Resistance Profiling of Predominant Non–E. coli Enterobacteriaceae Isolated From Humans, Food Animals, and the Environment in the Fako Division of Cameroon
Abstract
The impact of the current global rising resistance of Enterobacteriaceae to antibiotic agents is of great concern. Detecting and monitoring resistance in these pathogens in humans, animals, and the environment and taking appropriate actions based on results obtained are indispensable to reverse this trend. This study is aimed at contributing to the fight against resistance of predominant non–Escherichia coli Enterobacteriaceae in the Fako Division of Cameroon through a one-health approach. Freshly collected human feces, rectal swabs from pigs, cloacal swabs from chicken, cow intestinal content, and environmental samples were cultured. Isolates were identified using API 20E. Predominant non–E. coli isolates (Enterobacter spp., 65.0%; Salmonella spp., 11%; and Citrobacter spp., 9.9%) were confirmed by polymerase chain reaction (PCR). Antibiotic susceptibility profiles of these isolates were determined by the Kirby-Bauer disc diffusion technique, while the resistant genes were detected by PCR. The quinolones (norfloxacin, 94.7%, and ofloxacin, 91.2%), carbapenem (imipenem, 96%), aminoglycoside (amikacin, 95.5%), and chloramphenicol (91.3%) were the most active drugs. Penicillins (amoxicillin, 24.7%; ampicillin, 21.2%; and amoxicillin–clavulanic acid, 19.9%) were the most inactive drugs. However, isolates showed the highest rate of intermediate susceptibility (48.6%) to cefepime. Out of the 226 isolates, 214 (94.7%) showed resistance to at least one antibiotic agent. Multidrug resistance was found in 54 (25.2%) of the isolates. The predominant antibiotypes were AXR AMR AMCR (25, 11.1%), AXR AMR AMCR AZMR (18, 8.4%), CAZR AXR AMR AMCR (12, 5.3%), and AXR AMCR AZMR (7, 3.1%). Isolates with these antibiotypes were from various sources and predominant genera. Plasmid-mediated quinolone resistance (PMQR) genes (acrA, acrB, qepA, and aac(6 ′ )-ib-cr) were detected in 97.8% (44/45) of isolates that showed resistance to at least one quinolone antibiotic, while the beta-lactamase genes, blaCMY-2 and blaCTX-M-1, were detected in 7.9% (5/63) and 22.0% (14/63), respectively, in isolates that showed resistance to cephalosporins. These isolates carrying these genes were from humans, food animals, and the environment. Of the 45 isolates, a total of 40 (88.9%) carried two or more PMQR genes, while 2 (0.6%) carried both bla genes (cocarriage). Five (17.9%) isolates out of the 28 screened for PMQR and beta-lactamase genes were positive for both sets of genes. Resistance to antibiotics was high with strains of the different genera carrying PMQR and beta-lactamase resistance genes circulating in humans, food animals, and the environment in the Fako Division of Cameroon.
1. Introduction
The family Enterobacteriaceae are gram-negative, nonspore-forming bacilli. They are part of the normal flora of the intestines of humans and animals and can also be found in the environment (soil, water, plants, and decaying matter) [1]. These bacteria are zoonotic [2] and constitute an important group of human and animal pathogens. They possess a high capacity for developing drug resistance owing to a matrix of plasmid-borne resistance genes they often carry and undergo mutations in the presence of a stressor such as antibiotics [3]. Most antibiotics used as growth enhancers and therapeutic or prophylactic agents in the livestock sector are equally used in human medicine [4], increasing their chances of being misused.
Misuse of antibiotics in the animal industry leads to indirect consumption by humans through their residues in meat products (especially in developing countries where meat consumption is still very high) and contaminated groundwater due to poor waste disposal from animal farms [4, 5]. These residues may produce several consequences, including developing antibiotic-resistant bacteria [6]. The high frequency of multidrug resistance in this family of bacteria especially to the high priority critically important antibiotics coupled with the insufficient discovery of new effective drugs makes these organisms a serious threat to public health [7]. In 2017, blood stream infections caused by carbapenem-resistant Enterobacteriaceae (CRE) accounted for a 25.7% mortality rate in China [8], and the World Health Organisation rates Enterobacteriaceae as the fifth cause of mortality after malaria, HIV-AIDS, neonatal diseases, and lower respiratory diseases in Cameroon [9]. Therefore, understanding the antibiotic resistance patterns of these organisms is important for the clinical management of infections they cause.
In Cameroon, studies have investigated Enterobacteriaceae from food animals [10–12], clinical samples [13–15], and environmental samples [16–18]. Bissong et al. [19] compared the susceptibility of Escherichia coli from clinical and zoonotic sources and reported higher resistance rates in clinical than in zoonotic samples. A systematic review and meta-analysis by Mouiche et al. [11] presented data on the resistance profile of Enterobacteriaceae and non-Enterobacteriaceae from some studies carried out in different regions of Cameroon. None of these studies investigated these organisms following the one-health concept. There is more data on E. coli than on other members of the Enterobacteriaceae in our study area, which is the reason why our study focused on non–E. coli Enterobacteriaceae. The scarcity of research data on antibiotic resistance profiles for species of non–E. coli Enterobacteriaceae makes empirical treatment challenging as most clinical settings in our study area lack laboratory facilities for antimicrobial susceptibility testing. Establishing the occurrence and antibiotic resistance profiles of these potential pathogens in humans, animals, and the environment is essential for establishing effective treatment, prevention, and control measures. In the face of this dilemma, our study is aimed at producing data on the susceptibility pattern and antibiotic resistance genes of non–E. coli Enterobacteriaceae in isolates from humans, food animals, and the environment. Such information will help measure the degree of resistance stemming from these pathogens and identify effective antibiotics for clinical and veterinary care.
2. Materials and Methods
2.1. Study Design
This was a cross-sectional study in which the participating health facilities, abattoirs, and animal farms were selected by convenience sampling. Sample collection spanned from March to September 2020. Non–E. coli Enterobacteriaceae were isolated, and antibiotic resistance profiles as well as resistance genes of predominant isolates were investigated.
2.2. Study Site
This study was conducted in Buea, Limbe, Mutengene, and Tiko, which are major towns of the Fako Division, Cameroon. Administratively, Fako is one of the six divisions of the Southwest Region of Cameroon. The headquarters are in Limbe. Fako Division also harbours Buea, the regional headquarters of the Southwest Region. Figure 1 shows the location of Fako Division and the sites where samples were collected. With regard to its health structure, Fako Division has four health districts, namely, Limbe, Tiko, Buea, and Muyuka.
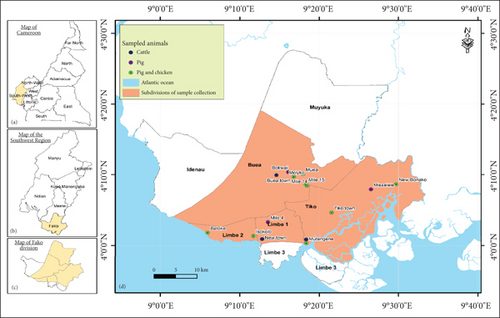
2.3. Sample Collection
Freshly produced stool samples were collected from humans at the Limbe Regional Hospital, Mutengene Sub-Divisional Medical Centre, Tiko CDC Central Clinic, and the Buea Regional Hospital. A sterile swab was inserted 2–3 cm into the rectum of pigs, cloacae of chicken, and intestine of cattle during slaughter, rotated three to five times clockwise and withdrawn. Surfaces in the hospital environment (sink, bedside cupboard, ward table, doorknobs, bed rail, and floor) were swabbed. Water samples were collected at different points in streams flowing in Buea, Mutengene, Tiko, and Limbe using a sterile syringe. Samples were also collected from knives, cutlasses, soil, and the floor at slaughterhouses and from drinkers, feeders, drinking water, soil, and the floor of pig and poultry farms by swabbing their surfaces. The sample collection respected aseptic techniques.
One–two grams of human stool, swabs carrying samples, and 2 mL of water sample were placed in 10 mL of selenite F broth and in nutrient broth and transported to the laboratory following standard procedures [20].
2.4. Bacteriological Analysis
Samples were incubated for 18–24 h at 37°C, after which a loop full of each broth culture was aseptically streaked on cysteine lactose electrolyte deficient (CLED) agar. Plates were incubated for 18–24 h at 37°C for isolation of Enterobacteriaceae. All large, elevated, yellow, opaque colonies with a centre more intense yellow on yellowish medium were considered E. coli [21] and eliminated from the study. Every other colony was streaked on nutrient agar plates to obtain pure cultures which were subjected to oxidase testing, catalase testing, and gram staining. The gram-negative, oxidase-negative, and catalase-positive bacilli were then identified using analytical profile index (API) 20E (bioMérieux, Marcy-l′Étoile, France) following the manufacturers’ instructions. Predominant isolates were confirmed with PCR, using Bio-Rad T100 TM Thermal Cycler.
2.4.1. Confirmation of the Identity of Isolates
To confirm the identity of isolates obtained with API 20E, PCR was done for 30 randomly selected Enterobacter isolates and for 10 each of Salmonella and Citrobacter isolates. Bacterial DNA was extracted using the SDS chloroform protocol described by Sambrook and Russell [22]. Amplification was achieved by conventional PCR in a 20 μL final reaction mixture. Each reaction tube contained 10 μL master mix, 8 μL nuclease-free water, 0.5 μL of each primer (forward and reverse), and 1 μL DNA template. Amplification conditions are presented in Table 1. Amplicons were loaded on agarose gel alongside a 1-kb Plus Ladder (Thermo Fisher Scientific), and electrophoresis was performed at 120 V and 400 mA for 45 min. Detection was done with the Gel Doc XR+ imaging system (Bio-Rad).
| Organism | Target gene | Primer sequence | PCR cycling parameters | Amplicon size (bp) | Reference | |
|---|---|---|---|---|---|---|
| Genus-specific amplification | ||||||
| 1 | Salmonella serotypes | 16S rDNA |
|
|
324 | Lin et al., [23] |
| 2 | Citrobacter spp. | 16S rRNA |
|
|
529 | Anbazhagan et al., [24] |
| 3 | Enterobacter spp. | dnaJ |
|
|
714 bp | Hernandez-Alonso, [25] |
2.5. Antibiotic Susceptibility Testing (AST)
Predominant Enterobacteriaceae isolates were subjected to AST by the Kirby-Bauer disc diffusion technique as described by Bauer et al. and CLSI [26, 27]. Two to three colonies of pure isolates were emulsified in sterile normal saline in a bijou bottle and adjusted to a density of 0.5 McFarland standard which is approximately equal to 1.5 × 108 bacteria/mL. A uniform lawn of standard suspension of bacterial isolates was made on Mueller–Hinton agar. The following antibiotic discs (Bioanalyse, Ve Tic. Ltd., Sti, Yenimahalle Turkey) were tested: amoxicillin–clavulanic acid (AMC), 30 μg; amoxicillin (AX), 30 μg; ampicillin (AM), 30 μg; imipenem (IPM), 10 μg; cefixime (CFM), 5 μg; ceftazidime (CAZ), 30 μg; cefotaxime (CTX), 30 μg; ceftriaxone (CRO), 5 μg; cefepime (FEP), 10 μg; aztreonam (ATM), 30 μg; chloramphenicol C, 30 μg; azithromycin (AZM), 15 μg; tetracyclines (TEs), 30 μg; cotrimoxazole (SXT), 25 μg; norfloxacin (NOR), 10 μg; nalidixic acid (NA), 30 μg; ciprofloxacin (CIP), 5 μg; ofloxacin (OFX), 5 μg; and amikacin (AK), 30 μg. The antibiotics used were selected based on local practice and literature [28, 29]. Discs were placed at least 25 mm apart and 10 mm from the edge of the plate, to avoid overlapping of the zones of inhibition.
Plates were incubated for 18–24 h at 37°C, and the diameters of the zones of inhibition were measured and recorded. Isolates were either classified as sensitive, intermediate, or resistant to each of the antibiotics based on the interpretive category for zone diameter breakpoints achieved using the CLSI M100-Ed 31 [27] and the British Society for Antimicrobial Chemotherapy [28] (for AX).
2.6. Screening for Resistance Genes
Isolates resistant to quinolones and cephalosporins were screened for plasmid-mediated quinolone resistance (PMQR) and the beta-lactamase resistance (bla) genes, by conventional PCR using specific primers (Table 2). The PMQR genes screened were the qepA, acrA, acrB and aac(6 ′ )-ib-cr, while the beta-lactamase genes screened were the blaCMY-2 and blaCTX-M-1. Bacterial plasmid was extracted with the miniprep protocol as described by Birnboim and Doly [29], and the amplification was done in a 20 μL final reaction volume using the amplification conditions shown on Table 2. Amplicons were detected as described under bacteriological analysis.
| Target gene | Primer sequence | PCR cycling parameters | Expected band size (bp) | Reference | |
|---|---|---|---|---|---|
| Beta-lactamase genes | |||||
| 1 | blaCTX-M-1 (ESBL) |
|
|
766 | [30] |
| 2 | blaCMY-2 (AmpC) |
|
|
870 | [30] |
| Quinolone genes | |||||
| 3 | qepA |
|
|
403 | [31] |
| 4 | acrA |
|
|
157 | [31] |
| 5 | acrB |
|
|
64 | [31] |
| Aminoglycoside and fluoroquinolone gene | |||||
| 6 | aac(6 ′ )-ib-cr |
|
|
611 | [31] |
2.7. Statistical Analysis
Data was expressed as percentages. The analysis of variance (ANOVA) was used to compare differences in the distribution of isolates among the various sources. The level of significance was set at p ≤ 0.05.
3. Results
3.1. Summary of Samples Collected
A total of 2042 samples were collected as follows: 976 (47.8%) from humans, 743 (36.4%) from animals, and 323 (15.8%) from the environment (Table 3). The distribution of environmental samples is presented in Table 4.
| Locality | Source | |||||
|---|---|---|---|---|---|---|
Human n(%) |
Chicken n (%) |
Pig n (%) |
Cow n (%) |
Environment n (%) |
Total n (%) |
|
| Buea | 186 (9.1) | 112 (5.5) | 42 (2.1) | 86 (4.2) | 135 (6.6) | 571 (28.0) |
| Mutengene | 282 (13.8) | 60 (2.9) | 12 (0.6) | 33 (1.6) | 36 (1.8) | 423 (20.7) |
| Tiko | 338 (16.6) | 186 (9.1) | 35 (1.7) | 0 (00.0) | 62 (3.0) | 621 (30.4) |
| Limbe | 170 (8.3) | 81 (4.0) | 57 (2.8) | 29 (1.4) | 90 (4.4) | 427 (20.9) |
| Total | 976 (47.8) | 449 (22) | 146 (7.1) | 148 (7.2) | 323 (15.8) | 2042 (100.0) |
- Note: n = number of isolates.
| Locality | Source | |||||
|---|---|---|---|---|---|---|
Hospital n (%) |
Stream n (%) |
Slaughterhouse n (%) |
Pig farm n (%) |
Poultry n (%) |
Total n (%) |
|
| Limbe | 19 (5.9) | 13 (4.0) | 15 (4.6) | 17 (5.3) | 18 (5.6) | 82 (25.4) |
| Buea | 19 (5.9) | 23 (7.1) | 19 (5.9) | 15 (4.6) | 19 (5.9) | 95 (29.4) |
| Mutengene | 8 (2.5) | 14 (4.3) | 16 (5.0) | 19 (5.9) | 15 (4.6) | 72 (22.3) |
| Tiko | 12 (3.7) | 20 (6.2) | 0 (0.0) | 21 (6.5) | 21 (6.5) | 74 (22.9) |
| Total | 58 (18.0) | 70 (21.7) | 50 (15.5) | 72 (22.3) | 73 (22.6) | 323 (100.0) |
- Note: p = 0.93; n = number of isolates.
3.2. Prevalence of Non–E. coli Enterobacteriaceae
Out of the 2042 samples collected, a total of 260 non–E. coli isolates belonging to 16 genera (Table 5) were identified using API 20E. Species identification was achieved for 156 (60.0%) of the isolates. Most (97, 37.3%) of the isolates were from humans, while the least were from pigs (24, 9.2%). There was no significant difference in the distribution of the isolates among the different sources (p = 0.7).
| Genus | Source | Total n (%) |
||||
|---|---|---|---|---|---|---|
Human n (%) |
Chicken n (%) |
Pig n (%) |
Cow n (%) |
Environment n (%) |
||
| Cedecea spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.4) |
| Chryseomonas spp. | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Citrobacter spp. | 13 (5.0) | 3 (1.2) | 7 (2.7) | 0 (0.0) | 2 (0.8) | 26 (10.0) |
| Enterobacter spp. | 68 (26.1) | 31 (11.9) | 11 (4.2) | 24 (9.2) | 37 (14.2) | 171 (65.8) |
| Escherichia spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.2) | 3 (1.1) |
| Hafnia spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 2 (0.8) |
| Klebsiella spp. | 2 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.2) | 6 (2.3) |
| Morganella spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.4) |
| Pantoea spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (2.3) | 6 (2.3) |
| Proteus spp. | 0 (0.0) | 3 (1.2) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 6 (2.3) |
| Providencia spp. | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Rahnella spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 2 (0.8) |
| Raoultella spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.4) |
| Salmonella spp. | 12 (4.6) | 6 (2.3) | 6 (2.3) | 3 (1.2) | 2 (0.8) | 29 (11.2) |
| Serratia spp. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.2) | 13 (5.0) |
| Tatumella spp. | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Total | 97 (37.3) | 46 (17.7) | 24 (9.2) | 27 (10.4) | 66 (25.4) | 260 (100) |
- Note: Genus names in bold were the predominant ones. These were the only genera involved in downstream experiments. p = 0.7; n = number of isolates.
The predominant genera were Enterobacter (171, 65.8%), Salmonella (29, 11.2%), and Citrobacter (26, 10%), these three accounting for 11.1% (226) of the isolates, while the predominant species of these genera were Enterobacter cloacae (64, 41.0%), Citrobacter freundii (23, 14.7%), Enterobacter sakazakii (14, 9.0%), and Salmonella Typhimurium (9, 5.8%).
3.3. AST
Susceptibility testing of the predominant isolates (226), that is, Enterobacter spp. (171), Salmonella spp. (29), and Citrobacter spp. (26) to 19 antibiotic agents revealed that two quinolones (NOR, 94.7%, and OFX, 91.2%), a beta-lactamase (IPM, 96%), an aminoglycoside (AK, 95.5%), and chloramphenicol (91.3%) were the most active drugs, while isolates were most resistant to AX (24.7%), AM (21.2%), and AMC (19.9%). FEP however showed a very high rate (48.6%) of intermediate susceptibility.
Enterobacter species showed highest resistance to CAZ (38%), AX (76.2%), AM (70.2%), AMC (70.2%), SXT (29.2%), ATM (24%), NA (24.1%), and AZM (25.7%) (Table 6). Citrobacter species showed highest resistance to AX (69.2%) and AMC (65.4%). Other drugs to which isolates had increasing resistance were FEP (21.1%), CFM (23.1%), CTX (28%), ATM (23.1%), and NA (23.1%). Salmonella were most resistant to AX (69%), AM (65.5%), and AMC (75.9%). Other drugs to which Salmonella showed high rates of resistance were AZM (41.4%), CAZ (34.5%), CFM (31%), ATM (31%), CTX (24.1%), and NA (24.1%). No Salmonella isolate showed resistance or intermediate susceptibility to OFX (Table 6).
| Antibiotic | Enterobacter spp. | Citrobacter spp. | Salmonella spp. | Cum S | Cum I | Cum R | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n S (%) | n I (%) | n R (%) | Total | n S (%) | n I (%) | n R (%) | Total | n S (%) | n I (%) | n R (%) | Total | n (%) | n (%) | n (%) | |
| Cefepime (FEP) | 43 (41.0) | 45 (42.9) | 17 (16.2) | 105 | 2 (10.5) | 13 (68.4) | 4 (21.1) | 19 | 5 (35.7) | 9 (64.3) | 0 (0.0) | 14 | 50 (36.2) | 67 (48.6) | 21 (15.2) |
| Cefixime (CFM) | 100 (58.5) | 23 (13.5) | 48 (28.1) | 171 | 16 (61.5) | 4 (15.4) | 6 (23.1) | 26 | 16 (55.2) | 4 (13.8) | 9 (31.0) | 29 | 132 (58.5) | 31 (13.7) | 63 (27.9) |
| Ceftazidime (CAZ) | 92 (53.8) | 14 (8.2) | 65 (38.0) | 171 | 16 (64.0) | 2 (8.0) | 7 (28.0) | 25 | 13 (44.8) | 6 (20.7) | 10 (34.5) | 29 | 121 (53.8) | 22 (9.8) | 82 (36.4) |
| Cefotaxime (CTX) | 122 (71.8) | 16 (9.4) | 32 (18.8) | 170 | 19 (73.1) | 3 (11.5) | 4 (15.4) | 26 | 18 (62.1) | 4 (13.8) | 7 (24.1) | 29 | 159 (70.7) | 23 (10.2) | 43 (19.1) |
| Ceftriaxone (CRO) | 123 (71.9) | 18 (10.5) | 30 (17.5) | 171 | 19 (76.0) | 1 (4.0) | 5 (20.0) | 25 | 21 (72.4) | 2 (6.9) | 6 (20.7) | 29 | 163 (72.4) | 21 (9.3) | 41 (18.2) |
| Amoxicillin (AX) | 39 (23.2) | 1 (0.6) | 128 (76.2) | 168 | 7 (26.9) | 1 (3.9) | 18 (69.2) | 26 | 9 (31.0) | 0 (0.0) | 20 (69.0) | 29 | 55 (24.7) | 2 (0.9) | 166 (74.4) |
| Ampicillin (AM) | 34 (19.9) | 17 (9.9) | 120 (70.2) | 171 | 7 (26.9) | 0 (0.0) | 19 (73.1) | 26 | 7 (24.1) | 3 (10.3) | 19 (65.5) | 29 | 48 (21.2) | 20 (8.9) | 158 (69.9) |
| Amoxiclav (AMC) | 33 (19.3) | 18 (10.5) | 120 (70.2) | 171 | 7 (26.9) | 2 (7.7) | 17 (65.4) | 26 | 5 (17.2) | 2 (6.9) | 22 (75.9) | 29 | 45 (19.9) | 22 (9.7) | 159 (70.4) |
| Imipenem (IPM) | 163 (95.3) | 7 (4.1) | 1 (0.6) | 171 | 25 (96.2) | 0 (0.0) | 1 (3.9) | 26 | 29 (100.0) | 0 (0.0) | 0 (0.0) | 29 | 217 (96.0) | 7 (3.1) | 2 (0.9) |
| Aztreonam (ATM) | 126 (73.7) | 4 (2.3) | 41 (24.0) | 171 | 19 (73.1) | 1 (3.9) | 6 (23.1) | 26 | 20 (69.0) | 0 (0.0) | 9 (31.0) | 29 | 165 (73.0) | 5 (2.2) | 56 (24.8) |
| Nalidixic acid (NA) | 114 (67.1) | 15 (8.8) | 41 (24.1) | 170 | 17 (65.4) | 3 (11.5) | 6 (23.1) | 26 | 21 (72.4) | 1 (3.5) | 7 (24.1) | 29 | 152 (67.6) | 19 (8.4) | 54 (24.0) |
| Ofloxacin (OFX) | 154 (90.1) | 2 (1.2) | 15 (8.8) | 171 | 25 (96.2) | 1 (3.9) | 0 (0.0) | 26 | 27 (93.1) | 1 (3.5) | 1 (3.5) | 29 | 206 (91.2) | 4 (1.8) | 16 (7.1) |
| Norfloxacin (NOR) | 160 (93.6) | 3 (1.8) | 8 (4.7) | 171 | 25 (96.2) | 0 (0.0) | 1 (3.9) | 26 | 29 (100.0) | 0 (0.0) | 0 (0.0) | 29 | 214 (94.7) | 3 (1.3) | 9 (4.0) |
| Ciprofloxacin (CIP) | 135 (79.9) | 19 (11.2) | 15 (8.9) | 169 | 20 (76.9) | 4 (15.4) | 2 (7.7) | 26 | 25 (86.2) | 3 (10.3) | 1 (3.5) | 29 | 180 (80.4) | 26 (11.6) | 18 (8.0) |
| Cotrimoxazole (SXT) | 117 (68.4) | 4 (2.3) | 50 (29.2) | 171 | 21 (80.8) | 0 (0.0) | 5 (19.2) | 26 | 24 (82.8) | 0 (0.0) | 5 (17.2) | 29 | 162 (71.7) | 4 (1.8) | 60 (26.6) |
| Chloramphenicol C | 142 (91.0) | 8 (5.1) | 6 (3.9) | 156 | 23 (95.8) | 1 (0.0) | 1 (4.2) | 25 | 24 (88.9) | 1 (3.7) | 2 (7.4) | 27 | 189 (91.3) | 9 (4.4) | 9 (4.4) |
| Azithromycin (AZM) | 127 (74.3) | 0 (0.0) | 44 (25.7) | 171 | 21 (80.8) | 0 (0.0) | 5 (19.2) | 26 | 17 (58.6) | 0 (0.0) | 12 (41.4) | 29 | 165 (73.0) | 0 (0.0) | 61 (27.0) |
| Amikacin (AK) | 163 (95.9) | 0 (0.0) | 7 (4.1) | 170 | 24 (96.0) | 0 (0.0) | 1 (4.0) | 25 | 27 (93.1) | 1 (3.5) | 1 (3.5) | 29 | 214 (95.5) | 1 (0.45) | 9 (4.0) |
| Tetracycline (TE) | 69 (79.3) | 4 (4.6) | 14 (16.1) | 87 | 13 (76.5) | 2 (11.8) | 2 (11.8) | 17 | 11 (91.7) | 0 (0.0) | 1 (8.3) | 12 | 93 (80.2) | 6 (5.2) | 17 (14.7) |
- Note: n = number of isolates.
- Abbreviations: S = sensitive, I = intermediate, R = resistant.
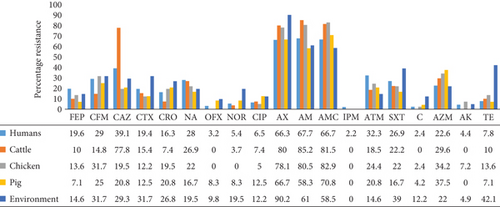
Generally, isolates from all sources had very high resistance rates to AM, AX, and AMC. Resistance to cephalosporins, ATM, SXT, and AZM was also high. Resistance to IPM (2.2%) was observed only among clinical isolates. The highest resistance rates to NOR (19.5%), CIP (12.5%), AX (90.2%), SXT (39%), chloramphenicol (12.2%), and TE (42.1%) were observed among environmental isolates (Figure 2).
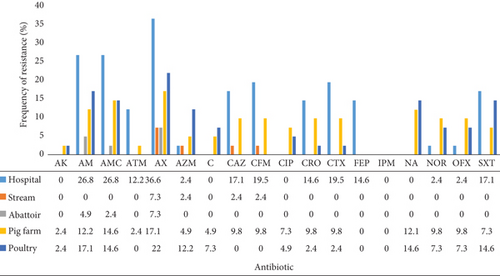
With regard to environmental isolates, resistance rates were highest among isolates from the hospital environment where the highest rates were observed for the penicillins and cephalosporins (Figure 3). This was followed by isolates from pig and poultry farms. Resistance was lowest among isolates from abattoir environment.
3.4. Antibiotypes
Out of the 226 isolates, 214 (94.7%) showed resistance to one or more antibiotic agents. Resistant strains were grouped into 131 antibiotypes (Supporting Information 1). The predominant antibiotypes were AXR AMR AMCR (25, 11.1%), AXR AMR AMCR AZMR (18, 8.4%), CAZR AXR AMR AMCR (12, 5.3%), and AXR AMCR AZMR (7, 3.1%). However, most (107, 47.3%) of the antibiotypes were observed only in single isolates. Antibiotype AXR AMR AMCR was detected in Enterobacter from chicken, pig, and environment; Citrobacter from human and pig; and Salmonella from chicken. AXR AMR AMCR AZMR was detected mostly in Enterobacter from chicken, human, and environment; Citrobacter from pig, and Salmonella from human and pig.
Multiresistance (resistance to ≥ 3 antibiotics of different classes) was seen in 54 (25.2%) isolates from various sources and genera (Supporting Information 1). Thirty-four (63.0%), 13 (24.1%), and 7(13.0%) isolates were resistant to 3, 4, and 5 antibiotic classes, respectively. Resistance to five antibiotic classes was observed only in Enterobacter spp. and Citrobacter spp. isolated from humans, chicken, and the environment (Tables 7 and 8).
| No. of antibiotic classes | Genera | |||
|---|---|---|---|---|
Enterobacter spp. n (%) |
Citrobacter spp. n (%) |
Salmonella spp. n (%) |
Total n (%) |
|
| 3 | 25 (46.3) | 2 (3.7) | 7 (13.0) | 34 (63.0) |
| 4 | 11 (20.4) | 1 (1.9) | 1 (1.9) | 13 (24.1) |
| 5 | 6 (11.1) | 1 (1.9) | 0 (0.0) | 7 (13.0) |
| Total | 42 (77.8) | 4 (7.4) | 8 (14.8) | 54 |
- Note: p = 0.08; n = number of isolates.
| No. of antibiotic classes | Source | |||||
|---|---|---|---|---|---|---|
Human n (%) |
Chicken n (%) |
Pig n (%) |
Cattle n (%) |
Env. n (%) |
Total n (%) |
|
| 3 | 15 (27.8) | 5 (9.3) | 2 (3.7) | 6 (11.1) | 6 (11.1) | 34 (63.0) |
| 4 | 4 (7.4) | 3 (5.6) | 1 (1.9) | 2 (3.7) | 3 (5.6) | 13 (21.1) |
| 5 | 2 (3.7) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 4 (7.4) | 7 (13.0) |
| Total | 21 (38.9) | 9 (16.7) | 3 (5.6) | 8 (14.8) | 13 (24.1) | 54 |
- Note: p = 0.39; Env. = environment; n = number of isolates.
3.5. Detection of Resistance Genes
3.5.1. PMQR Genes
PMQR genes were detected in 97.8% (44/45). The most prevalent gene was acrB (42, 93.3%) followed by the acrA (38, 84.4%) and aac(6 ′)-ib-cr (30, 66.7%), while the least was the qepA gene (2, 4.4%) which was detected only in humans (Table 9). The acrB gene was detected in isolates from all five sources, with highest detection rate in human 95.5% (21/22). The acrA gene was most prevalent 88.6% (31/35) among Enterobacter isolates, followed by Citrobacter isolates 75% (3/4) and least in Salmonella isolates 66.7% (4/6). It was detected in all five sources with the highest occurrence in chicken 100% (4/4%) and environment 100% (5/5). The aac(6 ′ )-ib-cr was detected in 75% (3/4) of Citrobacter isolates, 68.6% (24/35) of Enterobacter isolates, and 50% (3/6) of Salmonella isolates. It was detected in all five sources with the highest detection rate 81.8% (9/11) in cattle.
| Source | Genus | PMQR genes, N = 45 | bla genes, N = 63 | ||||||
|---|---|---|---|---|---|---|---|---|---|
aac(6 ′)-ib-cr n (%) |
qepA n (%) |
acrA n (%) |
acrB n (%) |
Total | blaCTX-M-1 n (%) |
blaCMY-2 n (%) |
Total | ||
| Human | Enterobacter | 10 (22.2) | 2 (4.4) | 13 (28.9) | 14 (31.1) | 39 | 4 (6.3) | 2 (3.2) | 6 |
| Salmonella | 3 (6.7) | 0 (00.0) | 3 (6.7) | 4 (8.9) | 10 | 2 (3.2) | 1 (1.6) | 3 | |
| Citrobacter | 3 (6.7) | 0 (00.0) | 2 (4.4) | 3 (6.7) | 8 | 0 (00.0) | 0 (00.0) | 0 | |
| Chicken | Enterobacter | 1 (2.2) | 0 (00.0) | 3 (6.7) | 2 (4.4) | 6 | 0 (00.0) | 0 (00.0) | 0 |
| Salmonella | NT | NT | NT | NT | / | NT | NT | / | |
| Citrobacter | 0 (0.0) | 0 (00.0) | 1 (2.2) | 1 (2.2) | 2 | 0 (00.0) | 0 (00.0) | 0 | |
| Pig | Enterobacter | 2 (4.4) | 0 (00.0) | 1 (2.20) | 1 (2.2) | 4 | 0 (00.0) | 0 (00.0) | 0 |
| Salmonella | 0 (00.0) | 0 (00.0) | 0 (00.0) | 1 (2.2) | 1 | 0 (00.0) | 0 (00.0) | 0 | |
| Citrobacter | NT | NT | NT | NT | / | NT | NT | / | |
| Cattle | Enterobacter | 9 (20) | 0 (00.0) | 9 (20) | 10 (22.2) | 28 | 6 (9.5) | 2 (3.2) | 8 |
| Salmonella | 0 (0.0) | 0 (00.0) | 1 (2.2) | 1 (2.2) | 2 | 0 (00.0) | 0 (00.0) | 0 | |
| Citrobacter | NT | NT | NT | NT | / | NT | NT | / | |
| Environment | Enterobacter | 2 (4.4) | 0 (00.0) | 5 (11.1) | 5 (11.1) | 12 | 2 (3.2) | 0 (00.0) | 2 |
| Salmonella | 0 (00.0) | 0 (00.0) | 0 (00.0) | 0 (00.0) | 0 | 0 (00.0) | 0 (00.0) | 0 | |
| Citrobacter | NT | NT | NT | NT | / | NT | NT | / | |
| Total | 30 (66.7) | 2 (4.4) | 38 (84.4) | 42 (93.3) | 14 (22.2) | 5 (7.9) | |||
- Note: p = 0.03.
- Abbreviation: NT = not tested.
3.5.2. The Beta-Lactamase Resistance Genes
The blaCTX-M-1 gene was more frequently detected (14, 22.2%) than the blaCMY-2 gene (5, 7.9%). These genes were detected in isolates from human, cattle, and environmental origin. The blaCTX-M-1 gene was detected in Enterobacter and Salmonella isolates. It was most prevalent in Enterobacter spp. 12/14 (85.7%) when compared to Salmonella spp. 2/10 (20%). A similar prevalence was seen in humans 6/14 (42.9%) and cattle 6/14 (42.9%), which was higher when compared to environmental isolates 2/14 (14.3%). The blaCMY-2 gene was detected in Enterobacter 4/5 (80.0%) and Salmonella spp. 1/5 (20.0%). Among the sources involved, the blaCMY-2 gene was detected only in isolates from humans 3/5 (60.0%) and cattle 2/5 (40.0%), and not the environment.
3.6. Cocarriage of Resistance Genes
A total of 80 isolates were involved in the screening of the PMQR and the beta-lactamase genes. Forty-five and 63 of these isolates were tested for PMQR and beta-lactamase genes, respectively, while 28 were tested for both sets. Forty-one (51.3%) isolates carried two or more resistance genes as illustrated in Table 10. Among these, 14 isolates 14/41 (34.1%) carried 2 genes, and 22 (53.7%) carried 3 genes, while 6 (14.6%) carried 4 genes. A total of five (17.9%) isolates of the 28 screened for both the PMQR and the beta-lactamase genes carried both sets of genes.
| SN | Source | Genus | qepA | acrA | acrB | aac(6 ′)-ib-cr | blaCMY-2 | blaCTX-M-1 | No. of genes positive |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Human | Enterobacter | − | + | + | + | NT | NT | 3 |
| 2 | Human | Salmonella | − | + | + | + | − | − | 3 |
| 3 | Human | Enterobacter | − | + | + | + | − | + | 4 |
| 4 | Human | Citrobacter | − | + | + | + | − | − | 3 |
| 5 | Human | Citrobacter | − | + | + | + | NT | NT | 3 |
| 6 | Human | Enterobacter | − | + | + | + | − | − | 3 |
| 7 | Human | Enterobacter | − | + | + | + | − | − | 3 |
| 8 | Human | Enterobacter | − | − | − | − | − | − | 0 |
| 9 | Chicken | Enterobacter | − | + | − | + | NT | NT | 2 |
| 10 | Human | Enterobacter | − | + | + | − | NT | NT | 2 |
| 11 | Human | Enterobacter | − | + | + | + | − | − | 3 |
| 12 | Chicken | Enterobacter | − | + | + | − | NT | NT | 2 |
| 13 | Human | Enterobacter | + | + | + | + | − | − | 4 |
| 14 | Human | Salmonella | − | + | + | + | − | − | 3 |
| 15 | Human | Salmonella | − | + | + | + | + | − | 4 |
| 16 | Chicken | Citrobacter | − | + | + | − | NT | NT | 2 |
| 17 | Human | Citrobacter | − | − | + | + | − | − | 3 |
| 18 | Human | Salmonella | − | − | + | − | − | − | 1 |
| 19 | Human | Enterobacter | + | + | + | + | − | − | 4 |
| 20 | Human | Enterobacter | − | + | + | + | − | − | 3 |
| 21 | Pig | Salmonella | − | − | + | − | NT | NT | 1 |
| 22 | Human | Enterobacter | − | − | + | − | − | − | 1 |
| 23 | Cattle | Enterobacter | − | + | + | + | − | − | 3 |
| 24 | Cattle | Enterobacter | − | + | + | + | − | − | 3 |
| 25 | Cattle | Salmonella | − | + | + | − | − | − | 2 |
| 26 | Cattle | Enterobacter | − | + | + | + | NT | NT | 3 |
| 27 | Cattle | Enterobacter | − | + | + | + | − | − | 3 |
| 28 | Pig | Enterobacter | − | + | + | + | NT | NT | 3 |
| 29 | Cattle | Enterobacter | − | + | + | + | − | + | 4 |
| 30 | Cattle | Enterobacter | − | + | + | + | − | + | 4 |
| 31 | Cattle | Enterobacter | − | + | + | + | − | − | 3 |
| 32 | Cattle | Enterobacter | − | − | + | + | − | − | 2 |
| 33 | Cattle | Enterobacter | − | + | + | − | − | − | 2 |
| 34 | Cattle | Enterobacter | − | + | + | + | NT | NT | 3 |
| 35 | Human | Enterobacter | − | + | + | + | − | − | 3 |
| 36 | Human | Enterobacter | − | + | + | − | NT | NT | 2 |
| 37 | Human | Enterobacter | − | + | + | + | − | − | 3 |
| 38 | Human | Enterobacter | − | + | + | − | − | − | 2 |
| 39 | Environment | Enterobacter | − | + | + | − | NT | NT | 2 |
| 40 | Chicken | Enterobacter | − | + | + | − | NT | NT | 2 |
| 41 | Environment | Enterobacter | − | + | + | + | NT | NT | 3 |
| 42 | Pig | Enterobacter | − | − | − | + | NT | NT | 1 |
| 43 | Environment | Enterobacter | − | + | + | − | NT | NT | 2 |
| 44 | Environment | Enterobacter | − | + | + | − | − | + | 3 |
| 45 | Environment | Enterobacter | − | + | + | + | NT | NT | 3 |
| 46 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 47 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 48 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 49 | Human | Enterobacter | NT | NT | NT | NT | − | + | 1 |
| 50 | Human | Salmonella | NT | NT | NT | NT | − | − | 0 |
| 51 | Human | Citrobacter | NT | NT | NT | NT | − | − | 0 |
| 52 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 53 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 54 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 55 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 56 | Human | Enterobacter | NT | NT | NT | NT | − | + | 1 |
| 57 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 58 | Human | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 59 | Human | Salmonella | NT | NT | NT | NT | − | + | 1 |
| 60 | Cattle | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 61 | Cattle | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 62 | Cattle | Enterobacter | NT | NT | NT | NT | − | + | 1 |
| 63 | Cattle | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 64 | Cattle | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 65 | Cattle | Enterobacter | NT | NT | NT | NT | + | + | 2 |
| 66 | Cattle | Enterobacter | NT | NT | NT | NT | − | + | 1 |
| 67 | Cattle | Salmonella | NT | NT | NT | NT | − | − | 0 |
| 68 | Cattle | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 69 | Cattle | Enterobacter | NT | NT | NT | NT | + | + | 2 |
| 70 | Human | Enterobacter | NT | NT | NT | NT | − | + | 1 |
| 71 | Human | Salmonella | NT | NT | NT | NT | − | + | 1 |
| 72 | Human | Enterobacter | NT | NT | NT | NT | + | − | 1 |
| 73 | Human | Enterobacter | NT | NT | NT | NT | + | − | 1 |
| 74 | Environment | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 75 | Environment | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 76 | Environment | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 77 | Environment | Enterobacter | NT | NT | NT | NT | − | + | 1 |
| 78 | Environment | Salmonella | NT | NT | NT | NT | − | − | 0 |
| 79 | Environment | Enterobacter | NT | NT | NT | NT | − | − | 0 |
| 80 | Environment | Enterobacter | NT | NT | NT | NT | − | − | 0 |
- Abbreviation: NT = not tested.
Of the 14 isolates that carried two genes, most (12, 85.7%) were Enterobacter spp., and one isolate each (7.1%) was Salmonella and Citrobacter spp. With regard to source, the majority were from cattle (5, 35.7%) and chicken (3, 14.4%). For the 22 isolates carrying 3 genes, 17 (77.3%) were Enterobacter spp., 3 (13.6%) Citrobacter spp., and 2 (9.1%) Salmonella spp. The majority (12, 54.5%) were from human and cattle (7, 31.8%). None was observed among chicken isolates. Of the six isolates that carried four genes, most (5, 83.3%) were Enterobacter spp., while one (16.7%) was Salmonella spp. Most (4, 66.7%) of these isolates were collected from human.
Among the 45 isolates screened for the PMQR genes, 40 (88.9%) carried two or more genes as shown in Table 10. Two isolates (4.4%) were positive for all PMQR genes. These were Enterobacter species isolated from human. Twenty-five (55.6%) carried 3 genes each, while 14 (31.1%) carried 2 genes each. Whereas of the 63 isolates screened for the beta-lactamase genes, only 2 (0.6%) carried both genes. These isolates were of the genus Enterobacter, isolated from cattle sampled in Mutengene and Limbe. Among the isolates for which both PMQR and beta-lactamase genes were investigated, those positive for any of the beta-lactamase genes were also positive for at least two PMQR genes.
4. Discussion
Members of the Enterobacteriaceae family are zoonotic and cause diseases such as urinary tract infection, abscesses, septicaemia, enteric fever, gastroenteritis, and meningitis in humans and animals [32]. These organisms being part of the bacterial commensals of food animals remain a potential threat to mankind as they constantly spread from these sources to the environment [4]. Even though the infections they cause are usually self-limiting in immunocompetent humans, they cause serious health problems to immunocompromised persons requiring prompt treatment with effective antibiotics. Carbapenem-resistant strains have emerged lately and have devastating consequences on both human and veterinary heath including the healthy population [33].
In this study, non–E. coli Enterobacteriaceae made up 12.7% (260/2042) of all bacteria isolated from the different sources. The species of Enterobacter (171, 65.8%), Salmonella (29, 11.2%), and Citrobacter (26, 10.0%) genera were most frequently isolated. This low general prevalence could be explained by the fact that the gut of mammals is dominated by other bacterial species [34]. The relatively high prevalence of Enterobacter spp. and low prevalence of Citrobacter spp. did not agree with the results obtained by Otote et al. in Nigeria where Citrobacter spp. were more frequently isolated than Enterobacter spp. in clinical specimens [35]. No significant difference was seen in the distribution of these isolates across the various sources (Table 5), implying the use of whole stool, rectal swabbing, and swabbing environmental objects are all reliable techniques for the laboratory isolation of Enterobacteriaceae.
Antibiogram of predominant isolates revealed that the most active drugs were the quinolones (NOR and OFX), a beta-lactamase (IPM), an aminoglycoside (AK), and chloramphenicol. Isolates showed highest resistance to penicillins (AX, AM, and AMC). These results corroborate the findings of Leinyuy et al. who reported a marked susceptibility of Enterobacteriaceae isolated from chicken to IPM, AK, a reduced susceptibility to cephalosporins (CRO and CTX), and a marked resistance to penicillin (AX and AMC) [10]. The high resistance seen with penicillins could partly be due to the natural resistance exhibited by Enterobacter isolates against the latter [36]. TE is the most consumed antibiotic in the animal-rearing sector in Cameroon [37]. The overall susceptibility to this antibiotic of 80.2% could be an indication of good stewardship in both clinical and veterinary practice. The very high intermediate resistance rate to FEP (a fourth-generation cephalosporin) is a great call for concern as this indicates a shift towards resistance. It is challenging to explain this phenomenon given that it is a relatively new drug and does not exist in oral form, thus discouraging abusive use. However, it was observed that environmental isolates showed a leading resistance to more antibiotics when compared to those of the other sources. This was consistent with the findings of Tesfaye et al. [38]. This high resistance rate from environmental isolates could be a result of the high-frequency exchange of mobile genetic elements such as plasmids among bacterial isolates from diverse sources [39]. Although chloramphenicol showed an impressively high activity (91.3%) against the isolates, its use on both humans and food animals is discouraged in some countries, including Cameroon. This is owing to its known severe adverse effects, such as bone marrow toxicity and grey baby syndrome [40].
In this study, we observed a higher carriage of PMQR genes than beta-lactamase genes with the frequency of occurrence of the ESBL gene (blaCTX-M-1) higher than that of the ampC gene (blaCMY-2). Also, the frequency of carriage of acrA and acrB was markedly higher and similar compared to the other PMQR genes screened. This could be because they belong to the same family of efflux systems, the resistance-nodulation-cell division (RND) family that catalyze substrate efflux via a substrate/H+ antiport mechanism and are found in numerous gram-negative bacteria [41–43]. However, we observed that the general higher activity seen with quinolones compared to beta-lactams (cephalosporins) was in contrast with the frequency of detection of each set of the corresponding resistance genes 97.8% (44/45) for PMQR and 30.1% (19/63) for beta-lactamase genes. This suggests that these plasmid-borne quinolone resistance genes screened may not be very potent, while the resistance seen with beta-lactams stems from some other determinants. There was generally high-level cocarriage of resistance genes among isolates, most of them belonging to the genus Enterobacter particularly those of human origin (Table 10). This highlights the need for continuous surveillance of these genes as the efficacies of the quinolone and beta-lactamase antibiotics are at stake. Multiresistance (resistance to ≥ 3 antibiotics of different classes) was seen in 54 (25.2%) isolates. This is a further indication that several antibiotics are losing efficacy against members of the Enterobacteriaceae family. Most of the multiresistant strains were from humans (21, 38.9%) followed by the environment (13, 24.1%) and least from pigs (3, 5.6%). This suggests that the degree of antibiotic abuse is higher among humans when compared to the other sources.
A total of 131 antibiotypes were seen from the isolates with AXR AMR AMCR (25, 11.1%), AXR AMR AMCR AZMR (18, 8.4%), CAZR AXR AMR AMCR (12, 5.3%), and AXR AMCR AZMR (7, 3.1%), being the most common among the predominant Enterobacteriaceae and from the different sources. This could imply that the same strains circulate among humans, food animals, and the environment, most probably by means of cross-contamination. Also, strains of different genera having the same resistance patterns could imply the possibility of the exchange of genetic material such as plasmids [39] among them. Our finding show high levels of multidrug-resistant non–E. coli Enterobacteriaceae and high-level circulation of resistant strains between humans, animals, and the environment and the exchange of resistance markers, highlighting the potential risk of these strains becoming established in the Fako Division. This underscores the need for constant monitoring of the resistance patterns of these organisms in Fako.
5. Limitations
Microbial source tracking was based on antibiotype homology. Nucleic acid–based techniques such as multilocus sequence typing would be more reliable. The study could not extend to Muyuka subdivision due to insecurity linked to the sociopolitical crisis at the time of sample collection. This division is part of the Fako Division, and so the findings of this study may not be very accurate if generalised over the entire division. Positive control organisms were not available for resistance gene detection PCR experiments.
6. Conclusion
This study provides evidence of the buildup of antibiotic resistance among non–E. coli members of the Enterobacteriaceae family from humans, chickens, pigs, cattle, and the environment. The findings further predict the circulation of these bacterial species among the different sources investigated, hence the urgent need for the putting in place of an effective surveillance programme from a one-health perspective, which reports antimicrobial resistance patterns of these organisms circulating for appropriate government action.
Nomenclature
-
- FEP
-
- cefepime
-
- CFM
-
- cefixime
-
- CAZ
-
- ceftazidime
-
- CTX
-
- cefotaxime
-
- CRO
-
- ceftriaxone
-
- NA
-
- nalidixic acid
-
- OFX
-
- ofloxacin
-
- NOR
-
- norfloxacin
-
- CIP
-
- ciprofloxacin
-
- AX
-
- amoxicillin
-
- AM
-
- ampicillin
-
- AMC
-
- amoxicillin–clavulanic acid
-
- IPM
-
- imipenem
-
- ATM
-
- aztreonam
-
- SXT
-
- cotrimoxazole
-
- AZM
-
- chloramphenicol C, azithromycin
-
- AK
-
- amikacin
-
- TE
-
- tetracycline
-
- PMQR genes
-
- plasmid-mediated quinolone resistance
-
- bla
-
- beta-lactamase
-
- CLED
-
- cysteine lactose electrolyte deficient agar
-
- API 20E
-
- analytical profile index for Enterobacteriaceae
-
- PCR
-
- polymerase chain reaction
-
- CLSI
-
- Clinical and Laboratory Standards Institute
-
- BSAC
-
- British Society for Antimicrobial Chemotherapy
Ethics Statement
Administrative authorisation for research was obtained from the South West Regional Delegation of Public Health [34], and from the South West Regional Delegation of Fishery and Animal Husbandry [44]. Ethical clearance was obtained from the University of Buea, Faculty of Health Sciences Institutional Review Board [45], and from the University of Buea Institutional Animal Care and Use Committee (UB-IACUC) [46]. Also, local institutional authorities gave written authorisation before the sample collection from all the institutions involved in this study. A signed consent was obtained from every participant of this study after having explained its goal.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualisation: J.-F.T.K.A., C.E.N., S.W., and P.A.N.; project administration: C.E.N., J.-F.T.K.A., P.A.N., and S.W.; resources: C.E.N., J.-F.T.K.A., S.W., and M.R.; methodology: C.E.N., J.-F.T.K.A., P.A.N., and S.W.; investigation: C.E.N. M.M.N., and M.E.; software: C.E.N.; formal analysis: C.E.N.; visualization: C.E.N.; writing—original draft: C.E.N.; writing—review and editing: D.L.N., P.A.N., S.W., and J.-F.T.K.A.; validation: P.A.N., S.W., and J.-F.T.K.A.; supervision: S.W., J.-F.T.K.A., and P.A.N.; data curation: D.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for this research.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
All data obtained during this study is available and can be shared for relevant purpose.




