Nutrient Profiling of Some Terrestrial Plant Leaves for Their Prospective Use as Nonconventional Ingredients in Carp Diets
Abstract
The present study was performed to investigate the basic chemical composition and nutritional value of 10 terrestrial plant leaves (viz. Mangifera indica, Litchi chinensis, Artocarpus heterophyllus, Ziziphus mauritiana, Albizia lebbeck, Tamarindus indica, Cicer arietinum, Bauhinia acuminata, Delonix regia and Peltophorum pterocarpum) abundant in tropical and subtropical environments. The leaf meals were evaluated comprehensively, encompassing proximate composition (% dry matter), amino acids, fatty acids, antinutritional factors (ANFs), antioxidants and antibacterial potential using established methodologies. The crude protein (CP) content of the leaves ranged from 10.92% to 20.74% (w/w), with the highest content observed in A. lebbeck leaves. The crude lipid (CL) and crude fibre (CF) contents varied between 1.23%–5.38% and 13.73%–24.63%, respectively (w/w). The protein-to-energy ratio (P/E) ranged between 32.32 and 60.70 mg/kcal, with significantly higher (p < 0.05) P/E in A. lebbeck compared to the other leaves. Dominant fatty acids in the evaluated plant leaves included palmitic acid (C16:0), oleic acid (C18:1n9c) and linolenic acid (C18:3n3). Considerable amounts of essential amino acids (EAAs) and nonessential amino acids (NEAAs) were recorded in the plant leaves, with maximum quantities detected in A. lebbeck and A. heterophyllus, respectively. Notably, substantial amounts of mineral elements were also detected in the plant leaves. Z. mauritiana was identified as a rich source of iron (Fe), copper (Cu) and manganese (Mn), while the highest calcium (Ca), phosphorus (P) and magnesium (Mg) levels were recorded in A. heterophyllus. Furthermore, the aqueous extracts of the leaves exhibited antioxidant and antibacterial potentials, with the maximum activities for both observed in T. indica. Analyses of ANFs indicated the presence of trypsin inhibitor (TI) (1.62–3.69 mg/g), tannin (21.25–61.25 mg/g), phytic acid (1.74–5.42 mg/g), cellulose (14.53%–21.37%) and hemicellulose (5.48%–10.56%) in the leaves. The study conclusively proposes the utilization of leguminous plants, particularly A. lebbeck, B. acuminata and P. pterocarpum, as potential nonconventional ingredients after eliminating or deactivating the major ANFs. This information on nutrients and ANFs of the less explored terrestrial plant leaves can be used to optimize low-cost carp diet formulation to ensure the economic profitability of fish farmers in the region.
1. Introduction
The consumption of fish and other aquatic products constitutes ~26% of the animal proteins consumed in the least developed countries, in contrast to 11% in the developed countries [1]. As a result, fish farming plays a pivotal role in the livelihood of farmers and contributes to food security for the growing human population. The feed cost constitutes a significant portion of the production expenses in commercial aquaculture, making it an essential requirement [2]. Commonly used fish feed ingredients are fishmeal (FM), animal by-products, soybean meal, seeds of leguminous plants and diverse oil cakes that fulfil the protein and lipid requirements. Additionally, de-oiled rice bran, wheat bran, corn and maize meal are predominantly utilized as carbohydrate sources. Notably, the global price of conventional fish feed ingredients has experienced a substantial increase during the first two decades of the twenty-first century [2]. Consequently, the rising demand and subsequent high cost of traditional fish feed ingredients have prompted the exploration of novel, cost-effective alternatives to sustain the continued expansion of farmed fish production [3–5]. Plant feedstuffs, such as leaf meal or seed meal, are considered potentially viable alternatives for inclusion in fish feeds [4]. Plant seeds of the family Leguminosae and various oil cakes rich in protein and minerals have been evaluated as components of fish (and livestock) feed in previous studies and have become conventional practice at present [6–9]. In contrast, the nutritional potential of terrestrial plant leaves, grasses, freshwater macrophytes and algae is yet to be adequately studied [10–13].
In nature, herbivorous and omnivorous fish species (e.g. carp) are known to feed on aquatic vegetation. Thus, it is apparent that despite being usually low in protein content, plant leaves can satisfy the nutritional requirements of the carps to a large extent. In this context, research on using browse plants as sources of nutrients for fish and livestock has been rekindled over the past few decades [4, 14, 15]. As recorded in previous studies, leaves of subabul (Leucaena leucocephala) [16], cassava (Manihot esculenta) [17], alfalfa (Medicago sativa) [18], mulberry (Morus alba) [19] and drumstick (Moringa oleifera) [20] were used as components of feed for diverse fish species and achieved limited success. However, the nutritional potential and functional properties of terrestrial plants abundant in tropical and subtropical climatic conditions have not been fully explored due to inadequate scientific knowledge. Some terrestrial plant leaves have been considered alternative sources of protein, vitamins and n-3/n-6 fatty acids [21–24]. Additionally, the leaves of different plants contain numerous minerals that serve as catalysts for different biochemical reactions and are vital components for metabolism, growth and development [25]. In addition to major nutrients (e.g. protein, carbohydrate and lipid), diverse terrestrial leaf meals contain a wide variety of secondary metabolites (e.g. alkaloids, phenolic compounds and terpenoids) with antinutritional, antioxidant, antibacterial and anti-inflammatory/immunomodulatory properties [26–29]. While leaf meals are criticized for the imbalance of essential amino acids (EAA), several contain some of the EAA and essential n-3/n-6 polyunsaturated fatty acids (PUFAs). Therefore, a comprehensive evaluation of major nutrients, minerals, fatty acids and amino acids compositions, along with the antioxidant and antibacterial potential of terrestrial plant leaves, is essential for their selection as prospective feed ingredients for fish or other animals. Furthermore, it is important to consider the presence of endogenous antinutritional factors (ANFs) such as tannin, trypsin inhibitor (TI), phytic acid, cellulose and hemicellulose that act as feeding deterrents and thereby limit the application of plant feed ingredients in the diets [3, 30]. Thus, while appraising the nutritive value of the plant-derived feed ingredients, an account of the ANFs is necessary owing to their adverse effects on growth performance, digestibility, mineral metabolism and health status of the fish.
The nutrient accumulation in plant feedstuffs is primarily influenced by factors such as nutrient supply (e.g. carbon, nitrogen and phosphorus [P]), environmental conditions (e.g. temperature, light and salinity) and the specific species/variety being considered [13]. As a result, significant variations in nutrient compositions exist between and within the plant species grown in different environments. Given the abundant resources of terrestrial plants, it is hypothesized that nutrient profiling and strategic exploitation of the nutrient-rich plant leaves could present a new avenue for cost-effective and sustainable alternatives to traditional feed ingredients [12]. Therefore, the present study aimed to assess the nutritional potential of commonly available and underexplored terrestrial leaf meals, considering their potential utilization as a component of fish feed. In this study, we evaluated 10 commonly available plant leaves for their proximate composition, minerals, fatty acids, amino acids, ANFs, antioxidant capacity and antibacterial potential to determine their suitability for use in carp diets.
2. Materials and Methods
2.1. Collection of Leaves
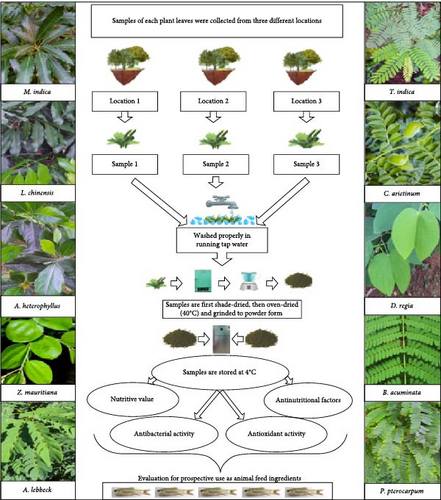
The study involved the manual harvesting of three sets of fresh leaves from each of the 10 locally available terrestrial plants in the districts of Birbhum (23°53′ N; 87°34′ E), East Burdwan (23°13′ N; 87°51′ E) and Hooghly (22°53′ N; 88°23′ E), West Bengal, India. The terrestrial plants are taxonomically identified as mango (Mangifera indica, Himsagar variety), litchi (Litchi chinensis, Bombai variant), jackfruit (Artocarpus heterophyllus), Indian jujube (Ziziphus mauritiana), Indian siris (Albizia lebbeck), tamarind (Tamarindus indica), chickpea (Cicer arietinum, Pusa-372 variety), white bauhinia (Bauhinia acuminata), royal poinciana (Delonix regia) and yellow poinciana (Peltophorum pterocarpum) with the help of the Taxonomy and Biosystematics Section (Department of Botany) of our university. Fresh leaves, amounting to approximately 1 kg of wet biomass, were hand-plucked during the months of November and December from three different locations and promptly transported to the laboratory. Leaves were rigorously washed in running tap water (to remove dust and debris), afterwards with distilled water and blotted dry. Subsequently, samples were dried under shaded conditions (24 h) and oven-dried at 40–60°C until crunchiness came. Each sample was ground to powder form by a laboratory blender and stored in an airtight container at 4°C, awaiting further analysis. The systematic sampling and processing strategy employed for the collection of leaves from the terrestrial plants is visually elucidated in Figure 1.
2.2. Proximate Analysis
The analyses of the dried leaves were conducted in triplicate to mitigate potential erroneous conclusions resulting from individual variances across different locations. Samples collected from three distinct locations were treated as replicates for each plant. The methods outlined by the Association of Official Analytical Chemists [31] were employed to assess the proximate compositions of the dried leaves. To determine the moisture content of the powdered leaves, they were initially dried at 100 ± 5°C (30 min), followed by additional drying at 60°C until a constant weight was achieved. The difference between sun-dried powdered leaves and moisture content represented dry matter. The estimation of crude protein (CP) (N% × 6.25) was carried out through micro-Kjeldahl digestion and distillation (KjelTRON, Tulin Equipments, Chennai, India), while crude lipid (CL) was determined by extracting the residue in 50−60°C petroleum ether using a Socsplus apparatus (Pelican Equipments, Chennai, India). The determination of crude fibre (CF) involved assessing the loss due to ignition of the dried fat-free residue after digestion with H2SO4 (1.25%) and NaOH (1.25%) in a Fibraplus apparatus (Pelican Equipments, Chennai, India). The ash content of the samples was determined by ignition at 550°C (±5°C) in a muffle furnace until a constant weight was reached. Nitrogen-free extract (NFE) was computed by subtracting the sum of values for moisture, ash, CP, CL and CF from 100 [32]. Total carbohydrate (%) was calculated as the sum of CF and NFE values. Total free amino acids (TFAAs) and total free fatty acids (TFFAs) were measured according to Moore and Stein [33] and Cox and Pearson [34], respectively. The gross energy of the plant leaves was determined using an adiabatic bomb calorimeter (Lab-X, Kolkata, India). Furthermore, the protein-to-energy ratio (P/E) was calculated as milligram of protein to kilocalorie per 100 g sample.
2.3. Determination of Antinutritional Components
The ANFs were analysed using various methods. Tannin was extracted by soaking in distilled water overnight and then boiling at 100°C for 30 min. The tannin content was determined spectrophotometrically using the Folin–Denis reagent [35]. Phytic acid was extracted in 2.4% HCl, and the phytate content was determined using a modified Wade reagent (0.03% FeCl3, 6H2O + 0.3% sulfosalicylic acid) following Vaintraub and Lapteva [36]. The sodium phytate (phytic acid sodium salt, HiMedia Laboratories Pvt. Ltd., Mumbai, India) was used as the standard. Benzoyl-dl-arginine-p-nitroanilide (BAPNA) was used as a substrate to determine TI following Smith et al. [37] with minor modifications. Cellulose content was determined spectrophotometrically using an anthrone reagent [38]. Hemicellulose was determined by subtracting the acid detergent fibre from the neutral detergent fibre [39]. Detailed methodologies for estimating the ANFs were described elsewhere [40, 41].
2.4. Mineral Analysis
The mineral components of the plant leaves were extracted after Issac and Johanson [42]. Manganese (Mn), copper (Cu), zinc (Zn), iron (Fe) and magnesium (Mg) were analysed using an atomic absorption spectrophotometer (Lab India, AA5000) with standard references. Sodium (Na) and potassium (K) were detected using flame photometry, while calcium (Ca) and P were estimated using biochemical methods as per Oser et al. [43].
2.5. Amino Acid Analysis
The analysis of the amino acid profile of the dried leaves involved the use of a high-performance liquid chromatography (HPLC) system. Weighed samples (50 mg) were hydrolysed with 6 N HCl in glass tubes. An anaerobic condition was maintained within the sealed tubes using nitrogen gas, and the tubes were kept overnight at 105°C for 12 h [44]. The samples were then neutralized with an equal volume of 6 N NaOH to make them acid-free, derivatized with O-phthalaldehyde and subsequently injected into an HPLC system (1260 Infinity II Amino Acid Analysis System, Agilent Technologies) equipped with a reversed-phase C18 column (InfinityLab Poroshell 120 HPH column) and a UV detector. The chromatogram peaks were matched with the retention time of the standard amino acids, the area (%) was calculated, and the contents of specific amino acids were expressed as percentage (%) of protein. Additionally, tryptophan was determined spectrophotometrically following the method of Sastry and Tammuru [45].
2.6. Fatty Acid Analysis
The dried powdered samples underwent extraction for fatty acid analysis in accordance with the method described by Folch, Lees and Stanley [46] using chloroform:methanol (2:1, v/v) solvent containing 0.01% butylated hydroxyl anisole as an antioxidant. Fatty acid methyl esters (FAMEs) of the lipid fractions were prepared through transmethylation with boron trifluoride in methanol [47]. The FAMEs were quantified by injecting 1 µL (50:1 split ratio) into a gas chromatograph (Perkin Elmer; CLARUS 480) equipped with a flame ionization detector operated by TotalChrom software. The FAME peaks were identified by comparing their respective retention times with those of the certified C4-C24 FAME standard (Supelco, Cat. No. 47885-U, Sigma–Aldrich, 178, USA) [48].
2.7. Analysis of Antioxidant Potential
The EC50 (effective concentration causing 50% effect/half maximal effective concentration) values of all the plant–leaf extracts and standard ascorbic acid were calculated graphically by plotting percent inhibition versus concentration.
2.8. Analysis of Antibacterial Potential
The antibacterial potential of the plant leaves was evaluated by conducting a disc diffusion assay using crude aqueous extracts against five common fish pathogenic bacteria [51]. The bacteria included Aeromonas salmonicida MTCC-1945 (AS), Aeromonas hydrophila MTCC-1739 (AH), Pseudomonas putida MTACC-1072 (PP), Pseudomonas fluorescens MTCC-103 (PF) and Aeromonas veronii KT737240 (AV). The antibacterial properties of the plant extracts were analysed following the standard methods [52]. Crude extracts of the dried powdered leaves were made with sterile distilled water, and stock solutions (20 mg/mL) were used to soak (100 μL) blank sterile 6-mm paper discs (Himedia, India) and dried within a laminar flow. Pure cultures of the pathogenic bacteria were grown in Tryptone Soya Broth (Himedia, India), colony-forming units (CFUs) versus OD600 were established, and the concentration of the bacteria was adjusted to 108 CFU/mL and inoculated (20 μL) over the sterilized nutrient agar media plates. The sterile paper discs soaked with the leaf extracts were placed gently on the inoculated agar plates and incubated overnight (24 h; 37°C) [53]. Gentamicin and chloramphenicol (10 μg/disc) were used as the positive control, while a blank disc served as the negative control. The diameter of the zone of inhibition (halo) produced by the pathogenic strains was measured and presented as inhibition scores [54]: 6–10 mm, low, 1; 11–20 mm, moderate, 2; 21–25 mm, high, 3; and >25 mm, very high; 4.
2.9. Statistical Analysis
The experimental data were represented as mean ± standard error (n = 3) and analysed using one-way analysis of variance (ANOVA). The post hoc Tukey test (p < 0.05) was employed to identify significant differences among the plant species. Additionally, principal component analysis (PCA) and Pearson’s correlation matrix were used to elucidate the relationship between the parameters. Both ANOVA and PCA were conducted using the R-Studio 3.6.3 [55], while Pearson’s correlation matrix was computed using PAST 4.06 [56].
3. Results
3.1. Proximate Analysis
The study revealed significant variations in proximate composition among different plant species (Table 1). The moisture content of the dried plant leaves exhibited a narrow range of variation (5.16%–7.23%). Notably, significantly lower moisture content was recorded in the leguminous plants such as A. lebbeck, T. indica, C. arietinum, B. acuminata, D. regia and P. pterocarpum (5.16%–5.47%) compared to other species. Consequently, the leaves of leguminous plants displayed the highest dry matter content (94.53%–94.84%). Furthermore, leguminous plants exhibited significantly higher CP contents, with values ranging from 16.64% to 20.74%. A. lebbeck exhibited the highest CP content (20.74%) followed by B. acuminata (19.43%), while M. indica displayed the lowest CP content (10.92%). A. heterophyllus exhibited the highest CL content (5.38%), whereas the lowest CL content was displayed by L. chinensis (1.23%). The ash content ranged between 8.38% and 13.41%, with Z. mauritiana demonstrating the highest ash content (16.73%) among the studied leaves.
| Parameter | Mangifera indica | Litchi chinensis | Artocarpus heterophyllus | Ziziphus mauritiana | Albizia lebbeck | Cicer arietinum | Tamarindus indica | Bauhinia acuminata | Delonix regia | Peltophorum pterocarpum |
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | 07.23 ± 0.12c | 06.62 ± 0.14bc | 06.53 ± 0.20b | 06.51 ± 0.16b | 05.16 ± 0.11a | 05.47 ± 0.14a | 05.37 ± 0.10a | 05.28 ± 0.10a | 05.31 ± 0.08a | 05.34 ± 0.15a |
| Dry matter | 92.77 ± 0.12a | 93.38 ± 0.14ab | 93.47 ± 0.20b | 93.49 ± 0.16b | 94.84 ± 0.11c | 94.53 ± 0.13c | 94.63 ± 0.10c | 94.82 ± 0.09c | 94.69 ± 0.08c | 94.66 ± 0.15c |
| Crude protein | 10.92 ± 0.25a | 13.13 ± 0.10b | 13.54 ± 0.06b | 13.72 ± 0.06b | 20.74 ± 0.16g | 16.64 ± 0.10c | 16.81 ± 0.20cd | 19.43 ± 0.10f | 18.10 ± 0.16e | 17.45 ± 0.21de |
| Crude lipid | 02.61 ± 0.08bc | 01.23 ± 0.09a | 05.38 ± 0.06g | 01.58 ± 0.05a | 02.47 ± 0.15b | 02.99 ± 0.09c | 03.97 ± 0.08d | 04.89 ± 0.09f | 04.90 ± 0.10f | 04.44 ± 0.10e |
| Crude fibre | 18.38 ± 0.10e | 24.63 ± 0.16g | 17.43 ± 0.06d | 16.61 ± 0.15c | 20.14 ± 0.16f | 13.79 ± 0.17a | 15.09 ± 0.10b | 18.64 ± 0.11e | 13.73 ± 0.17a | 16.34 ± 0.11c |
| Ash | 10.62 ± 0.10c | 12.48 ± 0.14e | 11.31 ± 0.07d | 16.73 ± 0.06g | 10.40 ± 0.12c | 09.07 ± 0.13b | 08.38 ± 0.13a | 11.59 ± 0.13d | 10.60 ± 0.09c | 13.41 ± 0.15f |
| NFE | 50.24 ± 0.12g | 41.91 ± 0.13b | 45.80 ± 0.10e | 44.86 ± 0.10d | 41.12 ± 0.32b | 52.05 ± 0.10h | 50.38 ± 0.06g | 40.27 ± 0.09a | 47.36 ± 0.12f | 43.03 ± 0.12c |
| TC | 68.62 ± 0.10h | 66.54 ± 0.11g | 63.23 ± 0.03d | 61.46 ± 0.05c | 61.19 ± 0.10bc | 65.84 ± 0.07f | 65.47 ± 0.08e | 61.67 ± 0.04c | 61.09 ± 0.05b | 59.37 ± 0.06a |
| OM | 89.38 ± 0.10e | 87.52 ± 0.14c | 88.69 ± 0.07d | 83.27 ± 0.06a | 89.60 ± 0.12e | 90.93 ± 0.13f | 91.62 ± 0.13g | 88.41 ± 0.13d | 89.40 ± 0.09e | 86.59 ± 0.15b |
| Caloric valueα | 338.33 ± 2.77bcd | 336.73 ± 1.60bc | 347.93 ± 1.39cd | 313.53 ± 4.45a | 342.33 ± 2.12bcd | 341.53 ± 3.49bcd | 351.93 ± 3.20de | 363.93 ± 2.88e | 347.13 ± 1.60cd | 332.09 ± 3.12b |
| P/E ratio€ | 32.32 ± 0.22a | 39.15 ± 0.56b | 38.93 ± 0.60b | 43.74 ± 0.45c | 60.70 ± 0.17f | 48.73 ± 0.77d | 47.91 ± 0.62d | 53.40 ± 0.53e | 52.16 ± 0.38e | 52.58 ± 0.37e |
| TFFA | 0.34 ± 0.03c | 0.22 ± 0.01a | 0.49 ± 0.03f | 0.26 ± 0.01ab | 0.32 ± 0.01bc | 0.37 ± 0.02cd | 0.41 ± 0.02de | 0.43 ± 0.01def | 0.45 ± 0.03ef | 0.44 ± 0.01ef |
| TFAA | 0.32 ± 0.03a | 0.33 ± 0.02a | 0.34 ± 0.01ab | 0.37 ± 0.01abc | 0.50 ± 0.03d | 0.42 ± 0.01bd | 0.44 ± 0.02d | 0.47 ± 0.01d | 0.46 ± 0.02d | 0.44 ± 0.02cd |
- Note: Results are mean ± standard error (n = 3). Different superscripts in the same row signify statistical differences (p < 0.05).
- Abbreviations: NFE, nitrogen-free extract; OM, organic matter; P/E, protein-to-energy ratio; TC, total carbohydrate; TFAA, total free amino acid; TFFA, total free fatty acid.
- αkcal/100 g.
- €mg protein/kcal.
The total carbohydrate content (NFE + CF) was significantly higher (p < 0.05) in M. indica (68.62%) compared to the other plants studied, while L. chinensis had the highest CF content (24.63%), followed by A. lebbeck (20.14%). The lowest total carbohydrate content was recorded with P. pterocarpum (59.37%). The CF content was minimal in D. regia (13.73%), and it did not differ significantly (p < 0.05) from C. arietinum (13.79%). NFE ranged between 40.27% and 52.05%, with the highest value observed in C. arietinum. Furthermore, the calculated values of organic matter ranged from 83.27% (Z. mauritiana) to 91.62% (T. indica). In terms of energy content, B. acuminata exhibited the highest gross energy (363.93 kcal/100 g), followed by T. indica (351.93 kcal/100 g) and A. heterophyllus (347.93 kcal/100 g). Although the highest P/E ratio (mg protein/kcal) was noticed in A. lebbeck (60.70), considerable P/E values were recorded for B. acuminata, D. regia and P. pterocarpum (52.16–53.40). On the other hand, M. indica showed the lowest P/E ratio (32.32).
TFAAs varied within the range of 0.32%–0.50%, with A. lebbeck exhibiting the maximum TFAAs (0.50%), although not significantly different (p < 0.05) from other studied leguminous plants. TFFAs ranged between 0.22% and 0.49%.
3.2. Analysis of ANFs
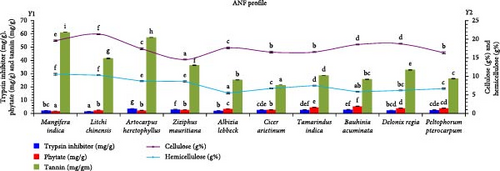
The analysis revealed substantial ANFs in the plant leaves, as depicted in Figure 2. The highest tannin content was recorded in M. indica (61.25 mg/g), while C. arietinum (21.25 mg/g) was evidenced with the lowest tannin content. In addition, considerable amounts of tannin were detected in B. acuminata (25.72 mg/g) and A. lebbeck (25.32 mg/g). The maximum TI and phytic acid contents were represented by A. heterophyllus (3.69 mg/g) and B. acuminata (5.42 mg/g), respectively. Cellulose contents ranged between 14.53% (Z. mauritiana) and 21.37% (L. chinensis) among the investigated plant leaves. Subsequently, M. indica exhibited the highest hemicellulose content (10.56%), although it did not significantly differ from L. chinensis.
3.3. Mineral Analysis
Among the macrominerals (Table 2), Na content exhibited variations within 2.26–15.88 mg/g range, with the highest concentration observed in A. lebbeck. The maximum amount of K was noticed in T. indica (14.98 mg/g), followed by A. heterophyllus (11.54 mg/g) and B. acuminata (10.32 mg/g). The Ca content ranged between 6.13 and 21.94 mg/g, with the highest value recorded in A. heterophyllus, followed by A. lebbeck (20.53 mg/g) and Z. mauritiana (18.82 mg/g). Additionally, A. heterophyllus exhibited the highest levels of Mg (6.33 mg/g) and P (4.63 mg/g). Among the trace minerals, Z. mauritiana displayed the highest concentrations of Fe (244.03 µg/g), Cu (5.61 µg/g) and Mn (127.3 µg/g). Furthermore, the maximum Zn content was noticed with C. arietinum (79.27 µg/g), followed by A. heterophyllus (46.27 µg/g) and Z. mauritiana (44.17 µg/g).
| Sl. No. | Plants | Macro minerals (mg/g) | Trace minerals (µg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Sodium (Na) |
Potassium (K) |
Calcium (Ca) |
Phosphorus (P) |
Magnesium (Mg) | Iron (Fe) |
Zinc (Zn) |
Copper (Cu) |
Manganese (Mn) |
||
| 1 | Mangifera indica | 2.26 ± 0.14a | 6.56 ± 0.22c | 6.13 ± 0.18a | 2.30 ± 0.11a | 2.62 ± 0.06a | 82.07 ± 0.46c | 22.40 ± 0.10c | 0.80 ± 0.05a | 42.37 ± 0.26b |
| 2 | Litchi chinensis | 4.41 ± 0.19b | 4.57 ± 0.25b | 9.84 ± 0.10c | 2.75 ± 0.08abc | 3.73 ± 0.13bc | 76.25 ± 0.86b | 16.83 ± 0.73b | 1.41 ± 0.08b | 63.17 ± 0.41e |
| 3 | Artocarpus heterophyllus | 13.61 ± 0.58f | 11.54 ± 0.16f | 21.94 ± 0.24i | 4.63 ± 0.15f | 6.33 ± 0.11g | 124.32 ± 1.32e | 46.27 ± 1.07g | 3.18 ± 0.15d | 56.23 ± 0.59d |
| 4 | Ziziphus mauritiana | 9.23 ± 0.38de | 6.47 ± 0.22c | 18.82 ± 0.23g | 2.83 ± 0.14bcd | 3.26 ± 0.15ab | 244.03 ± 1.01i | 44.17 ± 0.23g | 5.61 ± 0.10f | 127.3 ± 1.18h |
| 5 | Albizia lebbeck | 15.88 ± 0.53g | 8.76 ± 0.11d | 20.53 ± 0.45h | 4.15 ± 0.07f | 4.97 ± 0.14ef | 175.07 ± 0.75h | 10.67 ± 0.09a | 2.07 ± 0.08c | 72.89 ± 0.41g |
| 6 | Cicer arietinum | 5.23 ± 0.11b | 2.37 ± 0.11a | 17.09 ± 0.15f | 3.34 ± 0.06de | 4.04 ± 0.17cd | 34.71 ± 0.32a | 79.27 ± 0.71h | 2.28 ± 0.08c | 68.03 ± 0.53f |
| 7 | Tamarindus indica | 8.62 ± 0.17cd | 14.98 ± 0.19g | 8.59 ± 0.09b | 2.95 ± 0.08cde | 5.55 ± 0.29f | 127.87 ± 0.93e | 22.29 ± 0.19c | 4.59 ± 0.09e | 46.29 ± 0.74c |
| 8 | Bauhinia acuminata | 7.41 ± 0.09c | 10.32 ± 0.27e | 15.51 ± 0.18e | 3.39 ± 0.10e | 3.11 ± 0.05ab | 145.9 ± 0.72g | 27.27 ± 0.31d | 2.34 ± 0.03c | 54.61 ± 0.66d |
| 9 | Delonix regia | 10.23 ± 0.11e | 3.23 ± 0.19a | 13.38 ± 0.14d | 2.58 ± 0.08abc | 5.57 ± 0.15fg | 140.13 ± 0.81f | 32.28 ± 0.10e | 1.6 ± 0.05b | 34.37 ± 0.58a |
| 10 | Peltophorum pterocarpum | 13.13 ± 0.22f | 4.71 ± 0.18b | 12.71 ± 0.28d | 2.33 ± 0.11ab | 4.65 ± 0.14de | 104.23 ± 1.18d | 39.14 ± 0.26f | 2.22 ± 0.08c | 46.19 ± 0.55c |
- Note: Results are mean ± standard error (n = 3). Different superscripts in the same column signify statistical differences (p < 0.05).
3.4. Amino Acid Analysis
The analysis revealed that A. lebbeck contained the highest sum of EAAs (ƩEAA), while A. heterophyllus exhibited the maximum nonessential amino acids (ƩNEAA) compared to the other leaf meals (Table 3). It is noteworthy that cystine and tyrosine can be derived from methionine and phenylalanine; hence, they are categorized as EAAs. The quantity of ƩEAA was relatively higher than the ƩNEAA in all leguminous plants, compared to the other studied terrestrial leaf meals. Valine (8.68 g/100 g protein), histidine (8.58 g/100 g protein), methionine (3.95 g/100 g protein), tryptophan (2.83 g/100g protein), arginine (7.99 g/100 g protein), tyrosine (3.59 g/100 g protein) and cysteine (2.01 g/100 g protein) among the EAA, along with serine (5.41 g/100 g protein) among the NEAA were dominant in the A. lebbeck leaf meal. The maximum amount of isoleucine was detected in C. arietinum (6.21 g/100 g protein), although it was not significantly different from M. indica (6.18 g/100 g protein) and A. lebbeck (6.04 g/100 g protein). Furthermore, a higher proportion of leucine (9.65 g/100 g protein), phenylalanine (3.57 g/100 g protein) and threonine (3.54 g/100 g protein) were detected in P. pterocarpum, Z. mauritiana and C. arietinum, respectively. Regarding NEAA, B. acuminata exhibited the highest amount of alanine (5.11 g/100 g protein) and glycine (4.83 g/100 g protein), while A. heterophyllus contained dominant levels of glutamic acid (12.31 g/100 g protein) and proline (9.47 g/100 g protein). L. chinensis represented the maximum amount of aspartic acid (11.48 g/100 g protein) and asparagine (2.86 g/100 g protein). Evaluation of the plant leaves also indicated variation in the sulphur-containing amino acids, methionine and cysteine, ranging between 1.08–3.95 and 0.45–2.01 g/100 g of protein, respectively. Furthermore, the leaf meals exhibited the presence of some unusual amino acids, which were notably rich in hydroxyproline.
| Amino acids | Mangifera indica | Litchi chinensis | Artocarpus heterophyllus | Ziziphus mauritiana | Albizia lebbeck | Cicer arietinum | Tamarindus indica | Bauhinia acuminata | Delonix regia | Peltophorum pterocarpum |
|---|---|---|---|---|---|---|---|---|---|---|
| Essential amino acids | ||||||||||
| Arginine | 3.95 ± 0.08a | 4.23 ± 0.23a | 3.62 ± 0.11a | 3.53 ± 0.16a | 7.99 ± 0.08d | 5.25 ± 0.06b | 5.85 ± 0.08bc | 6.56 ± 0.29c | 5.99 ± 0.10bc | 5.81 ± 0.12bc |
| Histidine | 6.15 ± 0.08bc | 5.91 ± 0.16b | 7.48 ± 0.16de | 1.26 ± 0.12a | 8.58 ± 0.21f | 7.48 ± 0.19de | 6.73 ± 0.22cd | 7.69 ± 0.14e | 7.98 ± 0.11ef | 7.94 ± 0.14ef |
| Isoleucine | 6.18 ± 0.25d | 3.43 ± 0.10ab | 2.96 ± 0.09a | 3.95 ± 0.06bc | 6.04 ± 0.13d | 6.21 ± 0.07d | 5.95 ± 0.08d | 4.51 ± 0.03c | 4.22 ± 0.12c | 4.09 ± 0.14c |
| Leucine | 5.85 ± 0.05b | 4.22 ± 0.10a | 3.81 ± 0.10a | 4.16 ± 0.12a | 3.92 ± 0.07a | 6.19 ± 0.08bc | 5.96 ± 0.08b | 6.85 ± 0.18c | 6.39 ± 0.11bc | 9.65 ± 0.25d |
| Lysine | 2.87 ± 0.04a | 3.27 ± 0.11ab | 3.03 ± 0.13a | 3.91 ± 0.17c | 5.40 ± 0.23de | 4.86 ± 0.11d | 4.20 ± 0.04c | 5.84 ± 0.11e | 3.92 ± 0.08c | 3.66 ± 0.03bc |
| Methionine | 1.08 ± 0.07a | 1.14 ± 0.15a | 1.80 ± 0.10b | 1.58 ± 0.04ab | 3.95 ± 0.11g | 2.88 ± 0.16de | 3.19 ± 0.06ef | 3.49 ± 0.14fg | 2.33 ± 0.05c | 2.57 ± 0.09cd |
| Phenylalanine | 0.19 ± 0.06a | 0.18 ± 0.05a | 1.23 ± 0.07c | 3.57 ± 0.18e | 2.42 ± 0.07d | 1.32 ± 0.06c | 1.13 ± 0.09c | 2.51 ± 0.11d | 0.76 ± 0.04b | 0.32 ± 0.06a |
| Threonine | 0.44 ± 0.07a | 1.22 ± 0.08b | 1.37 ± 0.09b | 1.12 ± 0.09b | 0.63 ± 0.12a | 3.54 ± 0.19d | 3.42 ± 0.08d | 0.67 ± 0.05a | 2.33 ± 0.13c | 2.54 ± 0.07c |
| Tryptophan | 0.20 ± 0.09a | 0.43 ± 0.07a | 1.20 ± 0.07bc | 0.35 ± 0.08a | 2.83 ± 0.10e | 1.21 ± 0.09bc | 0.95 ± 0.07b | 1.32 ± 0.10bc | 1.41 ± 0.05c | 2.12 ± 0.04d |
| Valine | 3.87 ± 0.09b | 3.93 ± 0.11b | 2.07 ± 0.03a | 2.06 ± 0.08a | 8.68 ± 0.17f | 5.64 ± 0.17de | 4.19 ± 0.07b | 4.94 ± 0.13c | 5.37 ± 0.09cd | 6.27 ± 0.24e |
| Cysteine | 0.55 ± 0.08ab | 0.45 ± 0.10a | 0.61 ± 0.12abc | 0.97 ± 0.04bcd | 2.01 ± 0.09f | 0.89 ± 0.16abc | 1.05 ± 0.06cd | 1.60 ± 0.06e | 1.40 ± 0.11de | 1.58 ± 0.04e |
| Tyrosine | 1.77 ± 0.16bc | 1.20 ± 0.06a | 1.37 ± 0.05ab | 2.07 ± 0.14c | 3.59 ± 0.12e | 2.86 ± 0.09d | 3.14 ± 0.08de | 2.98 ± 0.08d | 3.04 ± 0.07d | 3.10 ± 0.11de |
| ∑EAA | 33.1 | 29.61 | 30.55 | 28.53 | 56.04 | 48.33 | 45.76 | 48.96 | 45.14 | 49.65 |
| Nonessential amino acids | ||||||||||
| Alanine | 3.54 ± 0.08b | 2.96 ± 0.12a | 3.16 ± 0.07ab | 2.71 ± 0.05a | 4.18 ± 0.07c | 4.25 ± 0.11c | 4.33 ± 0.11c | 5.11 ± 0.12d | 4.14 ± 0.21c | 4.55 ± 0.08c |
| Asparagine | 1.34 ± 0.07b | 2.86 ± 0.05c | 0.44 ± 0.08a | 0.30 ± 0.03a | 1.23 ± 0.06b | 0.51 ± 0.04a | 0.45 ± 0.04a | 1.42 ± 0.06b | 2.64 ± 0.11c | 0.48 ± 0.08a |
| Aspartic acid | 8.45 ± 0.08d | 11.48 ± 0.11e | 8.27 ± 0.10d | 8.26 ± 0.07d | 4.45 ± 0.08b | 5.65 ± 0.13c | 4.39 ± 0.10b | 4.26 ± 0.07b | 4.20 ± 0.12b | 3.43 ± 0.12a |
| Glutamic acid | 8.60 ± 0.16e | 3.4 ± 0.11a | 12.31 ± 0.06f | 4.63 ± 0.11b | 4.60 ± 0.16b | 4.32 ± 0.07b | 6.63 ± 0.13d | 8.45 ± 0.08e | 5.27 ± 0.14c | 3.54 ± 0.07a |
| Glutamine | 1.27 ± 0.06b | 0.67 ± 0.06a | 1.32 ± 0.11b | 1.32 ± 0.06b | 1.35 ± 0.07b | 2.63 ± 0.11c | 2.44 ± 0.06c | 1.22 ± 0.09b | 2.43 ± 0.11c | 2.53 ± 0.11c |
| Glycine | 2.44 ± 0.19a | 2.64 ± 0.05ab | 3.16 ± 0.08b | 2.55 ± 0.08a | 4.77 ± 0.13e | 4.18 ± 0.15cd | 3.93 ± 0.12c | 4.83 ± 0.07e | 4.70 ± 0.07de | 4.53 ± 0.12de |
| Proline | 6.34 ± 0.14d | 6.34 ± 0.09d | 9.47 ± 0.10f | 8.49 ± 0.09e | 3.30 ± 0.09b | 3.46 ± 0.14b | 4.63 ± 0.04c | 4.45 ± 0.08c | 3.16 ± 0.07b | 2.22 ± 0.09a |
| Serine | 2.60 ± 0.10a | 3.06 ± 0.14ab | 3.25 ± 0.08bc | 2.96 ± 0.04ab | 5.41 ± 0.09f | 3.64 ± 0.13c | 4.78 ± 0.09e | 4.17 ± 0.09d | 4.90 ± 0.07e | 5.21 ± 0.14ef |
| ∑NEAA | 34.58 | 33.41 | 41.38 | 31.22 | 29.29 | 28.64 | 31.58 | 33.91 | 31.44 | 26.49 |
| UAA | ||||||||||
| Citrulline | 2.41 ± 0.11e | 1.35 ± 0.04bcd | 1.45 ± 0.08cd | 3.42 ± 0.12f | 1.02 ± 0.06ab | 0.73 ± 0.06a | 2.43 ± 0.06e | 1.65 ± 0.09d | 0.65 ± 0.05a | 1.19 ± 0.09bc |
| Norvaline | 0.59 ± 0.08a | 0.43 ± 0.05a | 1.06 ± 0.08b | 0.53 ± 0.06a | 1.45 ± 0.08cd | 2.12 ± 0.11e | 1.53 ± 0.06d | 0.59 ± 0.09a | 1.13 ± 0.06bc | 1.14 ± 0.06b |
| Hydroxyproline | 13.14 ± 0.14b | 16.09 ± 0.15d | 14.01 ± 0.09c | 17.30 ± 0.22e | 11.22 ± 0.09a | 13.27 ± 0.08b | 14.19 ± 0.18c | 11.07 ± 0.17a | 13.26 ± 0.12b | 14.48 ± 0.13c |
| Sarcosine | 3.66 ± 0.14c | 2.37 ± 0.09b | 1.18 ± 0.06a | 3.18 ± 0.13c | 1.23 ± 0.03a | 2.16 ± 0.08b | 0.85 ± 0.09a | 1.36 ± 0.09a | 3.54 ± 0.16c | 2.19 ± 0.10b |
- Note: Results are mean ± standard error (n = 3). Different superscripts in the same row signify statistical differences (p < 0.05).
- Abbreviations: EAA, essential amino acid; NEAA, nonessential amino acid; UAA, unusual amino acid.
3.5. Fatty Acid Analysis
The fatty acid composition of various leaf meals was analysed, revealing that the total sum of SFA, MUFA and PUFA followed the PUFA > SFA > MUFA order in most cases (Table 4). However, exceptions were observed in Z. mauritiana and A. lebbeck, where the order of the fatty acids was SFA > PUFA > MUFA. Notably, the highest total SFA area percentage was recorded in Z. mauritiana (45.39% of total fatty acid), closely followed by A. lebbeck (44.66%). Conversely, SFA content was the lowest in D. regia (23.46%). Among the SFA, palmitic acid (C16:0) was predominant in the evaluated leaf meals (15.8%–39.78% of total fatty acid), representing its maximum value in Z. mauritiana. Among the MUFA, a considerably higher proportion was noticed in L. chinensis (30.26%), followed by T. indica (24.68%) and A. heterophyllus (22.76%). Oleic acid (C18:1) was identified as the most abundant MUFA in the studied samples, with the highest amount exhibited by L. chinensis (28.67%).
| Fatty acid | Area percentage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mangifera indica | Litchi chinensis | Artocarpus heterophyllus | Ziziphus mauritiana | Albizia lebbeck | Cicer arietinum | Tamarindus indica | Bauhinia acuminata | Delonix regia |
Peltophorum pterocarpum | |
| pterocarpumTridecanoic acid (C13:0) | 0.24 ± 0.08a | 0.98 ± 0.15c | 0.19 ± 0.09a | — | 0.63 ± 0.03bc | 0.48 ± 0.04ab | 0.18 ± 0.04a | 0.86 ± 0.08c | 0.45 ± 0.05ab | 0.38 ± 0.09ab |
| Myristic acid (C14:0) | 1.24 ± 0.10c | — | 0.96 ± 0.07bc | 0.22 ± 0.04a | 1.67 ± 0.09d | 0.12 ± 0.05a | 1.24 ± 0.06c | 2.41 ± 0.06e | 0.26 ± 0.04a | 0.68 ± 0.04b |
| Pentadecanoic acid (C15:0) | 0.01 ± 0.01a | 0.01 ± 0.01a | — | 0.16 ± 0.07ab | 0.24 ± 0.03b | 0.32 ± 0.07b | 0.01 ± 0.01a | 0.27 ± 0.07b | 0.16 ± 0.03ab | 0.003 ± 0.003a |
| Palmitic acid (C16:0) | 30.26 ± 0.51f | 23.75 ± 0.38d | 16.36 ± 0.37a | 39.78 ± 0.47g | 30.34 ± 0.17f | 27.26 ± 0.13e | 21.28 ± 0.34c | 15.8 ± 0.31a | 18.82 ± 0.25b | 19.59 ± 0.23bc |
| Stearic acid (C18:0) | 2.46 ± 0.12d | 3.71 ± 0.15e | 4.56 ± 0.14f | 1.16 ± 0.04a | 7.81 ± 0.26g | 2.32 ± 0.03cd | 2.14 ± 0.09bcd | 4.36 ± 0.14ef | 1.88 ± 0.13bcd | 1.60 ± 0.12ab |
| Arachidic acid (C20:0) | 1.26 ± 0.12cd | 0.84 ± 0.02bc | 0.16 ± 0.07a | 0.64 ± 0.11ab | 1.04 ± 0.20bcd | 1.84 ± 0.09e | 3.27 ± 0.08f | 1.02 ± 0.03bcd | 1.89 ± 0.08e | 1.49 ± 0.05de |
| Tricosanoic acid (C23:0) | 0.69 ± 0.10bc | 1.52 ± 0.06e | 1.19 ± 0.06cde | 1.08 ± 0.06cd | 1.12 ± 0.05cde | 0.68 ± 0.15bc | 0.49 ± 0.10b | 0.88 ± 0.08bc | 0.01 ± 0.01a | 1.38 ± 0.08de |
| Lignoceric acid (C24:0) | 2.48 ± 0.21de | 3.70 ± 0.30f | 2.57 ± 0.08e | 2.35 ± 0.03de | 1.81 ± 0.12cd | 0.22 ± 0.10a | 1.34 ± 0.07bc | 1.46 ± 0.03c | 0.59 ± 0.04a | 0.74 ± 0.08ab |
| ∑SFA | 38.63 | 34.50 | 25.99 | 45.39 | 44.66 | 33.24 | 29.94 | 27.06 | 23.46 | 25.86 |
| Myristoleic acid (C14:1) | 0.18 ± 0.05ab | 0.01 ± 0.01a | 0.47 ± 0.05bcd | 0.34 ± 0.06abcd | 0.23 ± 0.02abc | 0.43 ± 0.03bcd | 0.59 ± 0.10d | 0.28 ± 0.04abcd | 0.56 ± 0.13cd | 0.44 ± 0.08bcd |
| Pentadecanoic acid (C15:1) | 0.64 ± 0.13c | 0.02 ± 0.01a | — | 0.17 ± 0.04ab | 0.43 ± 0.02bc | 0.12 ± 0.06ab | 0.23 ± 0.03ab | — | 0.54 ± 0.08c | 0.38 ± 0.04bc |
| Palmitoleic acid (C16:1) | 0.18 ± 0.08ab | — | 0.01 ± 0.01a | 0.41 ± 0.10bcd | — | 0.26 ± 0.03abc | 0.13 ± 0.04a | 0.49 ± 0.06bcd | 0.58 ± 0.04cd | 0.69 ± 0.14d |
| Oleic acid (C18:1n9c) | 19.46 ± 0.25e | 28.67 ± 0.12h | 20.71 ± 0.12f | 14.56 ± 0.33bc | 13.67 ± 0.09b | 18.58 ± 0.27de | 22.06 ± 0.29g | 12.19 ± 0.10a | 15.56 ± 0.06c | 17.86 ± 0.35d |
| Elaidic acid (C18:1n9t) | 0.46 ± 0.03ab | 0.63 ± 0.13b | 0.58 ± 0.04b | 0.66 ± 0.05b | 0.39 ± 0.05ab | 0.24 ± 0.03a | 0.41 ± 0.03ab | 0.24 ± 0.08a | 0.39 ± 0.02ab | 0.44 ± 0.08ab |
| Nervonic acid (C24:1) | 0.76 ± 0.05a | 0.96 ± 0.17ab | 1 ± 0.05ab | 1.28 ± 0.08b | 1.33 ± 0.12b | 0.66 ± 0.04a | 1.26 ± 0.05b | 1.28 ± 0.05b | 2.09 ± 0.12c | 1.26 ± 0.06b |
| ∑MUFA | 21.68 | 30.26 | 22.76 | 17.42 | 16.05 | 20.29 | 24.68 | 14.48 | 19.72 | 21.07 |
| Linoleic acid (C18:2n6c) | 4.68 ± 0.14a | 5.10 ± 0.18a | 12.10 ± 0.18de | 6.35 ± 0.06b | 17.90 ± 0.39f | 11.26 ± 0.24cd | 12.35 ± 0.12e | 10.57 ± 0.07c | 4.56 ± 0.06a | 6.23 ± 0.12b |
| Linolenic acid (C18:3n3) | 31.42 ± 0.17d | 29.03 ± 0.21c | 37.79 ± 0.29f | 27.28 ± 0.14b | 20.11 ± 0.19a | 32.56 ± 0.12e | 32.01 ± 0.15de | 45.03 ± 0.25g | 51.42 ± 0.23h | 45.68 ± 0.31g |
| Arachidonic acid (C20:4n6) | 3.59 ± 0.11d | 0.89 ± 0.04a | 1.36 ± 0.08a | 3.56 ± 0.23d | 1.28 ± 0.07a | 2.25 ± 0.11b | 1.02 ± 0.06a | 2.86 ± 0.09c | 0.84 ± 0.06a | 1.16 ± 0.09a |
| ∑PUFA | 39.69 | 35.02 | 51.25 | 37.19 | 39.29 | 46.07 | 45.38 | 58.46 | 56.82 | 53.07 |
| PUFA/SFA | 1.02 | 1.01 | 1.97 | 0.81 | 0.88 | 1.39 | 1.52 | 2.16 | 2.42 | 2.05 |
| ∑n3 | 31.42 | 29.03 | 37.79 | 27.28 | 20.11 | 32.56 | 32.01 | 45.03 | 51.42 | 45.68 |
| ∑n6 | 8.27 | 5.99 | 13.46 | 9.91 | 19.18 | 13.51 | 13.37 | 13.43 | 5.4 | 7.39 |
| n3/n6 | 3.80 | 4.84 | 2.81 | 2.75 | 1.04 | 2.41 | 2.39 | 3.35 | 9.52 | 6.18 |
- Note: Results are mean ± standard error (n = 3). Different superscripts in the same row signify statistical differences (p < 0.05).
While considering PUFA, α-linolenic acid (C18:3n3) emerged as the major fatty acid (n-3 fatty acid), demonstrating its maximum concentration in D. regia (51.42%), followed by P. pterocarpum (45.68%) and B. acuminata (45.03%). Notably, PUFA content was the maximum in B. acuminata (58.46%).
3.6. Antioxidant Activity
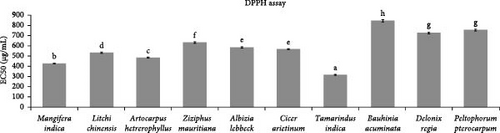
The study demonstrated that T. indica exhibited the highest free radical scavenging activity, followed by M. indica. The lowest activity was exhibited by B. acuminata. The order of free radical scavenging activity observed was T. indica > M. indica > A. heterophyllus > L. chinensis > C. arietinum > A. lebbeck > Z. mauritiana > D. regia > P. pterocarpum > B. acuminata (Figure 3). The EC50 values of the standard ascorbic acid and the plant–leaf extracts were 36.64 μg/mL, 314.92 μg/mL, 427.05 μg/mL, 484 μg/mL, 533.32 μg/mL, 566.38 μg/mL, 584.64 μg/mL, 633.02 μg/mL, 726.18 μg/mL, 756.24 μg/mL and 846.24 μg/mL, respectively.
3.7. Antibacterial Activity
Considerable antipathogenic activities were exhibited by T. indica and B. acuminata, with a cumulative inhibition score of ≥10. T. indica demonstrated the highest total score of 12. Conversely, C. arietinum and Z. mauritiana displayed the least activity (Table 5).
| Sl. No. | Plants | Pathogenic bacteria | |||||
|---|---|---|---|---|---|---|---|
| AH | AV | AS | PF | PP | Total score | ||
| 1 | Mangifera indica | 1 | — | 2 | 2 | 1 | 6 |
| 2 | Litchi chinensis | — | 1 | 1 | 2 | 1 | 5 |
| 3 | Artocarpus heterophyllus | 1 | — | 2 | 1 | 1 | 5 |
| 4 | Ziziphus mauritiana | — | — | 1 | 1 | 1 | 3 |
| 5 | Albizia lebbeck | 1 | 1 | — | 1 | 1 | 4 |
| 6 | Cicer arietinum | — | 1 | 1 | — | 1 | 3 |
| 7 | Tamarindus indica | 3 | 2 | 3 | 2 | 2 | 12 |
| 8 | Bauhinia acuminata | 2 | 1 | 2 | 2 | 3 | 10 |
| 9 | Delonix regia | — | 2 | 1 | 3 | 2 | 8 |
| 10 | Peltophorum pterocarpum | 2 | 1 | 1 | 1 | 3 | 8 |
| 11 | Gentamicin | 3 | 3 | 4 | 4 | 4 | 18 |
| 12 | Chloramphenicol | 2 | 3 | 4 | 3 | 2 | 14 |
- Note: Zones of inhibition (halo diameter) are presented as scores. Gentamicin and chloramphenicol were served as the standards. 1, low (6–10 mm); 2, moderate (11–20 mm); 3, high (21–25 mm); 4, very high (>25 mm); “-” no inhibition. Data represent the mean value of three observations.
- Abbreviations: AH, A. hydrophila; AS, A. salmonicida; AV, A. veronii; PF, P. fluorescens; PP, P. putida.
3.8. PCA and Correlation Matrix Analysis
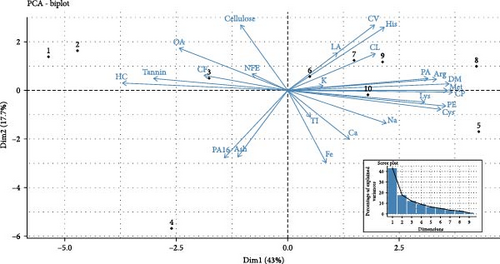
The analysis revealed that the first two principal components (PC1 and PC2) captured 60.70% of variability present in the dataset, with PC1 (Dim1) explaining 43% of the total variance along the X axis and PC2 (Dim2) explaining 17.7% along the Y axis (Figure 4). The Y axis effectively discriminated leguminous plants (A. lebbeck, T. indica, C. arietinum, B. acuminata, P. pterocarpum and D. regia) from the other four plants (M. indica, L. chinensis, A. heterophyllus and Z. mauritiana). Conversely, M. indica, L. chinensis, A. heterophyllus and Z. mauritiana exhibited higher levels of ash, oleic acid, NFE, CF and certain ANFs such as tannin and cellulose.
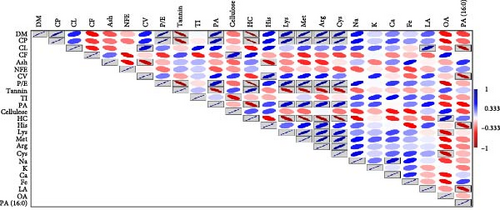
The correlation matrix highlighted CP as a pivotal parameter of feed/feed ingredients, showing significant positive correlations with the dry matter, P/E, amino acids (histidine, lysine, arginine, cysteine and methionine) and phytic acid (Figure 5). Conversely, tannin and hemicellulose displayed significant negative correlations with CP. Moreover, CL exhibited significant positive correlations with caloric value, while palmitic acid was negatively correlated with phytic acid. P/E showed positive correlations with dry matter, CP, amino acids and phytic acid whereas negative correlations with tannin and hemicellulose. Additionally, cellulose demonstrated a significant positive correlation with CF. Tannin displayed negative correlations with dry matter, CP, P/E ratio, phytic acids and certain amino acids (histidine, lysine, arginine, cysteine and methionine).
4. Discussion
Evaluating the nutritional value of alternative plant-derived ingredients is crucial to provide the farmers with optimal dietary choices for fish production. Compared to oilcakes, seed meals and aquatic macrophytes, leaf meals from terrestrial plants have received inadequate attention in the context of fish nutrition [4]. This study aims to appraise the nutritional value of some terrestrial plant leaves to determine their potential importance as fish feed ingredients. In feed formulation, both the quality and quantity of the protein are among the most important criteria to consider for any ingredient. The present study recorded extensive variability in the composition of terrestrial leaf meals. The CP levels in the evaluated terrestrial leaf meals ranged from 10.92% to 20.74%, wherein leguminous plants were recorded with comparatively higher CP levels (>16.5%) with A. lebbeck exhibiting the highest (20.74%). These findings are consistent with previous reports of CP levels of 19.1% and 20.5% in A. lebbeck grown in semiarid and arid regions, respectively [57]. In contrast, crop residues and forage grasses typically contain lower CP levels, ranging from 4% to 7% [58–60]. Apart from the high-protein seed meal and oilcakes (CP, ≃28%–35%), commonly used low-protein plant ingredients such as rice bran, wheat bran, wheat flour, corn meal and sorghum contain 9%–16% CP [13], which is lower than the CP content observed in the leguminous plant leaves studied in this research. The varying protein requirements of different fish species including major carps (25%–35% CP) can be met by blending high-protein oilcake/animal protein and low-protein vegetal meals [61].
Lipid is an essential dietary component, required at 5%–10% levels for the carps. The CL (ether extract) contents in the evaluated leaf meals ranged from 1.23% to 5.38%, which were lower than previous reports on tropical leguminous plants [62]. Furthermore, the leaf meals exhibited higher total carbohydrate contents (≃60%–68%) than those documented in preceding reports on terrestrial and aquatic plants [10, 13]. Carbohydrates are the vital energy sources in feeds, the dietary requirements of which may differ in varied fish species. For instance, herbivores can utilize dietary carbohydrates better than carnivores [63, 64]. Among the leaf meals studied, gross energy value was the maximum with B. acuminata (363.93 kcal/100 g), while the lowest value was observed in Z. mauritiana (313.53 kcal/100 g). Appraising the P/E ratio in feed and feed ingredients is essential, as a higher P/E ratio indicates better diet quality [10]. Leaf meals from the leguminous plants demonstrated a higher P/E ratio (60.70–52.16 mg protein/kcal) than the other leaf meals studied. Thus, considering high gross energy, elevated carbohydrate levels (60%–65%), considerable CP levels (>16.5%) and a moderate P/E ratio, the leaves of leguminous plants such as A. lebbeck, B. acuminata, D. regia and P. pterocarpum could be incorporated as the nonconventional feed ingredients for the formulation of low-cost carp diets.
Whenever an ingredient is analysed for its nutritive value, primary emphasis is given to the quality and quantity of the nutrients therein [13]. However, dietary utilization of the plant ingredients has been obstructed by various ANF substances within them [30]. These ANFs can affect the digestibility and bioavailability of the nutrients, thereby impacting fish growth [4]. For instance, tannins inhibit protease activity and form indigestible complexes with the dietary protein, reducing nutrient bioavailability [65]. Similarly, phytic acid chelates with proteins and certain minerals (viz. Ca, Na, Mg and P), forming phytate compounds, thereby impairing enzyme activities or reducing mineral bioavailability [66]. Nonstarch polysaccharides (NSPs) such as cellulose and hemicellulose are highly indigestible owing to the presence of β1–4 linkages, thus increasing the viscosity of the food and altering the gut microecosystem producing antinutritive effects [4]. Overall, the leguminous plants evaluated in the present study represented lower ANFs than the other plants. For example, leguminous plants like A. lebbeck (25.32 mg/g) and B. acuminata (25.72 mg/g) contained moderate levels of tannins in contrast to the elevated levels detected in M. indica (61.25 mg/g) and A. heterophyllus (57.24 mg/g). The amount of TI detected in the leaf meals, including the leguminous plants, was generally found to be less than 3 mg/g, which may not be alarming as fish could potentially compensate for dietary TI at this level (<5 mg/g) by augmenting trypsin production within them [30]. Dietary fibre consists of structural carbohydrates such as cellulose and hemicelluloses and lignin. The NSPs (sum of cellulose and hemicelluloses) in the leaf meals ranged between 23% and 32%, while CF contents varied between 13.73% and 24.63%. For example, CF content in L. chinensis (24.63%) was less than the obtained value of the NSPs (31.67%). Thus, this study noted an apparent discrepancy between the values of CF and NSPs in the leaf meals, citing that CF may not truly represent the total fibre content as a significant portion of the true fibres (cellulose, hemicellulose and lignin) may be lost during the acid as well as alkali digestion underestimating the fibre content [13, 67]. Hence, NSPs were evaluated in the present study, as they represent the major fibre fractions of the leaf meals. Further, the results of the study indicated that the leaf meals had a relatively higher proportion of cellulose and a lower level of hemicelluloses, which could undermine their nutritive value. Compared to hemicelluloses, cellulose is a less readily digestible component of the plant cell wall [24]. Consequently, fermentative degradations of the NSPs and other ANFs in vitro might be recommended to increase the incorporation of leaf meals in the formulation of fish feeds [4].
The assessment of leaf meals revealed considerable disparities in the macromineral and trace mineral compositions. These differences are likely attributable to phylogenic distinctions. Mineral elements are known to play crucial roles in vital physiological processes, including but not limited to neuromuscular function, skeletal structures (Ca and P), enzyme activity (metalloenzyme or cofactor) and hemostasis (Ca) [10]. Dietary K levels for common carp (Cyprinus carpio), grass carp and Nile tilapia (Oreochromis niloticus) were recommended as 0.9–12.4, 4.6 and 2.1– 3.3 g/kg diet, respectively. The requirement of Na in the diets of grass carp was advocated as 0.96 g/kg diet. In addition, Mg requirements may vary from 0.4 to 0.946 g/kg of diets among diverse fish species and prawns, Penaeus indicus [12, 68]. Among the trace minerals, Fe, Zn and Mn requirements were suggested to range within the 30–200, 15–79 and 12–25 mg/kg diet, respectively [12]. The findings of this investigation indicated that the evaluated leaf meals contained sufficient quantities of Ca, K, Na, P, Mg and other trace minerals, making them potential candidates for incorporation into feed. This study aligns with prior reports on A. lebbeck and other leguminous plant leaves describing rich mineral contents therein [57, 62].
The quality of feed protein is influenced by a balanced amino acid profile. While extensive research has been conducted on the amino acid compositions of various plant parts, plant leaves have been relatively understudied [69, 70]. Despite being deficient in overall protein content, leaves of leguminous plants were noticed to contain considerable amounts of EAAs and NEAAs. Notably, an apparent disparity between the CP contents and their corresponding total amino acid contents was recorded in the studied leaf meals. This observation is consistent with previous reports emphasizing that the Kjeldahl method used to determine CP content does not directly estimate the CP content but derives it from the total nitrogen content multiplied by the N–P factor, typically 6.25 in the case of plant products [71]. Thus, nonprotein nitrogen may contribute to the differences between CP and total amino acid contents in the leaf meals, as indicated elsewhere [13]. Indeed, higher plants contain substantial amounts of pigments (viz. chlorophyll-a, chlorophyll-b and carotenoids) that absorb light energy for photosynthesis, resulting in high levels of nonprotein nitrogen [72]. The biosynthesis of EAAs in animals is either absent or insufficient to support optimal growth. In fish, 10 EAAs are vital for their proper development: arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine [73]. Cystine and tyrosine can be produced from methionine and phenylalanine in animals. Consequently, the dietary requirement for these EAAs depends on the concentration of their precursor compounds in the diet. Therefore, these two amino acids are included under the EAA category [73, 74]. In order to support optimal growth and physiological processes, fish typically require 38.3% of indispensable amino acids, including arginine (5%), histidine (2%), isoleucine (3.3%), leucine (4.9%), lysine (5.2%), methionine + cysteine (3.5%), phenylalanine + tyrosine (6.2%), threonine (3.5%), tryptophan (0.9%) and valine (3.8%) [74]. Even though the leaves of the leguminous plants have lower protein percentages than the ideal level required for fish feed, all tested leguminous plants demonstrated higher proportions of total EAAs per 100 g of protein than the NEAAs. This suggests that these plants could be used to formulate diets for herbivorous or omnivorous carp species requiring comparatively less dietary protein. Further, the evaluated leaf meals displayed a substantial presence of naturally occurring unusual amino acids, for example, citrulline, hydroxyproline and norvaline. The rich amino acid profiles of the leguminous plants, especially A. lebbeck and B. acuminata leaf meals, were noticed in the present study. Notably, aspartic acid (3.43%–11.48% of CP) and glutamic acid (3.4%–12.31% of CP) were the most abundant amino acids among the leaf meals analysed. Previous studies have also reported the prevalence of both aspartic acid and glutamic acid in edible plant leaves across various species [69, 70, 75]. While plant parts typically lack sulphur-containing amino acids, namely, methionine and cystine [40], the considerable presence of methionine in the evaluated leaf meals is noteworthy. The presently reported study might indicate that the leaves of the leguminous plants could potentially serve as a source of EAAs and NEAAs if utilized in fish feed formulation.
The fatty acids play crucial roles as precursors of hormones and as energy reserves [69]. Leaves of the terrestrial plants evaluated in this study were dominated by PUFA compared to the SFA or MUFA (PUFA > SFA > MUFA), except Z. mauritiana and A. lebbeck where SFA was dominant (SFA > PUFA > MUFA). Previous studies also have recorded a higher proportion of PUFA in terrestrial plant leaves than SFA and MUFA [21, 76]. In general, palmitic acid (C16:0) and α-linolenic acid (ALA; C18:3n3) were identified as the predominant fatty acids in the plant leaves [75], which was consistent with the findings of the present report. Although ALA was the only n-3 PUFA detected in the evaluated leaf meals, its proportion was higher than the n-6 PUFA represented by linoleic acid (C18:2n6c) and arachidonic acid (C20:4n6). The n-6/n-3 PUFA ratio was consistently <1 in the presently reported study, aligning with preceding reports on plant leaves [12]. While freshwater teleosts typically have low n-3 PUFA, they can convert ALA to other long-chain PUFA like eicosapentaenoic acid (EPA; C20:5n3) and docosahexaenoic acid (DHA; C22:6n3) [77, 78]. Thus, the dietary applications of terrestrial plant leaves rich in ALA may help to satisfy the EPA and DHA requirements of cultured fish [12, 79]. However, it is important to note that the dietary requirement levels of the fatty acids may not be totally fulfilled for the fish because of the low lipid contents (CL 1.23%–5.38%) of the plant leaves appraised in this study.
The findings of this study demonstrate that the aqueous extracts of the leaves present a promising source of antioxidants that can shield cells from superoxide, hydroxyl and nitric oxide radicals. The antioxidant properties associated with these extracts could offer various health benefits, including but not limited to anticoagulation, anti-inflammatory and defence against neurodegenerative disorders [13]. The ability of both standard ascorbic acid and the leaf extracts to donate hydrogen was evident through the DPPH free radical scavenging activity, which was consistent with previous studies as indicated elsewhere [80–82]. This study is in line with previous research that demonstrated the potent antioxidant properties of T. indica [83, 84]. The antioxidant properties of the leaf aqueous extracts observed in this study might suggest their potential applications in fish feeds as natural and cost-effective sources of antioxidants.
Infections caused by bacterial pathogens are of great concern for fish farming. In accordance with the present report, previous studies have documented the antimicrobial activities of T. indica leaf extracts [85–87]. T. indica and B. acuminata leaf extracts with a cumulative inhibition score of ≥10 may demonstrate potential antipathogenic activities. Additionally, the leaf aqueous extracts evaluated in this study exhibited antagonistic effects against the fish pathogenic Gram-negative bacteria. Notably, the leaves of terrestrial plants contain a considerable amount of phenolic compounds, which have the potential to hydrolyse the lipopolysaccharide layer of the bacterial cell wall, thereby impacting their viability [88, 89]. Therefore, it is conceivable that the application of crude leaf aqueous extracts with antipathogenic activities could potentially reduce the reliance on antibiotics in aquafeed production.
Optimal fish growth necessitates a protein-rich feed with a balanced amino acid profile, a high P/E ratio and low levels of ANFs [4, 10]. Given the extensive data from nutritional and ANF profiling of plant leaves, statistical techniques are crucial for simplifying high-dimensional data while preserving variability. PCA addresses this issue effectively by revealing the potential candidates who meet these criteria [90]. The PCA biplot results indicated that the ordination of leguminous plants notably differed from the other four plants. Most significantly, A. lebbeck and B. acuminata exhibited positive correlations with CP, minerals (Ca, Fe, K and Na), P/E ratio, lysine, arginine and the sulphur-containing amino acid cysteine. Both plants were negatively correlated with the ANFs (tannins, hemicellulose and cellulose), suggesting their high potential as effective, protein- and mineral-rich fish feed ingredients with abundant amino acids. Additionally, assessing the strength of linear relationships between pairs of parameters is crucial for better understanding of the ingredients to be used in feed formulation. The correlation matrix quantifies these relationships [91]. Results indicated high CP content was associated with high dry matter, P/E ratio and EAAs (histidine, lysine, arginine, cysteine and methionine), underscoring their suitability as potent feed ingredients. However, an association of the high CP content with elevated phytic acid levels might indicate the need to adopt proper deactivation or elimination strategies to maximize the benefits of these plant resources [4]. Furthermore, negative correlations between CP and both tannins and hemicellulose suggest that plants with increased levels of these antinutrients might have lower protein quality and digestibility. Overall, the results from PCA and the correlation matrix will guide the selection of plant species based on their nutritional and ANF profiling, aiding in formulating nutritious and sustainable feed for the carps [13].
5. Conclusion
The present research offers a comprehensive analysis of the basic nutritional composition and ANFs in dried leaf meals from 10 terrestrial plant species abundant in tropical and subtropical regions. The PCA plot indicated the superior nutrient profile of leguminous plants, especially A. lebbeck and B. acuminata, characterized by higher levels of CP, CL, caloric value, P/E, EAAs and minerals. Consequently, it is suggested that the leaves of these two leguminous plants could potentially serve as substitutes for the low-protein vegetal meals (e.g. rice bran, wheat bran, wheat flour, corn meal and sorghum) used in the formulation of carp diets. In addition, leaf meals derived from leguminous plants exhibited antibacterial and antioxidant properties, as well as the presence of unsaturated fatty acids. Although substantial levels of ANFs were detected in the analysed leaf meals, it is proposed that a suitable processing method (e.g. fermentation) be employed prior to incorporating the leaf meals into the diets of nonruminants (e.g. fish). The variability in the nutrient and ANFs noted among the leguminous plants studied herein and those recorded in previous literature may be attributed to diverse environmental factors, including soil composition, temperature and rainfall, as well as differences in plant age and species. The current study did not assess the seasonal variation of nutrients and ANFs in the plant leaves. Therefore, future research is needed to gather comprehensive information in this area to ensure the best possible utilization of these plant resources. Apart from nutrient profiling, a cost–benefit analysis is deemed necessary before advocating terrestrial leaf meals as a component of aquaculture feeds. Compared to conventional vegetal meals, the natural harvesting of terrestrial plant leaves may involve the least expenditure, thus potentially reducing overall feed costs. Nevertheless, further investigations are imperative to determine the optimum inclusion level of terrestrial leaf meals and the most effective substitution ratio for conventional vegetal meals in the formulation of both commercial and/or farm-made fish feeds. Subsequent studies evaluating the acceptability, digestibility, feed conversion ratio and immune and stress responses in fish fed diets containing leaf meal are also recommended before advocating their incorporation into aquafeed formulations.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Dibyendu Acharya: investigation, methodology, software, data curation, formal Analysis, validation, writing–original draft, visualization. Zulhisyam Abdul Kari: formal analysis, validation, writing–review and editing. Lee Seong Wei: conceptualization, funding acquisition, supervision, writing–review and editing. Koushik Ghosh: conceptualization, funding acquisition, data curation, project administration, supervision, validation, writing–original draft, writing–review and editing. All authors have read and agreed to the final version of the manuscript.
Funding
This work was partially funded by the Department of Science and Technology and Biotechnology, Government of West Bengal, India (Project No. ST/P/S&T/2G-33/2017). The first author received research fellowship from the University Grants Commission, New Delhi, India.
Acknowledgments
The authors are obliged to the Department of Zoology, The University of Burdwan, West Bengal, India; the Department of Science and Technology (PURSE and FIST programmes), New Delhi, India; and the Faculty of Agro-Based Industry, Universiti Malaysia Kelantan for research support. Partial funding support from the Department of Science and Technology and Biotechnology (DSTBT), Government of West Bengal, India (Project No. ST/P/S&T/2G-33/2017) is gratefully acknowledged. The first author is indebted to the DSTBT and University Grants Commission, New Delhi, India, for the research fellowship (UGC-NET).
Open Research
Data Availability Statement
The data supporting the findings of this study will be made available by the authors upon reasonable request.




