Black Soldier Fly Meal as a Gastrointestinal Tract Microbiota Remodelling Factor: A New Natural and Sustainable Source of Prebiotic Substances for Fish?
Abstract
The microbiota of the gastrointestinal tract (GIT) plays a crucial role in the development, lifespan, physiology, barrier functions and immunity against pathogens in fish. One of the significant causes of fish mortality in production systems, which also generates huge financial losses, is pathogenic dieseases. Consequently, effectively managing disruptions in the microbiota could play a crucial role in mitigating economic losses across the sector. It was investigated the effect of an alternative raw material increasing inclusion—full-fat Hermetia illucens larvae meal on the composition of the gastrointestinal microbiome of Atlantic salmon. Thus, five experimental feeds were prepared: a control diet (CON) without the addition of black soldier fly full-fat meal (BSFM) and with 30% fish meal (FM); BSFM5, with 5% addition of full-fat BSFM and 27.1% FM; BSFM10, with 10% full-fat BSFM and 24.3% FM; BSFM15, with 15% addition of full-fat BSFM and 21.3% FM; and BSFM20, with 20% addition of full-fat BSFM and 18.6% FM. After 60 days of growth experiment on Atlantic salmon presmolts, the contents of two sections of the GIT, proximal and distal, were gently sampled and subjected to next-generation sequencing (NGS) to analyse the microbial populations. No significant differences were observed in the microbial compositions of the groups, irrespective of the intestinal section analysed. In the proximal and distal intestine, the CON group exhibited the highest number of distinct phyla. Increasing the inclusion of BSFM in the diet led to a reduction in the abundance of specific phyla. Similar results were noted at genus level. The BSFM5 and BSFM10 groups showed increase number in Enterococcus, while the Lactobacillus population were maintained at a high level. Due to abovementioned changes as well as the increased share of Bacilli populations, it may be concluded that up to 20% BSFM shares may have positive microbiota-modulating effects. Thus, BSFM may be considered not only as a nutrient source but a functional feed material in fish diets also, due to its prebiotic effects observed in the study.
Summary
- •
The black soldier fly full-fat meal (BSFM) was tested as 5%, 10%, 15% and 20% of the Atlantic salmon diet.
- •
Insect meals modulated the microbiome of the Atlantic salmon gastrointestinal tract (GIT).
- •
Microbiome modulation is possible due to the prebiotic properties of chitin and the regulation of the bacterial composition related to the presence of active compounds, antimicrobial peptides (AMPs) and lauric acid.
- •
BSFM may be considered as insect meal functional in terms of fish GIT microbiota modulation.
1. Introduction
Insect-derived products are increasingly being explored as a valuable alternative feed materials in aquafeeds primarily due to their high protein content. Compared to plant-derived components, insect production is characterized by reduced greenhouse gas emissions and a lower use of water and agricultural areas [1, 2]. While insect-based feed materials appear environmentally sustainable at this stage, it is essential to consider the overall sustainability of fish farming during production. The sustainability concerns associated with traditional fish meal (FM) and fish oil in aquaculture have been repeatedly highlighted in the scientific community [2, 3], and the fish-in fish-out (FIFO) indicator was developed to verify the environmental impact of replacing these components [4]. Studies have shown improved FIFO outcomes in fish fed diets supplemented with insect-derived products across various species [5–7]. Notably, an increase in the production of feed-purpose insects is observed. According to the 2021 International Platform of Insects for Food and Feed (IPIFF) report, more than 1 billion € has been invested in this sector since its establishment, and it is expected to reach 3 billion € by 2025. The total turnover of insect feed operators is expected to exceed 2 billion € per year by the end of the decade. The economic profitability of using feed with insect-derived products was demonstrated in Siberian sturgeon (Acipenser baerii) [5], and it was at least equal to FM for European seabass (Dicentrarchus labrax) [8]. Regarding the fast development of production and postprocessing technologies, the effects of commercially available insect meals must be continuously studied, not only in terms of sustainability and zootechnical parameters but also in case of the nutriphysiological and nutrimicrobiological aspects of gastrointestinal tract (GIT) function.
The microbial communities of the GIT of fish play a vital role in various aspects such as their growth, health, immune system and defence against harmful pathogens, as highlighted by several studies [9–11]. The composition of the microbiome influences digestion, which, in the context of fish farming, impacts their growth rate and how efficiently they utilize feed [12]. One of the significant causes of fish mortality in production systems, which also generates huge financial losses, is pathogenic diseases [13]. Consequently, effectively managing disruptions in the microbiota could play a crucial role in mitigating economic losses across sector. Various strategies, including probiotics, prebiotics, synbiotics, phytogenics and microbiota transplantation, have been suggested to maintain a healthy microbiota in fish, thereby enhancing production efficiency [12].
While the microbiota greatly influences digestive processes, alimentary factors also shape the population of bacteria in the GIT. Insect-derived products are increasingly considered innovative feed materials that may affect the proportion of microorganisms inhabiting the digestive tract. This interest stems from the bioactive compounds found in insects, such as chitin, lauric acid and antimicrobial peptides (AMPs) [14]. Among the insect species used in fish nutrition, the most common research object is Hermetia illucens (black soldier fly [BSF]), and its microbiota modulation properties have been studied in various salmonid species, including Atlantic salmon (Salmo salar) [15–17], rainbow trout (Oncorhynchus mykiss) [18–20] and brown trout (Salmo trutta m. fario) [6].
Due to the importance of the use of new protein sources in aquaculture nutrition, which would be environmentally sustainable while maintaining functional value in large-scale fish production, we investigated the impact of these components on the microbiome of the GIT of Atlantic salmon (S. salar). The present study evaluated the effect of the use of increasing the inclusion of full-fat H. illucens larval meal on the composition of the gastrointestinal microbiome. We tested hypothesis that the inclusion of these meals would positively modulate the bacterial composition of the Atlantic salmon GIT.
2. Materials and Methods
2.1. Ethical Statement
The methods and procedures employed in this study adhered to the guidelines outlined in Directive 2010/63/EU of the European Parliament and the Council dated 22 September 2010 concerning the protection of animals used for scientific purposes. Additionally, they were in accordance with the Polish legislation outlined in the law of 15 January 2015 concerning the protection of animals used for scientific purposes (Polish Journal of Laws 2015, Item 266). The study also followed the ethical standards and recommendations set forth by both the National Ethics Committee for Animal Experiments and the Local Ethics Committee for Animal Experiments at Poznań University of Life Sciences. The experimentation took place in facilities certified for animal research by the National Ethics Committee for Animal Experiments (authorized by the Ministry of Science and Higher Education, approved unit no. 0091), specifically, the Laboratory of Inland Fisheries and Aquaculture within the Faculty of Veterinary Medicine and Animal Science at Poznań University of Life Sciences.
2.2. Full-Fat Insect Meal and Experimental Diets
H. illucens biomass was sourced from HiProMine S.A. in Robakowo, Poland. BSF larvae were fed a blend of plant by-products, standardized in dry matter (DM) content through the inclusion of wheat middlings [21]. The insect biomass underwent freezing at −20°C followed by drying initially at 130°C for 1 h and then at 80°C for 23 h until reaching a constant weight, using a chamber air dryer from HiProMine S.A. in Robakowo, Poland. Subsequently, the prepared insects were homogenized to a particle size of less than 0.1 mm using a beater mill to produce a full-fat meal.
The formulation of the diet is guided by the ratio of amino acids to the total protein content, utilizing the N × 6.25 conversion method [22–24]. The presence of chitin in insect biomass may lead to the overestimation of crude protein. Therefore, a calculation based on the analysis of BSFM used for the present experiment was performed. All the experimental diets were isonitrogenous, isoenergetic and isolipidic (Table 1).
| Nutrients | CON | BSFM5 | BSFM10 | BSFM15 | BSFM20 |
|---|---|---|---|---|---|
| % of DM | |||||
| Crude protein | 45.00 | 45.01 | 45.04 | 45.04 | 45.02 |
| Crude fat | 20.00 | 20.05 | 20.08 | 20.00 | 20.02 |
| Nitrogen-free extracta | 19.40 | 19.41 | 19.28 | 19.37 | 19.41 |
| Ether extract | 9.26 | 9.05 | 8.83 | 8.59 | 8.35 |
| Crude fibre | 1.53 | 1.85 | 2.18 | 2.52 | 2.85 |
| Ca | 0.97 | 0.98 | 0.99 | 1.00 | 1.00 |
| P | 0.99 | 0.96 | 0.92 | 0.89 | 0.85 |
| Gross energy (MJ/kg) | 21.77 | 21.79 | 21.79 | 21.77 | 21.78 |
| Energy/protein ratio | 48.37 | 48.41 | 48.37 | 48.34 | 48.38 |
| Amino acid profile (% of crude protein) | |||||
| Arginine | 4.14 | 4.09 | 4.03 | 3.98 | 3.92 |
| Histidine | 2.31 | 2.33 | 2.35 | 2.37 | 2.39 |
| Lysine | 4.34 | 4.33 | 4.31 | 4.29 | 4.27 |
| Tryptophane | 0.78 | 0.75 | 0.72 | 0.69 | 0.66 |
| Phenylalanine + tyrosine | 4.77 | 4.86 | 4.94 | 5.02 | 5.10 |
| Methionine + cysteine | 1.79 | 1.77 | 1.76 | 1.74 | 1.72 |
| Threonine | 3.02 | 3.03 | 3.05 | 3.06 | 3.08 |
| Leucine | 6.20 | 6.17 | 6.13 | 6.09 | 6.05 |
| Isoleucine | 3.47 | 3.45 | 3.43 | 3.42 | 3.40 |
| Valine | 4.75 | 4.75 | 4.74 | 4.74 | 4.73 |
- Abbreviations: BSFM, black soldier fly full-fat meal; BSFM5, group with addition of 5% BSFM and 27.1% of FM; BSFM10, group with addition of 10% BSFM and 24.3% of FM; BSFM15, group with addition of 15% of BSFM and 21.3% of FM; BSFM20, group with addition of 20% of BSFM and 18.6% of FM; CON, control group, without the addition of BSFM and with 30% FM; FM, fish meal.
- aNitrogen-free extract = dry matter % – (crude protein % + crude fat % + crude fibre % + ash %).
The experimental diets were formulated and processed at the Experimental Station of Feed Production Technology and Aquaculture in Muchocin, Poland, utilizing a single-screw extruder (Metalchem S-60, Gliwice, Poland). Extrusion was conducted at 90°C in the cylinder and 110°C in the head, with a screw speed of 52 rpm and a 2 mm matrix size. Following drying and removal of dust particles through screening, the pellets were coated with fish oil at 40°C according to the specified recipe. Finally, the formulated diets were packed into plastic bags and stored at –18°C for preservation.
Five experimental feeds were prepared: a control diet (CON) without the addition of BSFM (crude protein 41.2%, crude fat 16.2%) and with 30% FM (crude protein 61.8%); BSFM5, with 5% addition of full-fat BSFM and 27.1% FM; BSFM10, with 10% full-fat BSFM and 24.3% FM; BSFM15, with 15% addition of full-fat BSFM and 21.3% FM; and BSFM20, with 20% addition of full-fat BSFM and 18.6% FM. The ingredient composition of the presmolt diets is presented in Table 2.
| Ingredients (g/kg) | CON | BSFM5 | BSFM10 | BSFM15 | BSFM20 |
|---|---|---|---|---|---|
| FMa | 300 | 271 | 243 | 213 | 186 |
| BSFMb | 0 | 50 | 100 | 150 | 200 |
| Blood cellsc | 95 | 95 | 95 | 95 | 95 |
| Yeasts | 60 | 60 | 60 | 60 | 60 |
| DDGS | 50 | 50 | 50 | 50 | 50 |
| Soybean meald | 90 | 90 | 90 | 90 | 90 |
| Wheat glutene | 120 | 120 | 120 | 120 | 120 |
| Wheat meal | 120 | 109 | 97 | 88 | 75 |
| Fish oilf | 129 | 119 | 109 | 98 | 88 |
| Lecithing | 10 | 10 | 10 | 10 | 10 |
| Premixh | 15 | 15 | 15 | 15 | 15 |
| Vitamin mixi | 1 | 1 | 1 | 1 | 1 |
| Choline chloride | 2 | 2 | 2 | 2 | 2 |
| Chalk | 5 | 5 | 5 | 5 | 5 |
| TiO2 | 3 | 3 | 3 | 3 | 3 |
| Total (g) | 1000 | 1000 | 1000 | 1000 | 1000 |
- Abbreviations: BSFM, black soldier fly full-fat meal; BSFM5, group with addition of 5% BSFM and 27.1% of FM; BSFM10, group with addition of 10% BSFM and 24.3% of FM; BSFM15, group with addition of 15% of BSFM and 21.3% of FM; BSFM20, group with addition of 20% of BSFM and 18.6% of FM; CON, control group, without the addition of BSFM and with 30% FM; FM, fish meal.
- aBrown fish meal, 62% total protein, 16% fat, Agro-Fish, Kartoszyno, Poland.
- bDried black soldier fly larvae, HiProMine S.A., Poland.
- cAP 301 P, 92% total protein, APC (GB) Ltd., Ings Road, Doncaster, UK.
- dSolvent extracted 45% crude protein, 1.8% crude lipid.
- eGluvital, Cargill, Poland.
- fAgro-fish, Kartoszyno, Poland.
- gBergaPure, deoiled lecithin, 97% pure lecithin, Berg + Schmidt GmbH & Co. KG, Hamburg, Germany.
- hPremix (g kg−1): vitamin A, 1,000,000 IU; vitamin D3, 200,000 IU; vitamin E, 1.5 g; vitamin K, 0.2 g; vitamin B1, 0.05 g; vitamin B2, 0.4 g; vitamin B12, 0.001 g; nicotinic acid, 2.5 g; inositol, 35 g; D-calcium pantothenate, 1.0 g; myoinositol, 500 IU; choline chloride, 7.5 g; folic acid, 0.1 g; methionine, 150.0 g; lysine, 150.0 g; Fe, 2.5 g; Mn, 6.5 g; Cu, 0.8 g; Co, 0.04 g; Zn, 4.0 g; J, 0.008 g; carrier > 1000.0 g.
- iVitamin mix (g kg−1): vitamin A, 50,000 IU; vitamin D3, 5000 IU; vitamin E, 30.0 mg; vitamin C, 100.0 mg.
Ingredients and experimental diets were analysed according to the AOAC [25] methodology for crude protein (976.05), ether extract (920.39), crude ash (920.153) and crude fibre (985.29). The DM content was analysed according to the ISO 6496 method. The gross energy content was analysed according to the ISO 9831 method using an adiabatic bomb calorimeter (KL 12Mn, Precyzja-Bit PPHU, Poland) standardized with benzoic acid. To determine the number of macroelements (C and P), the methodology described by Ptak et al. [26] was applied. The amino acid profile was determined using an AAA-400 automatic amino acid analyser (Ingos Ltd., Prague, Czech Republic) and ninhydrin for postcolumn derivatization. Before carrying out profile analyses, hydrolysis in 6 N HCl for 24 h at 110°C was applied according to the AOAC procedure (994.12) [25]. The chemical composition and amino acid profile of the ingredients are presented in Table 3, while the results for experimental diets can be found in Table 1.
| Nutrients | CON | BSFM |
|---|---|---|
| % of dry matter | ||
| Crude protein | 41.21 | 61.80 |
| Crude fat | 16.19 | 16.50 |
| Nitrogen-free extracta | 9.54 | 4.20 |
| Ash | 11.91 | 17.50 |
| Crude fibre | 12.06 | 0.71 |
| Ca | 1.32 | 3.92 |
| P | 2.35 | 2.16 |
| Amino acid profile (% of crude protein) | ||
| Indispensable amino acids (IAAs) | ||
| Arginine | 5.47 | 6.07 |
| Histidine | 3.25 | 2.09 |
| Isoleucine | 4.73 | 4.24 |
| Leucine | 7.83 | 7.48 |
| Lysine | 6.83 | 6.63 |
| Methionine | 2.13 | 2.53 |
| Phenylalanine | 4.76 | 3.07 |
| Threonine | 4.43 | 4.10 |
| Valine | 6.80 | 5.79 |
| Dispensable amino acids (DAAs) | ||
| Alanine | 8.22 | 6.87 |
| Aspartic acid | 7.30 | 9.40 |
| Cysteine | 0.77 | 9.59 |
| Glycine | 6.15 | 6.41 |
| Glutamic acid | 13.07 | 14.50 |
| Proline | 6.68 | 4.28 |
| Serine | 4.88 | 4.17 |
| Tyrosine | 6.71 | 3.00 |
- Abbreviations: BSFM, black soldier fly full-fat meal; FM, fish meal (brown fish meal, 62% total protein, 16% fat, Agro-Fish, Kartoszyno, Poland).
- aNitrogen-free extract = dry matter % – (crude protein % + crude fat % + crude fibre % + ash %).
2.3. Animal Handling and Nutritional Trial
At the Experimental Station of Feed Production Technology and Aquaculture in Muchocin, a total of 375 Atlantic salmon (S. salar) presmolts were housed. These fish were randomly divided into five experimental groups, and the trial was conducted in three replications (tanks) per diet, each containing 25 fish, within tanks with a water capacity of 260 L. The average initial fish weight was 201.3 g. The water supply originated from a natural watercourse and was filtered through gravel-filled filters before being distributed into two equalizing tanks, each with a capacity of 5 m3. Aeration was provided using Hiblow HP200 aerators from Hiblow, Japan, and water was distributed to the tanks via a network of pipes. Water parameters, including temperature (ranging from 14.2 to 16.3°C) and oxygen concentration (ranging from 7.8 to 8.4 mg/L), were monitored daily throughout the 60-day experiment. The photoperiod was maintained at L16:D8. Feeding was automated using belt feeders (FIAP Fishtechnik GmbH, Germany), with a discharge time of 12 h. The feed ratio was based on a feeding chart designed for Atlantic salmon, considering the average body weight and the water temperature [27]. Fish mortality and health conditions were monitored daily.
2.4. Sampling
At the conclusion of the experiment, five fish per treatment were randomly chosen for sampling. Animals were euthanized using an overdose of MS-222 [28] and decapitated for dissection. The contents of both the proximal and distal sections of the GIT were carefully extracted, placed in cryotubes containing 99% ethanol and promptly stored at –80°C. These samples were preserved for subsequent analysis of microbial populations using next-generation sequencing (NGS).
2.5. Bacterial DNA Extraction and 16S rRNA Sequencing
DNA extraction was conducted using a commercial kit (Sherlock AX, A&A Biotechnology, Poland) following the manufacturer’s instructions. The samples underwent mechanical lysis on FastPrep-24 Zirconia beads (A&A Biotechnology, Poland) and were subsequently subjected to enzymatic lysis to eliminate bacteria. To confirm the presence of bacterial DNA in the samples, real-time PCR was performed using an Mx3000P thermocycler (Stratagene, USA) with SYBR Green as the fluorochrome. Universal reaction primers (1055F 5′-ATGGCTGTCGTCAGCT-3′ and 1392R 5′-ACGGGCGGTGTGTAC-3′) were employed for the amplification of 16S rDNA. The temperature conditions of the reaction were 95°C, 3 min; 95°C, 15 s; 58°C, 30 s; 72°C, 30 s; and Tm 65°C → 95°C.
DNA quantification was carried out using a NanoDrop spectrophotometer and standardized to a concentration of 5 ng/µL. The study of microbial diversity involved sequencing the amplified V3–V4 region of the 16S rRNA gene using specific primer pairs. The PCR procedure comprised an initial denaturation step at 95°C for 3 min, followed by 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, final extension at 72°C for 5 min and a holding step at 4°C. The expected size of the PCR products after amplification was approximately 550 base pairs, as confirmed by Bioanalyzer analysis. Subsequently, the PCR products were purified using AMPure XP beads.
For library preparation, the purified PCR products were combined with sequencing adapters and dual indices using the Nextera XT Index Kit (Illumina, San Diego, CA, USA) as per the 16S Metagenomic Sequencing Library Preparation protocol (Illumina, San Diego, CA, USA). PCR with Nextera XT Index Primers was performed with initial denaturation at 95°C for 3 min, followed by eight cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, final extension at 72°C for 5 min and a holding step at 4°C. The resulting libraries were cleaned once more using AMPure XP beads and validated for the expected size on a Bioanalyzer trace, which was approximately 630 base pairs for the final library. Finally, the libraries were quantified using a fluorometric quantification method with dsDNA binding dyes, and individual concentrations were determined in nanomolar (nM) units based on the size of the DNA amplicons, as analysed by an Agilent Technologies 2100 Bioanalyzer.
For sequencing, each individual library was diluted to a concentration of 4 nM, denatured using 10 mM Tris buffer (pH 8.5) and supplemented with 20% (v/v) PhiX as an internal control. Aliquots containing 5 µL of diluted DNA were combined for pooling during library preparation for MiSeq analysis (Illumina, San Diego, CA, USA). Each sample underwent sequencing to generate more than 100,000 reads.
2.6. Metagenomic Analyses
Microbiome sequences were characterized based on the V3 and V4 amplicons and analysed using a database of 16S rRNA data. To amplify and prepare the library, specific sequences 341F and 785R were utilized. The PCR reaction conditions were carried out according to the specifications provided by the manufacturer for the Q5 Hot Start High-Fidelity 2X Master Mix. Sequencing was performed on the MiSeq platform using paired-end (PE) technology at 2 × 250 nucleotides with an Illumina v2 kit. The initial data analysis was conducted on the MiSeq instrument using MiSeq Reporter (MSR) v2.6 software. This analysis involved two main stages: automatic demultiplexing of samples and the generation of fastq files containing raw reads. Subsequently, the sequencing output provided a classification of reads at various taxonomic levels including kingdom, phylum, class, order, family, genus and species. Quality analysis of the sequences was performed through stringent quality control and filtration steps to obtain high-quality sequences. Valid sequences were selected from samples based on the barcode present at both ends of the sequence and were corrected for orientation using the primer sequences. All valid and filtered sequences were then clustered into operational taxonomic units (OTUs) based on a 97% similarity threshold in the 16S rRNA gene sequence identity. These OTUs were further analysed using basic local alignment search tool (BLAST) against the Greengenes database to determine their phylogenetic classification. The results were classified at multiple taxonomic levels including phylum, family and genus. Relative abundance profiles of the proximal and distal microbiota were established based on the abundance of OTUs belonging to different taxonomic groups.
2.7. Statistical Analysis
R studio software v1.4.14 was used to analyse the data obtained in this study. To determine the normality of the data distribution and equality of variances, the Shapiro–Wilk and Bartlett’s tests were used. In the case of a normal distribution, one-way ANOVA was used. When there were significant differences between treatments, further analysis was performed by correcting for Duncan’s post hoc test. For nonnormally distributed data, the Kruskal‒Wallis test was used. When there were significant differences between treatments, further analysis was performed by correcting Dunn’s post hoc test. The data are presented as the means ± standard error of the mean (SEM). The statistical significance level was set at p < 0.05. The packages used in the statistical analyses were Agricolae, Dplyr, FSA, Ggplot2, Rcmdr, Rcompanion, UpSetR and Vegan.
3. Results
3.1. GIT Microbiota Diversity
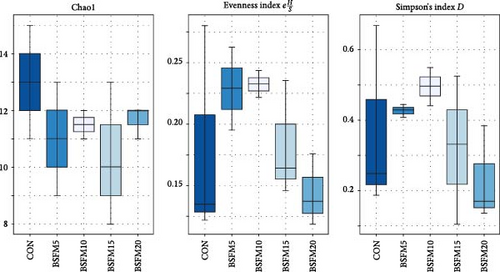
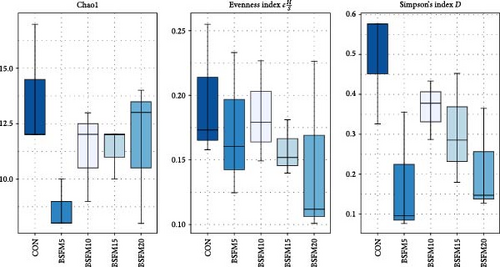
During the experiment, no mortality or health issues in fish were observed. The NGS analyses were conducted on a total of 25 samples, resulting in the generation of 2,455,779 raw sequence reads. Bacterial abundance was observed across all experimental groups, ranging from 99.92% to 100.00%. Additionally, traces of archaea were detected in the samples, comprising up to 0.02% of the total microbial composition. No significant differences (p > 0.05) were observed in the alpha diversity indices for the proximal and distal intestine microbial compositions of the groups, irrespective of the intestinal section analysed (Figures 1 and 2).
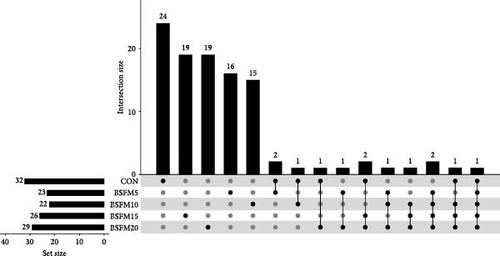
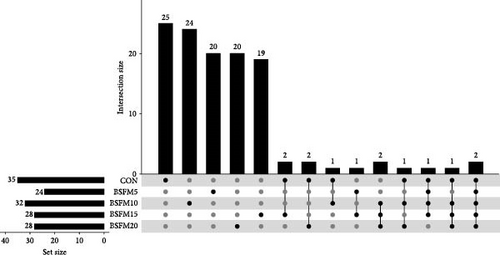
3.2. Microbial Population Intersections Among the Treatments
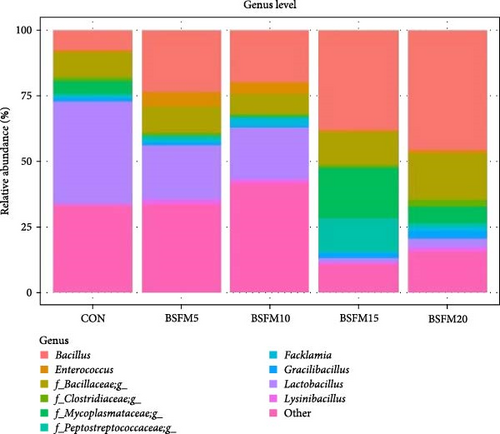
The majority of the identified OTUs are unique to specific experimental diets, with minimal overlap observed between groups. This pattern holds true for both the proximal and distal sections of the intestine. In the proximal intestine, the CON group exhibited the highest number of distinct phyla (24), while the group supplemented with 20% BSFM had 15 characteristic phyla. Similarly, in the distal intestine, the CON group displayed the highest number of specific phyla (25). Increasing the inclusion of BSFM in the diet led to a reduction in the presence of specific phyla. Only one phylum was shared among all experimental groups in the proximal region, while two phyla were shared in the distal region. Additionally, eight phyla in the proximal section and 10 phyla in the distal section were shared between the CON group and other experimental groups (Figure 3). Similar trends were observed at the family level (Figure 4), with the CON group exhibiting the largest number of specific families in both the proximal (59) and distal (51) sections. Increasing the proportion of BSFM in the diet correlated with an increase in microbiota diversity in both sections of the intestine, with a comparable effect size. Regarding genus-level analysis (Figure 5), the CON group displayed the highest number of specific microbiota in both the proximal (77) and distal (64) sections. There were 54 genera recorded for the proximal section and 55 genera for the distal section shared between the two experimental groups. In contrast, the number of genera unique to each group was lower, with 26 genera in the proximal section and 30 genera in the distal section.
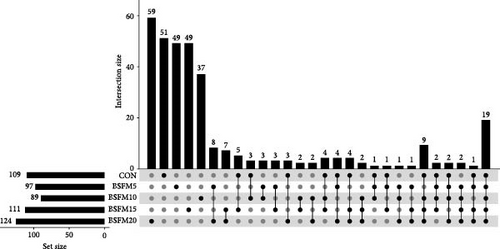
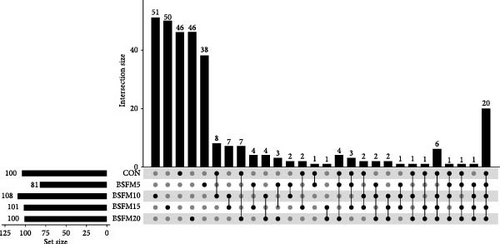
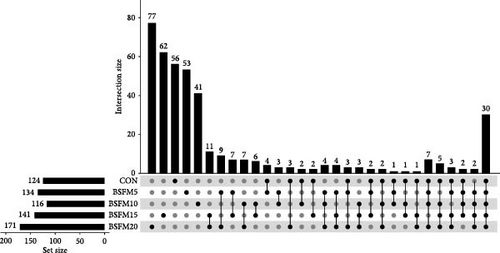
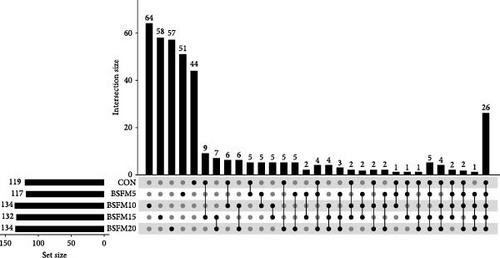
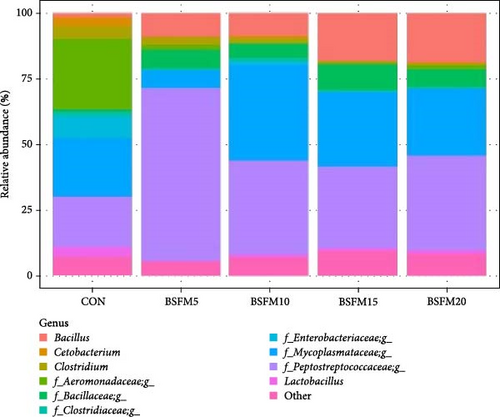
3.3. Relative Abundances of GIT Microbial Populations
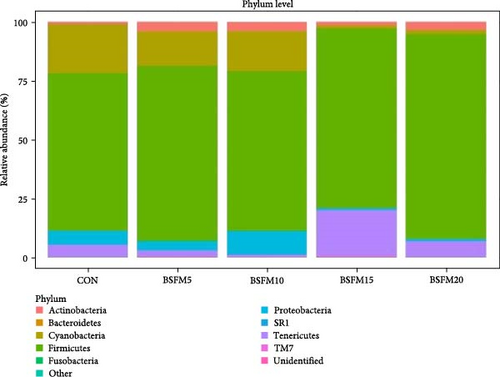
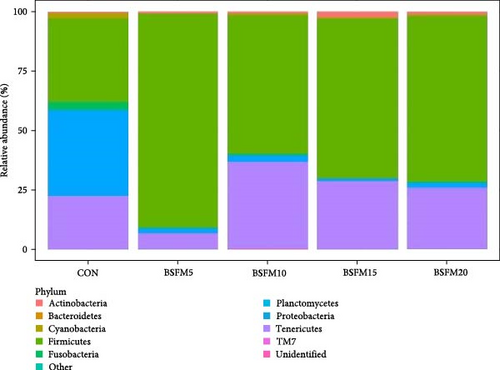
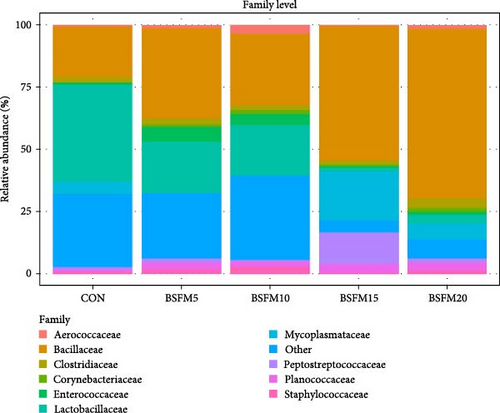
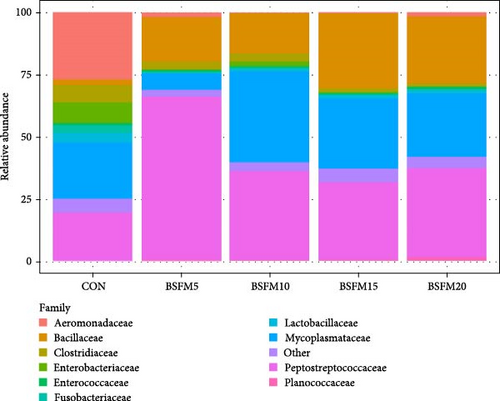
The relative abundances at three levels (phylum, family and genus) in the proximal and distal sections are presented in Figures 6–8. For the proximal region at the phylum level (Table 4), an increase in the amount of Actinobacteria was recorded in BSFM5, BSFM10 and BSFM20 (p = 0.001). Increasing insect meal inclusion led to a decrease in the Fusobacteria population (p = 0.041). At the family level, Corynebacteriaceae increased in abundance in response to the application of BSFM5, BSFM10 and BSFM20 (p < 0.001). BSFM10 showed an increase in the abundance of Staphylococcaceae. However, higher levels of Enterococcaceae were noted only in BSFM5 and BSFM10 (p = 0.042). Similar results were recorded at the genus level, where increases in Enterococcus were observed only in BSFM5 and BSFM10 (p = 0.042). In the case of Lactobacillaceae and Lactobacillus, no difference compared to CON was observed in BSFM5 and BSFM10 groups. Statistical analysis revealed no significant effect of the applied insect diets on the distal area microbiome (Table 5). Despite the lack of significant differences, Bacilliaceae family abundance increased from 20.08% in CON to 68.36% in BSFM20 treatment. while at the genus level, Bacillus increased from 7.78% in CON to 45% in BSFM20 in proximal intestine. Similarly in the distal part of the GIT at family level, numerical changes were observed in Bacillaceae which increased from 2.36% in CON to 30.06 and 27.13 in BSFM15 and BSFM20 groups, respectively. Bacillus population increased from 1.51% to 18.74 in BSFM 20. All groups with the use of BSFM resulted in Aeromonadaceae populations bellow 2%, while in CON it was 27.04%.
| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Microbiota | CON | BSFM5 | BSFM10 | BSFM15 | BSFM20 | SEM | p value |
| Phylum level | |||||||
| Actinobacteria | 1.09b | 4.06a | 3.87a | 1.68b | 3.32a | 0.37 | 0.001 |
| Bacteroidetes | 0.06 | 0.06 | 0.13 | 0.02 | 0.01 | 0.02 | 0.317 |
| Cyanobacteria | 20.41 | 14.65 | 16.94 | 1.13 | 1.89 | 3.70 | 0.051 |
| Firmicutes | 66.97 | 73.96 | 67.79 | 76.30 | 86.80 | 5.35 | 0.462 |
| Fusobacteria | 0.12bc | 0.32c | 0.02abc | <0.01ab | <0.01a | 0.05 | 0.041 |
| Other | 0.11 | 0.24 | 0.07 | 0.40 | 0.09 | 0.06 | 0.858 |
| Proteobacteria | 6.10 | 3.92 | 10.12 | 0.84 | 1.05 | 1.25 | 0.051 |
| SR1 | nr | nr | 0.0011 | nr | nr | 0.02 | 0.240 |
| Tenericutes | 5.08 | 2.74 | 0.91 | 19.59 | 6.82 | 3.33 | 0.839 |
| TM7 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | <0.01 | 0.453 |
| Unidentified | 0.03 | 0.02 | 0.03 | 0.02 | <0.01 | <0.01 | 0.473 |
| Family level | |||||||
| Aerococcaceae | 0.91 | 1.23 | 3.33 | 0.51 | 1.55 | 0.41 | 0.312 |
| Bacillaceae | 20.08 | 36.25 | 29.02 | 53.90 | 68.36 | 7.20 | 0.145 |
| Clostridiaceae | 2.01 | 2.37 | 1.77 | 1.67 | 3.67 | 0.32 | 0.238 |
| Corynebacteriaceae | 0.18b | 1.25a | 1.57a | 0.62b | 1.45a | 0.17 | <0.001 |
| Enterococcaceae | 0.76b | 5.64a | 4.60a | 0.67b | 1.44ab | 0.61 | 0.042 |
| Lactobacillaceae | 38.99a | 20.70a | 20.17a | 1.64b | 3.46ab | 6.39 | 0.042 |
| Mycoplasmataceae | 5.00 | <0.01 | 0.15 | 19.43 | 6.34 | 3.41 | 0.198 |
| Other | 20.23 | 14.65 | 16.91 | 1.02 | 1.81 | 3.70 | 0.051 |
| Peptostreptococcaceae | 0.72 | 1.44 | 0.54 | 12.68 | 1.37 | 2.81 | 0.339 |
| Planococcaceae | 2.76 | 2.76 | 2.15 | 3.30 | 3.36 | 0.49 | 0.592 |
| Staphylococcaceae | 0.80c | 1.81b | 2.82a | 0.60c | 1.37bc | 0.23 | 0.002 |
| Genus level | |||||||
| Bacillus | 7.78 | 23.58 | 19.93 | 38.13 | 45.66 | 5.29 | 0.083 |
| Enterococcus | 0.73b | 5.49a | 4.30a | 0.63b | 1.34ab | 0.58 | 0.042 |
| Bacillaceae_g | 9.38 | 10.02 | 7.64 | 12.57 | 17.51 | 2.23 | 0.229 |
| Clostridiaceae_g | 1.46 | 1.20 | 1.04 | 1.01 | 2.65 | 0.23 | 0.073 |
| Mycoplasmataceae_g | 5.00 | <0.01 | 0.15 | 19.43 | 6.34 | 3.41 | 0.198 |
| Peptostreptococcaceae_g | 0.71 | 1.40 | 0.26 | 12.64 | 1.34 | 2.80 | 0.228 |
| Facklamia | 0.86 | 1.18 | 3.26 | 0.48 | 1.50 | 0.40 | 0.312 |
| Gracilibacillus | 1.29 | 0.94 | 0.38 | 1.85 | 2.99 | 0.40 | 0.311 |
| Lactobacillus | 38.93a | 20.62a | 19.92a | 1.54b | 3.36ab | 6.40 | 0.042 |
| Lysinibacillus | 0.49 | 1.92 | 1.29 | 0.91 | 1.65 | 0.20 | 0.132 |
| Other | 33.37 | 33.64 | 41.83 | 10.81 | 15.66 | 6.18 | 0.103 |
- Abbreviations: BSFM, black soldier fly full-fat meal; BSFM5, group with addition of 5% BSFM and 27.1% of FM; BSFM10, group with addition of 10% BSFM and 24.3% of FM; BSFM15, group with addition of 15% of BSFM and 21.3% of FM; BSFM20, group with addition of 20% of BSFM and 18.6% of FM; CON, control group, without the addition of BSFM and with 30% FM; FM, fish meal; nr, not recorded.
- a,b,cValues within a row with different superscript letters differ significantly at p < 0.05. Values are presented as the means (n = 5/treatment), pooled standard error of the mean (SEM).
| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Microbiota | CON | BSFM5 | BSFM10 | BSFM15 | BSFM20 | SEM | p value |
| Phylum level | |||||||
| Actinobacteria | 0.52 | 0.77 | 0.74 | 2.35 | 1.24 | 0.39 | 0.867 |
| Chlamydiae | <0.01 | nr | 0.01 | 0.01 | <0.01 | <0.01 | 0.380 |
| Cyanobacteria | 2.40 | 0.18 | 0.82 | 0.56 | 0.63 | 0.32 | 0.287 |
| Firmicutes | 34.85 | 89.67 | 58.17 | 67.21 | 69.45 | 8.18 | 0.207 |
| Other | 0.12 | 0.09 | 0.17 | 0.14 | 0.06 | 0.03 | 0.675 |
| Planctomycetes | 0.03 | nr | 0.01 | <0.01 | 0.01 | 0.01 | 0.167 |
| Proteobacteria | 36.35 | 2.03 | 2.80 | 0.91 | 2.09 | 5.34 | 0.071 |
| Tenericutes | 22.27 | 6.84 | 36.62 | 28.73 | 25.86 | 7.61 | 0.406 |
| TM7 | 0.02 | <0.01 | 0.01 | <0.01 | 0.01 | <0.01 | 0.452 |
| Unidentified | 3.43 | 0.41 | 0.65 | 0.07 | 0.64 | 0.27 | 0.654 |
| Family level | |||||||
| Aeromonadaceae | 27.04 | 1.81 | 0.36 | 0.43 | 1.49 | 5.29 | 0.443 |
| Bacillaceae | 2.36 | 17.74 | 15.94 | 30.06 | 27.13 | 4.94 | 0.180 |
| Clostridiaceae | 6.81 | 3.51 | 3.25 | 1.57 | 1.29 | 1.10 | 0.633 |
| Enterobacteriaceae | 8.29 | 0.16 | 2.14 | 0.24 | 0.06 | 1.41 | 0.051 |
| Enterococcaceae | 0.87 | 0.58 | 0.52 | 0.84 | 0.74 | 0.17 | 0.994 |
| Fusobacteriaceae | 3.39 | 0.41 | 0.62 | 0.07 | 0.63 | 0.67 | 0.566 |
| Lactobacillaceae | 3.74 | 0.41 | 1.02 | 1.33 | 1.37 | 0.62 | 0.411 |
| Mycoplasmataceae | 22.24 | 6.66 | 36.43 | 28.40 | 25.50 | 7.64 | 0.406 |
| Other | 2.33 | 0.18 | 0.74 | 0.54 | 0.34 | 0.31 | 0.220 |
| Peptostreptococcaceae | 19.37 | 65.73 | 35.83 | 30.85 | 36.09 | 6.94 | 0.367 |
| Planococcaceae | 0.12 | 0.51 | 0.46 | 0.86 | 1.50 | 0.25 | 0.223 |
| Genus level | |||||||
| Bacillus | 1.51 | 8.86 | 8.78 | 18.23 | 18.74 | 3.28 | 0.228 |
| Cetobacterium | 3.39 | 0.41 | 0.62 | 0.07 | 0.63 | 0.67 | 0.566 |
| Clostridium | 4.84 | 2.93 | 1.92 | 0.76 | 0.56 | 1.01 | 0.598 |
| Aeromonadaceae_g | 27.04 | 1.81 | 0.36 | 0.43 | 1.49 | 5.29 | 0.443 |
| Bacillaceae_g | 0.68 | 7.25 | 5.62 | 9.76 | 6.61 | 1.48 | 0.165 |
| Clostridiaceae_g | 1.82 | 0.51 | 1.20 | 0.71 | 0.67 | 0.23 | 0.586 |
| Enterobacteriaceae_g | 8.22 | 0.15 | 1.21 | 0.19 | 0.05 | 1.42 | 0.335 |
| Mycoplasmataceae_g | 22.24 | 6.66 | 36.43 | 28.40 | 25.50 | 7.64 | 0.406 |
| Peptostreptococcaceae_g | 19.06 | 65.45 | 35.73 | 30.75 | 35.94 | 6.93 | 0.367 |
| Lactobacillus | 3.67 | 0.40 | 1.00 | 1.28 | 1.34 | 0.61 | 0.411 |
| Other | 7.53 | 5.57 | 7.13 | 9.42 | 8.47 | 4.83 | 0.214 |
- Abbreviations: BSFM, black soldier fly full-fat meal; BSFM5, group with addition of 5% BSFM and 27.1% of FM; BSFM10, group with addition of 10% BSFM and 24.3% of FM; BSFM15, group with addition of 15% of BSFM and 21.3% of FM; BSFM20, group with addition of 20% of BSFM and 18.6% of FM; CON, control group, without the addition of BSFM and with 30% FM; FM, fish meal.
- a,b,cValues within a row with different superscript letters differ significantly at p < 0.05. Values are presented as the means (n = 5/treatment), pooled standard error of the mean (SEM).
4. Discussion
This results confirm the hypothesis of positive microbiota modification by dietary application of BSFM into the diet of Atlantic salmon by propagation of Bacillus and Enterococcus strains. However, the observed effects of BSFM use on microbial populations have wide spectrum with no negative effects observed. This may be an indication for the use of BSFM not only as protein reach feed component but also as functional part of the diet for beneficial microbiota modulation. The findings revealed that two major phyla, Firmicutes and Cyanobacteria, dominated the microbiota of the proximal intestine across all experimental groups, constituting over 77% of the total microbial population. Additionally, Actinobacteria, Proteobacteria and Tenericutes were among the next most abundant taxa. These taxa largely align with the primary microbiota described in fish, as previously outlined by Ghanbari et al. [29]. However, clusters of Cyanobacteria and Tenericutes are less commonly characterized.
At the phylum level, the microbiota of the proximal intestine did not exhibit significant differentiation between the experimental groups. Nonetheless, a decrease in Fusobacteria concentration was observed in the BSFM20 group, while the percentage of Actinobacteria in the microbiome increased in groups with 5%, 10% and 20% BSFM inclusion. This increase in Actinobacteria population has been previously reported in Atlantic salmon [15, 17] and rainbow trout [18, 30]. At the family level, a decrease in the concentration of Lactobacillaceae was noted in the BSFM15 group. The introduction of BSFM at 5%, 10% and 20% levels in the feed had an impact on the microbiota, resulting in an increased abundance of Corynebacteriaceae, with a similar effect observed in the Enterococcaceae population, particularly in the 5% and 10% BSFM groups. Noteworthy was the observed numerical increase in the Bacillaceae population, although not statistically significant, indicating a significant biological effect, especially in the group with 20% BSFM. This trend was evident at the genus level, where the Bacillus group exhibited the highest abundance in the BSFM20 group. Moreover, BSFM5 and BSFM10 led to an increase in Enterococcus concentration, while BSFM15 caused a decrease in the Lactobacillus population.
In the distal part of the intestine, the main phyla were Firmicutes, Proteobacteria and Tenericutes, which accounted for more than 93% of the total population. The microbiota in this section was characterized by decreased diversity within the phyla. A numerical reduction in Cyanobacteria was also observed compared to the proximal section. Bacillaceae concentration upscaling was also observed in fish fed diets supplemented with increasing amounts of insect meal. These groups were also characterized by a decrease in the Aeromonadaceae population. The Mycoplasmataceae population was the lowest in the BSFM5 group, but the highest value of Peptostreptococcaceae was observed in this group, which was also noted at the genus level.
The alterations observed in the gut microbiota of fish fed diets containing BSFM can be attributed to the composition of the feed. This composition induces changes in the microbial population or microbes closely associated with the environment inhabited by the feed [17]. However, the majority of bacteria are unable to withstand the extreme environmental conditions, such as high temperatures and pressures, encountered during blanching, drying, extrusion and other postprocessing treatments. Microorganisms that can form spores have the greatest chance of surviving in unfavourable environmental conditions. This group included Bacillus [31], the concentration of which increased in both sections. Notably, these findings were confirmed by Li et al. [15], where the BSFM diet (14.75% inclusion) increased the relative abundance of this genus. The observed increase in the population of Enterococcus and Lactobacillus bacteria in the proximal intestine was a positive effect of the use of 5% and 10% BSFM in salmon feed. The Enterococcus genus is characterized by the presence of lactic acid bacteria (LAB) with antipathogenic properties [32]. Earlier studies on rainbow trout have validated that incorporating 20% BSFM in the diet leads to an elevation in the proportion of the Enterococcus/Lactobacillus group within the GIT microbiota [19]. All three groups of LAB, which were primarily increased in the proximal part of the intestine (Lactobacillus, Enterococcus and Bacillus), are characterized by probiotic properties and the potential for biocontrol maintenance in aquaculture [33]. The primary mechanism underlying the beneficial effects of LAB on the GIT microbiome is the production of bactericidal substances. These substances play a crucial role in inhibiting the growth of pathogenic bacteria and outcompeting pathogens for attachment to the intestinal epithelium. Additionally, they contribute to the preservation of intestinal balance by modulating immune pathways, resulting in the activation of local immune responses and enhancement of epithelial cell viability [34]. The observed growth of probiotic bacteria may have a significant impact on the reduced abundance of potentially pathogenic Aeromonadaceae. This effect was observed in the proximal intestine in fish fed a diet with BSFM, regardless of the inclusion level. The inhibition of pathogenic bacterial populations, such as Aeromonas salmonicida, by Bacillus and Lactobacillus species was reported previously in Atlantic salmon microbiota [35].
Based on the presented results, the authors of the paper investigated the causes of the modulatory effect of the gastrointestinal microbiota on the prebiotic properties of insect-derived products. Depending on the definition used, various criteria may be applied for identifying additives or feed materials as prebiotics. Probiotic may stimulate the growth of bacteria residing in the digestive tract, but any compound, substrate or nutrient that has nutritional effects on microorganisms in the GIT can also be called a prebiotic [36]. The unique chemical compounds, for example, chitin, lauric acid and AMPs, may explain the potential prebiotic insect meal mode of action.
The scientific literature concerning the content of AMPs in insect-derived products is limited. While research acknowledges their presence, the challenge lies in accurately determining AMPs, which hinders screening analyses. Additionally, there is a scarcity of information regarding the utilization of AMPs as functional additives in salmonid nutrition. Nonetheless, insights into their nutritional value can be inferred from data available for other species. The use of cecropin in turbot (Scophthalmus maximus) had a positive effect on growth performance and feed utilization and effectively mitigated intestinal enteritis in juveniles [37]. Ge et al. [38] reported that the inclusion of APSH-07 in a yellow croaker (Larimichthys crocea) diet had a positive effect on growth performance and stress reduction. The scientific literature emphasizes that these effects may be the result of a more favourable GIT microbiota occurring with AMP inclusion. For the lipid fraction, the fatty acid composition of BSF has been well studied, and it contains approximately 40% lauric acid [39]. However, the level can reach 52% [40]. Numerous studies described the antibacterial, antifungal [41] and antiviral [42] properties of this fatty acid. Do Couto et al. [43] showed that lauric acid reduced the growth of Aeromonas hydrophila and Ichthyophthirius multifiliis, which are common pathogens of fish.
Chitin, found abundantly in the exoskeletons of insects, is chemically classified as a dietary fibre component. Its notable feature is its strong affinity for water and its capacity to create bonds with other compounds, such as proteins. This bonding propensity might potentially diminish the nutritional efficacy of feeds containing insect meals [44]. It is possible to avoid the negative nutritional effect of this feature via the previously described application of the corrected Kp. Li et al. [15] suggested that the presence of chitin selectively promoted the growth of certain bacteria. Notably, many species from the Bacillus genus produce chitinase [45]. This statement is supported by the fact that Atlantic salmon fed feed containing 5% chitin were characterized by a high occurrence of Bacillus and Lactobacillus in the intestinal mucosa, and Bacillus exhibited the highest in vitro chitinase activity [35].
The interaction among these bioactive compounds likely underlies the observed changes highlighted in this study and corroborated by previous scientific findings. The ongoing expansion of large-scale insect cultivation offers a platform to further explore the functional attributes of these insects and integrate insect-derived products into animal diets as a sustainable alternative to environmentally detrimental raw materials.
5. Conclusions
This study affirms that incorporating BSFM into the diet of Atlantic salmon leads to modulation of the gastrointestinal microbiota. For the first time in the scientific literature we described the mechanism of BSFM-induced GIT microbiota structural modification by propagation of Bacillus and Enterococcus strains. The possible mode of action of this phenomenon may be attributed to presence of chitin, lauric acid and AMPs in this innovative feed material. It may be concluded that BSF meal ensured high representation of all the above-considered probiotic populations; thus, it may be considered as optimal insect meal dietary inclusion. Moreover it is worth considering to apply BSFM not only as nutrient source but also functional feed material in fish diets.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research was supported by the National Centre for Research and Development, no. POIR.01.01.01-00-0828/15, titled InnSecta: Innovative Technology of Feedstuffs Production Based on Insect Biomass, and the funds of Poznań University of Life Sciences. The publication was financed by the Polish Minister of Science and Higher Education as part of the Strategy of the Poznań University of Life Sciences for 2024−2026 in the field of improving scientific research and development work in priority research areas.
Acknowledgments
We gratefully acknowledge the contributions and logistical support provided by Poznań University of Life Sciences. The authors thank Silvia Nogales-Mérida, Krzysztof Florczyk and Jan Banaszak for their contribution to the technical parts of the insect meal and feed preparation, the growth experiment and the data analysis.
Open Research
Data Availability Statement
The data will be made available upon request.




