well (Supporting Information 1: Figure S1H). However, when PfIMC1f is knocked out, PfIMC1e is absent from the BC and instead shows cytoplasmic staining (Figure 2d). Western blots show that mislocalized PfIMC1e remains stably expressed (Supporting Information 1: Figure S1I). This suggests that these alveolins are recruited to the BC sequentially, with PfIMC1f recruiting PfIMC1e. Furthermore, it means that the PfIMC1f knockout, on its own, produces a double KO-like effect by preventing the recruitment of PfIMC1e, reinforcing our conclusion that these two proteins do not show functional redundancy.
2.4. The Alveolin Domain Recruits PfIMC1g to the IMC by Facilitating Interactions With PfIMC1c
We then tested whether alveolin–alveolin interactions are also important for recruitment to the IMC. We expressed our PfIMC1g alveolin domain construct in lines where the endogenous PfIMC1g or its putative partner PfIMC1c could be depleted using the TetR-DOZI inducible knockdown (iKD) system [32, 33].
With endogenous PfIMC1ciKD present (+anhydrotetracycline (ATc)), the PfIMC1g alveolin domain was localized properly, as expected. However, when endogenous PfIMC1ciKD is knocked down (−ATc), the PfIMC1g alveolin domain loses its IMC recruitment and instead becomes cytoplasmic (Figure 3a). Western blots indicate that the construct remains stably expressed despite its mislocalization (Supporting Information 1: Figure S1D). This is a confirmation that PfIMC1g and 1c are indeed interacting partners and that their interaction requires the alveolin domain. On the other hand, when endogenous PfIMC1g is either present (+ATc) or knocked down (−ATc), the PfIMC1g alveolin domain remains at the IMC (Figure 3b). To account for the possibility that residual amounts of PfIMC1g was responsible for IMC recruitment, we repeated this experiment in an PfIMC1g inducible knockout line (IMC1giKO), utilizing the insertion of loxP sites flanking the endogenous Pfimc1g locus [8, 31]. Upon the knockout of endogenous PfIMC1giKO, the PfIMC1g alveolin domain was again localized to the IMC (Figure 3c). This suggests that the PfIMC1g alveolin domain is recruited to the IMC through a pathway that does not require self-interactions with full-length PfIMC1g but does require interactions with PfIMC1c. Interestingly, when PfIMC1g was knocked down or knocked out, staining of the PfIMC1g alveolin domain at the IMC became fragmented (Figure 3d). Thus, while the alveolin domain of PfIMC1g absolutely requires PfIMC1c for localization, additional interactions with full-length PfIMC1g may stabilize the localization.
2.5. PfIMC1c Depletion Does Not Affect Recruitment of Full-Length PfIMC1g
A sequential recruitment pathway in which PfIMC1c is recruited to the IMC first and then recruits PfIMC1g would explain the dependence of PfIMC1g’s alveolin domain on PfIMC1c for recruitment. To test this possibility, we looked at whether PfIMC1c knockdown had any effect on the localization of full-length PfIMC1g.
To our surprise, depleting PfIMC1ciKD did not have any effect on the recruitment of in trans (Figure 3e) or endogenous full-length PfIMC1g (Figure 3f). This suggests that the N- and C- termini of PfIMC1g contain domains that, when combined with its alveolin domain, are sufficient for IMC localization. Since these experiments were conducted in a knockdown background, we cannot exclude the possibility that residual PfIMC1c is sufficient to recruit full-length PfIMC1g. However, neither depletion of PfIMC1giKD nor the knockout of PfIMC1giKO disrupts the recruitment of endogenous PfIMC1c (Figure 3g). So, an alveolin-independent recruitment pathway must exist, at least to recruit PfIMC1c, if not to recruit both alveolins.
2.6. Predicted Structure of Alveolins Provides Insights Into Multimerization and Role of the Alveolin Domain
To further explore the hypothesis that PfIMC1c and 1g are interacting partners, we compared their predicted structures as monomers with their predicted structures as multimers using AlphaFold and AlphaFold Multimer [34, 35]. On their own, PfIMC1g and 1c do not display much ordered secondary structure (Supporting Information 1: Figure S3A and B). However, when they are modeled as multimers with each other, they form a twisted β-ribbon structure (Supporting Information 1: Figure S3C). We also tested our theory that PfIMC1e needs both its alveolin domain (or an equivalent domain) and its NCR region to adopt a conformation that is stable and can interact with its BC partners. When modeled individually, the NCR region and alveolin domain of PfIMC1e are disordered (Supporting Information 1: Figure S3D and E)—much like PfIMC1g and 1c. However, when modeled together, they form a β-ribbon like the one formed by PfIMC1g and 1c (Supporting Information 1: Figure S3F). So, alveolin domains are likely disordered or misfolded as monomers and require interactions with additional monomers or domains to be able to adopt a stable conformation that allows them to bind their partner proteins.
If PfIMC1g and 1c are indeed unstructured as monomers, then a sequential recruitment model seems less likely. PfIMC1e, on the other hand, achieves the β-ribbon conformation using intramolecular interactions and can also form a β-ribbon when modeled with the PfIMC1f alveolin domain, which is in line with a model of sequential recruitment (Figure 4 and Supporting Information 1: Figure S3G). Our AlphaFold models suggest that the β-ribbon requires a specific partner and cannot form with just any other alveolin domain. While models of PfIMC1g dimerizing with 1c have high per-residue model confidence scores (pLDDT), our modeling does not support the ability of PfIMC1g to form dimers with itself or PfIMC1e. All such multimer predictions are largely unstructured and have low (pLDDT < 50) confidence (Supporting Information 1: Figure S3H and I). Models of the NCR region, on the other hand, suggest that it can attain an intramolecular β-ribbon conformation with both the PfIMC1e (70 < pLDDT < 95) and 1g (50 < pLDDT < 80) alveolin domains, though interactions with the PfIMC1g domain, as evaluated by model confidence, are less favorable (Supporting Information 1: Figure S3J). This is consistent with our IFA data which shows that the PfIMC1g alveolin domain can replace that of PfIMC1e without causing a loss in BC localization.
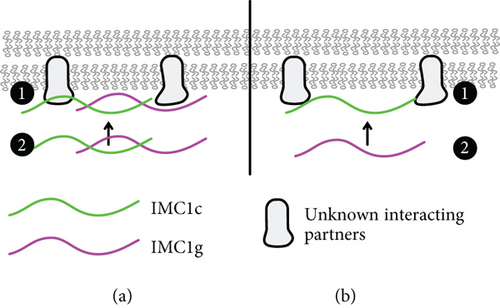
2.7. Alveolin–Alveolin Interactions Are Necessary for Filament Formation and Compartment Specificity
In this work, we assessed the role of the alveolin domain for the function and localization of asexual blood-stage alveolins. This is the first investigation to determine how alveolins localize to specific subcompartments in Plasmodium, yielding multiple unexpected results including direct evidence of alveolin–alveolin dependence.
We show that the alveolin domain of PfIMC1g is sufficient for recruitment to the IMC in a WT background. However, when PfIMC1c is depleted, the alveolin domain of PfIMC1g is no longer sufficient for recruitment. This confirms that PfIMC1g and PfIMC1c are interacting partners and further maps that interaction to the alveolin domain. Surprisingly, the recruitment of the full-length PfIMC1g was not affected by the depletion of PfIMC1c. PfIMC1c recruitment was also unaffected by the absence of PfIMC1g.
Based on this data, we propose two possible models for PfIMC1g recruitment to the IMC (Figure 4). In the first model, PfIMC1g and 1c are recruited through both alveolin-dependent and alveolin-independent pathways (Figure 4a). The alveolin-independent pathway would likely require the N- and C-termini of PfIMC1g and could be facilitated by palmitoylation or a yet to be identified interacting partner. Then, the alveolin–alveolin interactions we identified likely stabilize monomers of PfIMC1g and 1c into filaments at the IMC, meaning that if one is already present, the other can continue to localize to the IMC. The role of palmitoylation is likely since both PfIMC1g and PfIMC1c were palmitoylated in a genome-wide “palmitome” experiment [36]. In addition, PbIMC1g in the rodent malaria was shown to require palmitoylation for its IMC localization during the asexual blood stages [37]. The role of nonalveolin proteins is also likely. From previous work in Toxoplasma [22, 24] and coimmunoprecipitation (co-IP) experiments from our lab [8], we know that alveolin proteins interact with many nonalveolin proteins. To support this idea, we colocalized IMC1g with one of these nonalveolin IMC protein called PIC5, which was the top candidate in the IMC1g co-IP dataset, previously shown to localize to the IMC, and essential for proper cell division (Supporting Information 1: Figure S4) [38, 39]. IMC1g and PIC5 appear to colocalize properly, and, while these data increase our confidence in the IMC1g-PIC5 interaction, we still do not know if PIC5 is the interactor that initiates IMC1g recruitment. Thus, both palmitoylation- and nonalveolin-interacting partners likely contribute to the alveolin-independent recruitment pathway.
An alternative sequential recruitment model where PfIMC1c is recruited first and then recruits PfIMC1g would also fit our observations (Figure 4b). While PfIMC1c depletion did not affect PfIMC1g recruitment to the IMC, it is possible that the knockdown we employed for this depletion, despite being lethal, left enough residual protein to allow for recruitment of full-length PfIMC1g. Thus, this sequential recruitment cannot be ruled out without a PfIMC1c knockout. Furthermore, experiments exploring the dependence of the alveolin domain of PfIMC1c on PfIMC1g could clarify the relationship between these two proteins and how they are recruited to the IMC.
Interestingly, when PfIMC1g was depleted, staining of the PfIMC1g alveolin domain at the IMC became fragmented, but there was no change in PfIMC1c staining. This could indicate that, while not required for recruitment, full-length PfIMC1g is needed for proper architecture of the IMC, which has a downstream effect for the binding sites of the alveolin domain. It is also possible that without full-length PfIMC1g, the alveolin domain is recruited less efficiently. One might imagine that fibrils formed by the alveolin domain may have a suboptimal conformation or fewer binding sites when compared to hybrid fibrils that also contain the full-length protein. A change in the architecture or the distribution of unknown binding partners besides PfIMC1c could lead to a similar effect, affecting recruitment to the IMC or decreasing binding sites. So far, none of our data or modeling has provided evidence that PfIMC1g is forming homodimers. However, further experiments and modeling are necessary to rule out other types of interactions including higher order multimers and fibrils containing partner proteins as well as multiple copies of PfIMC1g.
The alveolin domain of PfIMC1g is both necessary and sufficient for recruitment to the IMC. For PfIMC1e, we identify an additional region of repeats, termed NCR region, that lies outside the alveolin domain that is important for BC recruitment. Indeed, the alveolin domain of PfIMC1e is not necessary nor sufficient for its localization. However, the combination of the alveolin and NCR domain is sufficient. We hypothesize that the alveolin domain of PfIMC1e acts as a stabilizing domain that allows the NCR to adopt the right conformation for BC binding. PfIMC1e and 1f show a very clear sequential recruitment pattern. So it is likely that the NCR region is facilitating interactions between the two proteins. However, additional experiments are needed to determine more robustly how and under what conditions NCRs are required for recruitment.
It also remains unclear from our data what defines, functionally, the alveolin domain of a protein. The NCR region, for example, could very well be considered a part of the alveolin domain of PfIMC1e if, instead of focusing on specific amino acid identities, we focused more on amino acid properties. We note that the alveolin domain of PfIMC1c contains enough repeats which deviate from the canonical consensus heptad of EKIVEVP in both amino acid identity and properties that it could also be considered noncanonical. Defining the characteristics of these proteins is key to our understanding of how they function, specialize, and evolve.
The hypothesis we put forward regarding the stabilizing role of the alveolin domain of PfIMC1e is consistent with our modeling of protein secondary structure using AlphaFold and AlphaFold Multimer. On their own, the alveolin domains of PfIMC1e, 1g, and 1c do not display any ordered secondary structure. However, when the alveolin domain of PfIMC1e is modeled alongside the NCR region, it attains a β-ribbon structure. The same is true when PfIMC1g and 1c are modeled as a heterodimer. This supports our hypothesis that alveolin domains are likely disordered or misfolded on their own and require interactions with additional monomers or domains to be able to bind their partner proteins and localize properly. While our modeling data suggests that these interactions must occur with specific partner proteins, we have not modeled multimers beyond dimers, and we have only tested a few putative protein partners. These models and experiments also have not mapped out the specific amino acids responsible for these interactions or alveolin–alveolin compatibility.
Overall, this study provides a first interrogation into the molecular interactions and organizing principles that govern alveolin function and localization in the Plasmodium falciparum asexual blood stages. Together, our results are in line with previous evidence that the alveolin domain is a key driver of filament formation and recruitment. Specifically, our data provide evidence that filament formation and recruitment are linked. All of the recruitment we observed to be mediated by the alveolin domain was traced back to alveolin–alveolin interactions. This leaves the question of how the alveolins are recruited to their specific compartments when those are devoid of alveolins and whether the alveolin domain mediates that recruitment as well by interacting with other protein partners. While in silico modeling provides some clues as to the conformation these proteins might adopt, much remains to be learned about alveolin structure.
3. Materials and Methods
3.1. Accession Numbers
The accession numbers for the genes analyzed in this study are as follows: PfIMC1g (PF3D7_0525800), PfIMC1c (PF3D7_1003600), PfIMC1f (PF3D7_1351700), and PfIMC1e (PF3D7_0304100).
3.2. Plasmid Construction [40]
All primer and gene block sequences are listed in Supporting Information 2: Table S1.
3.3. pAK60, 61, and 64 (Guide RNA Plasmids Targeting Pfbleb Locus)
The guides were annealed and ligated into BpiI-digested pRR216 (SpCas9 expression plasmid) to construct pAK60 (oJDD7004/7005), pAK61 (oJDD7006/7007), and pAK64 (oJDD5485/5486).
3.4. pAK86 (PfIMC1g Full Length in Trans Construct)
The Pfbleb 5 ′ homology region (HR) and 3 ′ HR region were amplified from Pf3D7 genomic DNA with oJDD6995/6996 and oJDD6997/6998, respectively. Codon-altered PfIMC1g flanked by inverse loxP511 and loxN sites and fused to a 1xV5 tag was amplified with oJDD7893/7895 and oJDD7893/7894. pGEM backbone was amplified from pCJM17 with oJDD6994/7003. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.5. pAK90 (PfIMC1g Alveolin Domain Only in Trans Construct)
The Pfbleb 5 ′ and 3 ′ HR regions were amplified from pAK87 with oJDD7393/8008. Codon-altered PfIMC1g alveolin domain, flanked by inverse loxP511 and loxN sites and fused to an smV5 tag, was amplified with oJDD8006/8007. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.6. pAK88 (PfIMC1g With PfIMC1e Alveolin Domain in Trans Construct)
The backbone containing Pfbleb 5 ′ and 3 ′ HR regions and the IMC1g N and C term were amplified from pAK86 with oJDD7924/7925. Codon-altered PfIMC1e alveolin domain flanked by inverse loxP511 and loxN sites and fused to a V5 tag was amplified with oJDD7926/7927 from pAK87. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.7. pAK87 (PfIMC1e Full Length in Trans Construct)
The backbone containing Pfbleb 5 ′ and 3 ′ HR regions was amplified using oJDD7393/7433. Codon-altered PfIMC1e flanked by inverse loxP511 and loxN sites and fused to an smV5 tag was amplified with oJDD7891/7892 from pPB23. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.8. pAK91 (PfIMC1e Alveolin Domain Only in Trans Construct)
The backbone containing Pfbleb 5 ′ and 3 ′ HR regions was amplified from pAK87 using oJDD7393/8008. Codon-altered PfIMC1e alveolin domain flanked by inverse loxP511 and loxN sites and fused to an smV5 tag was amplified with oJDD8009/8010 from pAK87. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.9. pAK89 (PfIMC1e With PfIMC1g Alveolin Domain in Trans Construct)
The backbone containing Pfbleb 5 ′ and 3 ′ HR regions as well as the N and C termini of IMC1e was amplified from pAK87 using oJDD7928/7929. Codon-altered PfIMC1g alveolin domain flanked by inverse loxP511 and loxN sites and fused to an smV5 tag was amplified with oJDD7930/7931 from pAK86. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.10. pAK102 (PfIMC1e NCR Domain Only in Trans Construct)
The backbone containing Pfbleb 5 ′ and 3 ′ HR regions was amplified from pAK91 using oJDD7393/8008. Codon-altered PfIMC1e NCR domain flanked by inverse loxP511 and loxN sites and fused to an smV5 tag was amplified with oJDD8065/8066 from pAK87. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.11. pAK103 (PfIMC1e Alveolin + NCR Domain in Trans Construct)
The backbone containing Pfbleb 5 ′ and 3 ′ HR regions was amplified from pAK91 using oJDD8068/8008. Codon-altered PfIMC1e sequencing flanked by inverse loxP511 and loxN sites and fused to an smV5 tag was amplified with oJDD8065/8067 from pAK87. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.12. pPB23 (PfIMC1eiKO With smV5 Tag and hDHFR Selectable Marker)
The PfIMC1e 5 ′ and 3 ′ HR regions were amplified from Pf3D7 genomic DNA with oJDD7684/7685 and oJDD7690/7691, respectively. Codon-altered PfIMC1e was amplified from synthetic gene block GB76 (IDT) with oJDD7686/7687. The pGEM backbone, smV5 tag, and hDHFR selectable markers were amplified from pAK79 with oJDD7682/7683 and 7688/7689, respectively. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.13. pAK101 (PfIMC1eiKO With smV5 Tag and BSD Selectable Marker)
The backbone containing PfIMC1e 5 ′ and 3 ′ HR regions and codon-altered IMC1e flanked by loxP sites and fused to an smV5 tag were amplified from pPB23 using oJDD7690/8047. BSD selectable marker was amplified with oJDD8051/8050. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.14. pPB24–26 (Guide RNA Plasmids Targeting PfIMC1e)
The guides were annealed and ligated into BpiI-digested pFN41 (SpCas9 expression plasmid with no selectable markers) to construct pPB24 (oJDD7692/7693), pPB25 (oJDD7694/7695), and pPB26 (oJDD6336/6337).
3.15. pPB77 (PfIMC1f With smHA Tag)
PfIMC1f 5 ′ and 3 ′ HR regions were amplified from pAK57 using oJDD8204/8205 and oJDD8208/8209, respectively. pGEM backbone and smHA cassette were amplified from pPB45 with oJDD8202/8203 and oJDD8206/8207, respectively. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.16. pRR255 (PfPIC5 With smV5 Tag)
PfPIC5 5 ′, 3 ′ HR, and codon-altered regions were amplified from 3D7 gDNA (or synthesized gene block, GB107) using oJDD5321/5322, oJDD5319/5320, and oJDD5323/5324, respectively. The three pieces were combined using overlapping PCR with oJDD5321/5324 and cloned into an smV5-containing plasmid (pRR190). The guide RNA was cloned into pRR216 with annealed oJDD5333/5334.
3.17. pAK104–106 (Guide RNA Plasmids Targeting the 3 ′ End of PfIMC1f)
The guides were annealed and ligated into BpiI-digested pRR216 (SpCas9 expression plasmid) to construct pAK104 (oJDD6464/6465), pAK105 (oJDD6466/6467), and pAK106 (oJDD6468/6469).
3.18. pAK35 (PfIMC1giKD With HA Tag)
pAK34 [8] was digested using PspOMI and NcoI and ligated to annealed oJDD6208/6209.
3.19. pAK73 (PfIMC1c With 2HA Tag)
pAK67 was digested using XmaI and NcoI and ligated to annealed oJDD7266/7267.
3.20. pAK109 (PfIMC1ciKD With smV5 Tag)
The backbone containing PfIMC1c 5 ′ and 3 ′ HR regions was amplified from pAK67 using oJDD8309/8310. The smV5 tag and 10xTet-TetRDOZI machinery were amplified with oJDD8311/8312 from pPG03. Fragments were assembled with the Golden Gate BsaI-HF v2 Assembly Kit (NEB).
3.21. The Following Plasmids Were Previously Described [8]
pBAM377 was used to knock out PfIMC1f, using guide RNA plasmids pBAM465 and pBAM553. pAK67 was used to tag PfIMC1c 2HA and the iKD machinery, using guide RNA plasmids pAK37, 38, and 39. pAK17 was used to tag PfIMC1g with 2HA and flank loxPint around its coding sequence, using guide RNA plasmids pRR189 and pAK30.
3.22. Protein Alignment and Analysis
Protein sequences were obtained from PlasmoDB, and their domains were analyzed using InterPro [28]. Alignments were carried out using ClustalW (MegaX), and diagrams were generated using ESPript 3. Repeats were identified using HHrepID using maximal number of MSA steps (3) and repeat family p value threshold e−2 [29, 30]. Protein structures were generated using AlphaFold.
3.23. Plasmodium Falciparum Culture
Parasite cultures were maintained as previously detailed [8]. Briefly, 3D7(pfs47) DiCre strains were cultured in RPMI 1640 supplemented with 25 mM HEPES, 0.21% sodium bicarbonate, 50 mg/L hypoxanthine, 0.5% AlbuMAX, and 2 mM choline chloride. Parasites were maintained in human O+ erythrocytes at 4% hematocrit under shaking conditions at 37°C.
3.23.1. Parasite Synchronization
Parasites were synchronized using Percoll density centrifugation and/or 5% (w/v) sorbitol selection as previously described [8].
3.23.2. Parasite Transfection
Homology-directed repair plasmid DNA (20 μg) was linearized and mixed with Cas9-targeting plasmids (20 μg each) and resuspended in 82 μL buffer P3 (Lonza) with 18 μL supplement solution (Lonza). Then, 10–30 μL of packed schizonts were mixed and electroporated with the Lonza Nucleofector 4D. Electroporated schizonts were mixed with 200 μL of packed RBCS in 1 mL of complete media and incubated at 37°C for 45 min before being transferred to a culture dish with complete media and 200 μL of packed RBCs. Parasites were cultured under drug pressure starting at 24 h posttransfection. The integration of each targeting construct was confirmed by a PCR. Individual transgenic clones were obtained by a limiting dilution.
3.23.3. Washout of ATc for Protein Knockdown
The depletion was achieved by washing a tightly synchronized ring culture three times in ATc-free RPMI. The washed culture was then divided into two dishes with (or without) 500 nM ATc.
3.23.4. Depletion by Inducible Knockout
A tightly synchronized ring culture was divided into two dishes with 100 nM RAPA or equivalent volume of DMSO control.
3.24. Reagents and Antibodies
3.24.1. Small Molecules
Small molecules used in this study include WR99210 (Jacobus Pharmaceuticals; 2.5 nM working concentration), ATc (Cayman chemical, 0.5 μM working concentration), and RAPA (Sigma, 100 nM working concentration).
3.24.2. Primary Antibodies
Commercially available antibodies include Sigma (rat anti-HA [clone 3F10]), Bio-Rad (mouse anti-V5), and Immunology Consultant Laboratories (rabbit anti-V5, RV5-45A-Z). Other antibodies were kindly provided by Julian Rayner at Cambridge Institute for Medical Research (rabbit anti-PfGAP45) [41] and by Anthony Holder at MRC National Institute for Medical Research (mouse anti-PfMSP1, clone 1E1) [42]. Rabbit anti-PfIMC1g has been described previously [8].
3.24.3. Secondary Antibodies
All secondary antibodies for immunofluorescence and western blots were obtained from Thermo Fisher and LI-COR, respectively.
3.25. Genomic DNA Extraction
Parasite genomic DNA was isolated from 5 to 10 mL of schizont-stage culture using the EZ-10 Spin Column Blood Genomic DNA Miniprep Kit (Bio Basic).
3.26. Growth Assays
Flow cytometry–based growth assays were performed as previously described [8]. Briefly, each strain was diluted to 0.25% parasitemia at 1% hematocrit; 100 μL of culture was collected 2 and 4 days after plating, stained with SYBR green, and measured by flow cytometry. One hundred thousand RBCs were counted for each condition, and the parasite multiplication rate was calculated as %RBCs infected at Day 4 divided by %RBCs infected at Day 2. Data represented as mean values of three independent biological replicates each with three technical replicates.
3.27. IFAs
IFAs were performed as previously described [8]. Briefly, parasites were sedimented on poly-d-lysine-coated coverslips, fixed in prewarmed 4% PFA, and permeabilized with 0.1% Triton X-100. Coverslips were then blocked for 1 h at room temperature or overnight at 4°C, incubated in primary antibodies for 1 h at room temperature or overnight at 4°C, incubated in secondary antibodies for 45 min at room temperature, and mounted on a slide with VECTASHIELD Vibrance with DAPI.
Cells were visualized on a Zeiss LSM880 with Airyscan or Zeiss LSM900 with Airyscan 2 (Plan Apo 63×/1.4 Oil DIC III) for superresolution microscopy. Dilutions for primary antibodies were as follows: mouse anti-V5 between 1:200 and 1:1000, rabbit anti-PfGAP45 1:5000, mouse anti-PfMSP1 1:500, rat anti-HA 3F10 between 1:100 and 1:500, and rabbit anti-IMC1g 1:2500.
3.28. Western Blots
Protocol was followed as previously described [43]. Parasites were isolated by lysing RBCs in 0.2% saponin in PBS with protease inhibitors (Pierce). Pelleted parasites were washed with PBS until the supernatant was clear; samples were then boiled in Laemmli buffer for 10 min. The equivalent of 1 mL of culture at 1% parasitemia was run on a 4%–20% mini-PROTEAN TGX gels (Bio-Rad) and transferred to nitrocellulose membranes. Membranes were blocked in Licor Odyssey blocking buffer, incubated with primary antibody, and then incubated in secondary antibodies diluted in blocking buffer. Membranes were scanned on a Licor Odyssey CLx imager system. The primary antibody dilutions are as follows: mouse anti-V5 (1:1000), rabbit anti-H3 (1:2500), and rat anti-HA (1:1000). The secondary antibody dilutions were all used at 1:10000.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ana Karla Cepeda Diaz: conceptualization, formal analysis, investigation, validation, visualization, writing – original draft, writing – review and editing. Peter S. Back: Formal analysis, investigation, validation, visualization, writing – original draft, writing – review and editing. Sreelakshmi K. Sreenivasamurthy: investigation and visualization. Jeffrey D. Dvorin: conceptualization, funding acquisition, supervision, writing – original draft, writing – review and editing. Ana Karla Cepeda Diaz and Peter Sungmin Back contributed equally to this study and are co-first authors.
Funding
This work was supported by grants from the National Institutes of Health/National Institute of Allergy and Infectious Diseases: R01 AI145941 (J.D.D.), F31 AI172110 (A.K.C.D.), and F32 AI186297 (P.S.B.).
Acknowledgments
We thank Julian Rayner and Anthony Holder for providing the primary antibodies and Ellen Knuepfer for providing the parasites.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
All data for this manuscript are presented in the article and the associated supporting files. Plasmids and parasite strains are available upon request.