Effects of Dietary Choline Chloride on Growth Performance, Antioxidant Properties, and Intestinal Function of Juvenile Bighead Carp (Aristichthys nobilis)
Abstract
Choline, an essential nutrient for aquatic animals, plays an important role in fish growth performance and health, but its effect on bighead carp is not yet known. To investigate the effects of choline chloride on growth performance, antioxidant properties, and intestinal function of juvenile bighead carp (Aristichthys nobilis), fish with an average weight of 1.77 ± 0.20 g were fed with different dietary choline chloride supplementation concentrations: 0 g/kg (control), 2, 4, 6, 8, and 10 g/kg for 66 days. The results indicated that dietary choline chloride supplementation at 6 g/kg improved the weight gain rate (WGR) and specific growth rate (SGR) (p < 0.05). Dietary choline chloride supplementation (4–10 g/kg) decreased serum in serum triglycerides (TGs), total cholesterol (T-CHO), and glucose (GLU) levels of juvenile bighead carp (p < 0.05). The malondialdehyde (MDA) level was significantly lower than the control group when choline chloride supplementation ranged from 6 to 10 g/kg. When choline chloride supplementation was at 6 g/kg, the activities of catalase (CAT) and superoxide dismutase (SOD) were significantly higher than the control group (p < 0.05). The levels of choline chloride supplementation (6–10 g/kg) increased the activity of intestinal digestive enzymes and enhanced the intestinal digestibility of juvenile bighead carp (p < 0.05). In addition, choline chloride levels did not affect the abundance of intestinal microbiota, and the composition of the dominant phylum genera was similar, including Ascomycetes, Actinobacteria, and thick-walled bacteria. At the genus level, there are Gemmobacter, ZOR0006, Peredibacter, and Mycobacterium, respectively. Overall, choline chloride supplementation (4–10 g/kg) could significantly improve the growth performance, antioxidant capacity, and digestive enzyme activity of juvenile bighead carp. Furthermore, broken-line regression analysis has identified 6.51 and 6.62 g/kg as the optimal levels of dietary choline chloride for juvenile bighead carp based on growth performance. Overall, appropriate dietary choline chloride could improve growth performance, antioxidant capacity, and intestinal function in bighead carp.
1. Introduction
Choline, also known as vitamin B4 [1], is essential for maintaining normal growth and development in animals. Choline, methionine, and betaine all have the role of providing methyl groups, of which choline is the most important methyl donor in the organism. Choline can be oxidized to betaine, which is then converted to methionine [2], thereby increasing the amount of essential amino acids and promoting growth.
More specifically, choline has been suggested to promote fish growth, as it has been reported that juvenile Perca flavescens [3], new Juvenile hybrid tilapia (Oreochromis niloticus × O. aureus) [4], Ctenopharyngodon Idella [5], Oreochromis mossambicus [6], and juvenile Seriola lalandi [7] had choline requirements of 0.60, 1.00, 1.14, 0.40, and 0.75 g/kg, respectively. The reason for this is that dietary choline chloride supplementation improves growth performance by increasing feed intake, liver capacity for metabolism and antioxidant function, and thus improving liver health. Additionally, the choline requirement of adult fish is lower than that of juvenile fish, as adult fish have fully developed organs and systems and can synthesize a small amount of choline [2]. However, when diets are deficient in methyl, the rate of choline synthesis is insufficient to meet the metabolic requirements of animals, including juvenile fish [8]. A deficiency of choline in the diet results in some adverse effects such as stunted growth, low feed efficiency, impaired fat metabolism and intestinal and renal hemorrhage in fish [2] which have been reported in red drum (Sciaenops ocellatus) [9], yellow perch (P. flavescens) [3], speckled catfish (Pangasius hypophthalmus) [10] hybrid tilapia (new jiffy tilapia × O. aureus) [4], and cobia (Rachycentron canadum) [11].
Choline is a precursor for the synthesis of phosphatidylcholine, nerve sphingomyelin, and carboxaldehyde phospholipids, which form phospholipids in the body that facilitate fat transport and prevent excessive fat deposition. This process is beneficial in the treatment of fatty liver and cirrhosis [12]. The administration of choline chloride-containing bait to Indian carp resulted in a notable decline in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, which implies an improvement in liver function [13]. Moreover, choline has the potential to avert oxidative stress by curbing the formation of reactive oxygen species (ROS) and enhancing the antioxidant capacity.
There is a paucity of published studies investigating the impact of choline on intestinal digestion and absorption in fish. The gut microbiota is regarded as a significant determinant of host health and nutrition [14]. Fish host provides shelter for the gut bacteria, which play an important role in the growth and development, nutrient absorption, and immunity of the host [15]. Some studies have demonstrated that fish gut microbes play a significant role in the digestive and absorptive processes, and are capable of producing various types of enzymes that cannot be synthesized by fish themselves [16] and the diversity and abundance of the gut bacteria of fish are influenced by the culture environment, nutritional type/dietary mode, and host species [17]. Whether choline affects fish microbial ecosystems has not been reported.
The bighead carp (Aristichthys nobilis) is one of the “four major Chinese carps” and also constitutes a significant freshwater economic fish species in China. As indicated in the 2023 China Fisheries Statistics Yearbook, the production of bighead carp reaches 3.3 million tonnes. Despite the extensive research on the nutritional requirements of choline for aquaculture species, there is a notable scarcity of studies examining the precise choline requirements for juvenile bighead carp. The objective of this study was to assess the effect of different levels of choline chloride on growth, serum biochemistry, immunological and intestinal enzyme activities, and gut microbiota in juvenile bighead carp, with a view to determining the dietary choline requirement.
2. Materials and Methods
2.1. Compound Feed Preparation
In the present study, a unidirectional gradient approach was utilized to formulate six distinct feeds, each containing varying concentrations (0.00%, 0.20%, 0.40%, 0.60%, 0.80%, and 1.00%) of choline chloride at a purity of 50%. The protein was derived from soybean meal, fish meal, and cotton meal, and the fat component consisted of an equimolar blend of soybean and fish oils. The experimental feed formulations and nutritional compositions are shown in Table 1. Each raw material over 40 mesh and trace ingredients were added using a step-by-step expansion method to ensure thorough mixing. The majority of the raw materials were mixed in a stepwise manner, adding fish oil and water, using a twin-screw extrusion machine into 2 mm diameter particles, natural air-drying crushed into powdered particles, and stored at −20°C for future use.
| Item | Dietary choline chloride added levels (g/kg) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 2.00 | 4.00 | 6.00 | 8.00 | 10.00 | |
| Ingredients (%) | ||||||
| Soybean meal | 36.50 | 36.50 | 36.50 | 36.50 | 36.50 | 36.50 |
| Fish meal | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| flour | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 |
| Cotton meal | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Mineral premixa | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premixb | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soybean oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Corn oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cellulose powder | 1.00 | 0.80 | 0.60 | 0.40 | 0.20 | 0.00 |
| Choline chloride | 0.00 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 |
| Calcium dihydrogen phosphate | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition (%) | ||||||
| Moisture | 8.56 | 7.48 | 7.57 | 7.37 | 8.63 | 8.41 |
| Crude protein | 43.57 | 43.02 | 43.08 | 43.21 | 43.49 | 43.48 |
| Crude lipid | 6.61 | 7.18 | 7.21 | 7.01 | 7.17 | 7.17 |
| Ash | 9.37 | 9.53 | 9.43 | 9.59 | 9.50 | 9.67 |
- aMineral premix (g/kg): MgSO4, 20 g; FeSO4·7H2O, 20 g; MnSO4·H2O, 2 g; CuSO4·5H2O, 4 g; Kal(SO4)2·12H2O, 6 g; ZnSO4·7H2O, 54 g; KCl, 64 g; CoCl2·6H2O, 0.4 g; KI, 0.6 g.
- bVitamin premix (g/kg): VA, 1.47 g; VD3, 0.022 g; VK, 8.9 g; VE, 44.5 g; VB1, 17.8 g; VB2, 17.8 g; VB6, 17.8 g; VB12, 0.018 g; biotin, 0.089 g; folic acid, 4.45 g; niacin, 89.12 g; Ca-D-pantothenate, 44.5 g; inositol, 89.12 g; cellulose, 575.05 g.
2.2. Management of Breeding Experiments
Experimental bighead carp were procured from Huanggang City, Hubei Province. Prior to the commencement of the experiment, fish underwent sterilization using a 30 mL/m3 concentration of povidone–iodine and were subsequently transferred to an indoor staging tank (2.00 × 1.50 × 1.50 m), where they were provided with a control diet for a duration of 2 weeks. A total of 900 healthy and well-specified fish with an initial body weight of (1.77 ± 0.20 g) were selected and randomly reared in 18 culture buckets (diameter 80.00 cm, height 60.00 cm), divided into six groups, with three replicates in each group and 50 fish in each replicate, and fed with the corresponding experimental diets.
During the experimental period, the feeding trials were conducted in a recirculating water culture system with aerators aerated to supplement the dissolved oxygen in the water, and the water used for aquaculture was aerated tap water, which conformed to the relevant provisions of the Standard Requirements for Fishery Water Quality of China (GB11607-1989). The water temperature was approximately 26–28°C, ammonia nitrogen was lower than 0.05 mg/L, nitrite was lower than 0.01 mg/L, and feeding was done twice a day (8:00 and 17:00). The overfeeding method was employed to ensure that the bighead carp reaches 80% of their full capacity. The amount of feeding was adjusted according to the weather and feeding conditions, and removal of feces was carried out by siphoning before feeding to prevent bighead carp from accidentally consuming feces during feeding. After 1 h of feeding, any remaining bait was extracted by the same method. The water was changed once or twice a day, according to its quality, with approximately 50% of the total volume replaced. The experimental period lasted 66 days.
2.3. Sample Collection
Once the experiment was complete, after 24 h of starvation, the number, final mass, and body length of fish in each bucket were recorded separately. Growth performance parameters, such as weight gain rate (WGR), specific growth rate (SGR), and carcass condition (condition factor [CF]), were further calculated. Five whole fish were retained from each bucket for body composition analysis. Ten fish from each bucket were dissected and weighed to calculate the viscerosomatic index (VSI) and liver body index (HSI). Blood was collected from all fish in each bucket by venipuncture using a sterile 1 mL syringe and stored in 1.5 mL centrifuge tubes, stored in a refrigerator at 4°C for 4 h, then centrifuged at 1382 × g for 10 min at 4°C in a tabletop high-speed centrifuge (TGL-20M). Subsequently, the supernatant was extracted and stored for the determination of serum biochemical and antioxidant indices. Five fish from each bucket were randomly dissected for the intestinal tissues, and then the intestines were rinsed with deionized water at 4°C and placed in 2 mL freezing tubes for the determination of intestinal digestive enzyme activity. Intestinal contents were collected from all fish in each bucket for analysis of the effect of choline chloride on intestinal microbes. The whole fish, serum, gut tissues, and gut contents collected were stored in a refrigerator at −80°C for preservation.
2.4. Index Analysis
2.4.1. Nutritional Composition
The nutritional composition of whole fish and feed was determined according to the method provided by the Association of Official Analytical Chemists [18]. After drying the water on the surface of the fish body and weighing it, it was placed in an electrically heated constant-temperature blast drying oven (DHG-9140A) and dried at 65°C, then it was taken out, weighed, and recorded, pulverized with a micro pulverizer, divided into sample bags and labeled well, and stored in a refrigerator at −20°C to be measured. Moisture was determined by the constant weight method at 105°C: first, the aluminum box was dried (1 h) in an oven (105 ± 2°C) and weighed, then the fish body and feed were put into the aluminum box and weighed, and dried (3 h) at the same temperature, weighed, and calculated. Crude protein was determined by the Kjeldahl method: the crude protein content was determined by Kjeldahl method: (1) weighing and digestion: weigh 0.5 g of sample, 3 g of mixed catalyst about, 10 mL of sulfuric acid into the Kjeldahl bottle, heating and digestion in the digestion furnace; (2) distillation: in the conical flask, add 10 mL sample decomposition solution and 40% sodium hydroxide dissolved in 10 mL distillation for 5 min; (3) titration: in the burette injected with 0.05 mol/L of the standard solution of HCI, with the prepared boric acid solution for titration, so that the solution from blue–green to gray–red. Crude fat was determined by the ether extraction method: weigh 1 g of the sample wrappedin a filter paper tube, put into the oven at 105°C drying (3 h), put into the extraction tube, add 80–100 mL of anhydrous ether extraction, so that the ether reflux, reflux about 60 times, and so the fat extraction is clean and removed from the filter paper tube to dry, and then put into the oven to dry for 2 h (105 ± 2°C), cooling and weighing, and calculation. And ash was determined by the burning to constant weight method at 550°C: put the crucible into the muffle furnace, burning 30 min (550 ± 20°C), then put into the desiccator to cool, weighing, in the crucible into 1 g or so of the sample, and then put into the muffle furnace burning (550 ± 20°C) for 3 h, cooling and weighing, record the data for calculation.
2.4.2. Haematological Characteristics
The serum samples were thawed at 4°C, and the following parameters were determined: total protein (TP), albumin (ALB), AST, ALT, alkaline phosphatase (ALP), total cholesterol (T-CHO), triglyceride (TG), and glucose (GLU) were determined by an automated biochemical analyzer (BX-3010, Sysmex, Tokyo, Japan), two parallels per bucket, a total of six parallels in a group, 200 μl of serum per tube was placed in the serum biochemistry instrument for determination. The reagents utilized were purchased from Sysmex Corporation (Tokyo, Japan).
2.4.3. Serum Antioxidant Indices
Serum samples were thawed at 4°C, and then blood samples from three barrels of fish per group were pooled to minimize variation, and then the antioxidant index was determined. The activity of superoxide dismutase (SOD) (SOD: A001-3-2) was determined by the WST-1 method, add 20 µl of the serum to be tested, then sequentially add 20 µl of enzyme working solution and substrate application solution (configured in advance according to the instructions), and then incubate at 37°C for 20 min before reading at 450 nm. Catalase (CAT) (CAT: A007-1-1) activity by the ammonium molybdate method, add 100 µl of serum to be tested, then add 1 mL of reagent I and 100 µl of reagent II sequentially, time the addition of reagent II and mix immediately, react accurately at 37°C for 1 min, then immediately add 1 mL of reagent III, followed by the addition of reagent IV and mixing, and then read at 405 nm. Malondialdehyde (MDA) activity by the thiobarbituric acid (TBA) method, add 100 µl of serum to be tested, then sequentially add 4 mL of working solution I mixing, 95°C water bath for 40 min, remove the water cooling, then 3500–4000 rpm, centrifugation for 10 min, take the supernatant at 532 nm to measure absorbance values. All kits were produced by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Refer to the instruction manual of the kit for the determination procedure, principle, and calculation formula.
2.4.4. Intestinal Digestive Enzyme Activity
Digestive enzyme activities include amylase, lipase, and trypsin activities, respectively. Amylase activity was determined by iodine–amylase colorimetric assay (C016-1-2). Intestinal tissue was thawed, and a saline solution was added in a 1:9 ratio (weight:volume) to make a 10% tissue homogenate, which was mechanically homogenized under ice-bath conditions at 3500 rpm/min and centrifuged for 10 min. Then take 100 µl of the supernatant of intestinal tissue homogenate in a test tube, add 0.5 mL of substrate buffer that has been prewarmed at 37°C for 5 min and mix well, and react accurately at 37°C for 7.5 min, and then add 0.5 mL of iodine application solution and 3 mL of double-distilled water, and then mix well and measure the OD value at the wavelength of 660 nm. Lipase activity by chemical turbidimetric assay (A054-1-1), intestinal tissue, and saline solution were added in a 1:4 ratio (weight:volume) to make a 20% tissue homogenate, which was mechanically homogenized under ice-bath conditions at 2500 rpm and centrifuged for 10 min. Firstly, add 25 µl of supernatant of intestinal tissue homogenate into the test tube, then add 25 µl of reagent IV, absorb 2 mL of prewarmed substrate buffer into the test tube, mix it quickly and time it, pour it into the cuvette quickly, turbidimetrically measure it at 420 nm and read the absorbance value of A1 in 30 s, then pour this colorimetric solution into the original test tube and set it into the accurate water bath at 37°C for 10 min, pour it quickly into the cuvette and read the absorbance value A2 at 10 min and 30 s, and find out the difference of absorbance between the two times. Trypsin (A080-2-2) activity is determined by hydrolysis of the substrate, which has a distinct absorption peak at a specific wavelength. Intestinal tissues were mechanically homogenized with homogenization medium (included in the kit) according to the weight–volume ratio of 1:9 in an ice bath, centrifuged at 2500 rpm for 10 min, 15 µl of supernatant was added into the substrate application solution (prepared and preheated in advance), the absorbance OD was measured at 253 nm after 20 min in a water bath at 37°C was noted as A1, then the reaction solution was poured into the original test tubes, and placed in a 37°C water bath for 20 min and the absorbance peaks were obvious at 20 min and 30 sec was noted as A2, and calculate. And soluble protein content of the tissues was determined by the Caumas Brilliant Blue assay [19], which was used for the calculation of specific viability, and the assay kits were purchased from Nanjing Bioengineering Institute (Nanjing, China).
2.4.5. Gut Microbiota
Following the extraction of total DNA from the gut microbes, the purity and concentration of the extracted DNA were determined using a spectrophotometer (NanoDrop2000), and DNA integrity was detected by agarose gel electrophoresis. Polymerase chain reaction (PCR) amplification was performed with forward primer 338F (5′-ACTCCTACGGGGAGGCAGCAG-3′) and reverse primer 806R (5′-GGACTACHVGGGGTWTCTAAT-3′). Amplification conditions: 98°C for 2 min, followed by 25 cycles of denaturation at 98°C for 15 s, 55°C for 30 s, and 72 °C for 30 s, and a final extension at 72 °C for 5 min. Once the reaction had reached completion, the PCR products were verified by 2% agarose gel electrophoresis, and purified by using the QIAEX II Gel Extraction Kit (QIAGEN). Subsequently, the PCR products were quantified by QuantiFluor-ST Blue Fluorescence Quantification System (Promega). Following this, high-throughput double-end sequencing by the Illumina MiSeq platform (Shanghai Maiorbio, China). The raw FASTQ files were assembled with QIIME and spliced under the default parameters of FLASH, followed by quality control of the spliced sequences with QIIME. The sequences were OTU clustered based on 97% similarity using UCHIME software to reject chimeras and then UPARSE software, each sequence was annotated for species classification using the RDP classifier. The Silva database (SSU123) was compared, and the comparison threshold was set to 70% to obtain its species taxonomic information used. Alpha diversity analysis was performed using Mothur software to calculate Chao1, Shannon, Ace, and Simpson indices.
2.5. Statistical Analysis
The data were tested for normality test before statistical analysis, and obtained from this experiment were analyzed by one-way analysis of variance (ANOVA) using SPSS 26.0 software, and Tukey’s multiple comparison method was employed to test the significance of differences. The data are presented as mean ± standard deviation (mean ± SD) with a significant difference level of p < 0.05 and a highly significant difference level of p < 0.01.
3. Results
3.1. Growth Performance and Morphology Index
The growth performance of juvenile bighead carp is illustrated in Table 2. The final mean weight (FBW), WGR, and SGR of bighead carps tended to increase and then decrease with the increasing dietary choline chloride supplementation levels. The control group exhibited significantly lower FBW, WGR, and SGR compared with 4 g/kg and 6 g/kg groups (p < 0.05), and the most notable difference was observed at 6 g/kg, which demonstrated a significantly higher than the control and other groups. No statistically significant difference was observed in CF among groups supplemented with choline chloride (p > 0.05). The VSI and HSI demonstrated a declining trend with the augmentation of choline chloride supplementation. The supplemental groups (2–10 g/kg) decreased VSI in comparison to the control group, while the HSI of the 8 g/kg and 10 g/kg groups also demonstrated a significant decline in relation to the control group (p < 0.05).
| Parameters | Dietary choline chloride added levels (g/kg) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 2.00 | 4.00 | 6.00 | 8.00 | 10.00 | |
| IBW (g) | 1.77 ± 0.06 | 1.78 ± 0.08 | 1.8 ± 0.12 | 1.77 ± 0.07 | 1.77 ± 0.02 | 1.75 ± 0.07 |
| FBW (g) | 6.52 ± 0.38a | 6.55 ± 0.11a | 7.41 ± 0.37b | 8.12 ± 0.19c | 6.67 ± 0.21a | 6.54 ± 0.10a |
| WGR (g)1 | 267.91 ± 9.84a | 271.00 ± 16.36a,b | 311.34 ± 8.32b | 360.20 ± 20.89c | 277.72 ± 14.63a,b | 274.25 ± 11.89a,b |
| SGR (%/day)2 | 1.97 ± 0.04a | 1.99 ± 0.07a | 2.14 ± 0.03b | 2.31 ± 0.07c | 2.01 ± 0.06a,b | 2.00 ± 0.05a,b |
| CF (g cm3)3 | 1.97 ± 0.08 | 1.89 ± 0.02 | 1.91 ± 0.03 | 1.88 ± 0.02 | 1.80 ± 0.03 | 1.82 ± 0.02 |
| VSI (%)4 | 11.15 ± 0.1c | 10.34 ± 0.47b | 10.23 ± 0.01b | 9.59 ± 0.25a | 9.47 ± 0.13a | 9.44 ± 0.32a |
| HSI (%)5 | 1.74 ± 0.17b | 1.65 ± 0.02a,b | 1.52 ± 0.12a,b | 1.50 ± 0.01a,b | 1.38 ± 0.1a | 1.42 ± 0.07a |
- Note: Means and pooled SEM are presented for each parameter. Means in the same row with different superscripts are significantly different (p < 0.05).
- Abbreviations: FBW, final mean weight; IBW, initial mean weight.
- 1Weight gain rate (WGR, %) = (FBW−IBW)/IBW × 100.
- 2SGR (specific growth, % per day) = [ln (FBW)–ln (IBW)]/day.
- 3CF (condition factor, g/cm3) = (g body weight)/(cm body length)3 × 100.
- 4VSI (viscerosomatic index) = 100 × (g viscera weight)/(g body weight).
- 5HSI (hepatosomatic index) = (g liver weight) × (g body weight).
Upon the application of broken-line analysis to delineate the correlation between WGR, SGR, and dietary choline chloride concentration, it was determined that the juvenile bighead carps achieved optimal growth when the dietary choline chloride concentration was set at 6.62 and 6.51 g/kg, respectively (Figure 1).
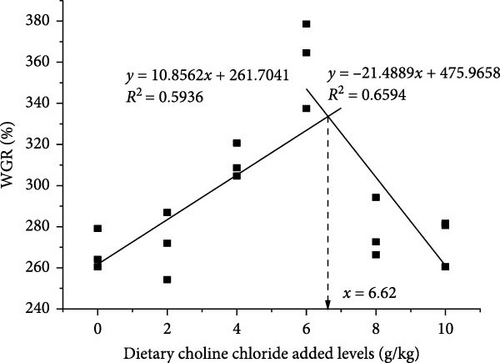
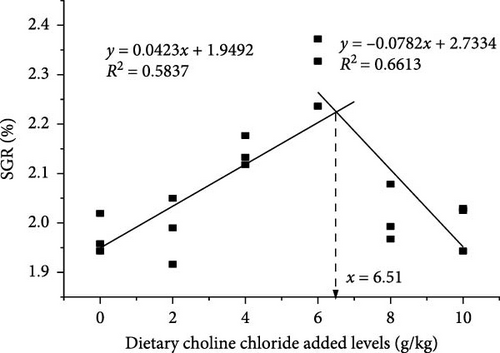
3.2. Nutritional Composition
The whole-body fish moisture, crude fat, and crude protein contents ranged from 66.17% to 69.19%, 39.41% to 41.28%, and 45.5% to 47.88%, respectively, and none of the above indexes were affected by the level of dietary choline chloride (p > 0.05). The content of whole-body ash was notably elevated in the 8 g/kg group compared to the control group (p < 0.05) (Table 3).
| Parameters | Dietary choline chloride added levels (g/kg) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 2.00 | 4.00 | 6.00 | 8.00 | 10.00 | |
| Moisture (g/kg) | 666.51 ± 4.13 | 667.54 ± 3.44 | 661.71 ± 19.91 | 691.92 ± 1.13 | 686.77 ± 10.95 | 674.23 ± 11.84 |
| Ash (g/kg) | 94.25 ± 1.15a | 97.22 ± 3.12a,b | 97.25 ± 0.44a,b | 93.60 ± 0.30a | 100.11 ± 3.23b | 95.08 ± 0.24a |
| Crude lipid (g/kg) | 205.64 ± 5.52 | 201.82 ± 8.31 | 207.75 ± 5.46 | 203.11 ± 4.45 | 196.63 ± 9.70 | 181.34 ± 27.19 |
| Crude protein (g/kg) | 556.88 ± 20.73 | 568.63 ± 2.14 | 548.45 ± 1.48 | 546.15 ± 0.47 | 571.26 ± 1.55 | 574.48 ± 1.81 |
- Note: Means in the same row with different superscripts are significantly different (p < 0.05).
3.3. Hematological Parameters
The levels of serum TP, ALB, and ALP did not exhibit significant differences between the groups (p > 0.05). TG level was significantly higher in the choline chloride concentrations were 4, 6, 8, and 10 g/kg than in the control and 2 g/kg groups (p < 0.05). T-CHO level in the 6 g/kg group was significantly lower than the control group (p < 0.05). The GLU level showed a tendency to decrease first then increase with the increase in choline chloride level, and was significantly lower in the 4 g/kg group than control and 10 g/kg groups (Table 4).
| Parameters | Dietary choline chloride added levels (g/kg) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 2.00 | 4.00 | 6.00 | 8.00 | 10.00 | |
| Biochemical indexes | ||||||
| TP (g/L) | 38.63 ± 2.61 | 34.64 ± 2.92 | 34.74 ± 5.17 | 34.17 ± 1.87 | 33.42 ± 4.5 | 32.32 ± 1.85 |
| ALB (g/L) | 14.61 ± 1.11 | 13.37 ± 0.99 | 13.28 ± 2.03 | 13.58 ± 0.28 | 12.81 ± 1.88 | 12.93 ± 0.53 |
| AST (U/L) | 63.50 ± 6.36 | 59.33 ± 11.15 | 55.67 ± 6.51 | 49.00 ± 4.58 | 47.50 ± 3.54 | 58.00 ± 14.18 |
| TG (mmol/L) | 5.55 ± 0.62a | 4.35 ± 0.21a | 4.09 ± 0.54b | 3.97 ± 0.24b | 4.05 ± 0.11b | 3.83 ± 0.50b |
| TCHO (mmol/L) | 4.70 ± 0.30a | 3.91 ± 0.18a,b | 3.78 ± 0.21a,b | 3.64 ± 0.47b | 4.60 ± 0.16a,b | 4.10 ± 0.13a,b |
| GLU (mmol/L) | 6.87 ± 1.64a | 6.26 ± 1.39a,b | 2.38 ± 0.39b | 3.31 ± 0.75a,b | 4.60 ± 1.58a,b | 7.11 ± 1.66a |
| Antioxidant indexes | ||||||
| MDA (nmol/mL) | 26.63 ± 0.11a | 22.02 ± 0.71b,c | 23.79 ± 0.91a,b,c | 21.79 ± 0.87b | 23.47 ± 1.07b | 20.25 ± 1.66c |
| CAT (U/mL) | 13.38 ± 0.27a | 16.53 ± 1.57a,b | 19.54 ± 2.04b,c | 24.84 ± 1.52c | 18.29 ± 1.95a,b | 17.06 ± 1.78a,b |
| SOD (U/mL) | 100.9 ± 0.08a | 103.41 ± 0.15b | 104.16 ± 0.51b,c | 105.33 ± 0.23c | 105.01 ± 0.13b,c | 103.57 ± 1.66b,c |
- Note: Means in the same row with different superscripts are significantly different (p < 0.05).
3.4. Antioxidant Function
When choline chloride supplementation ranged from 6 to 10 g/kg, the MDA level was significantly lower than the control group (p < 0.05). Serum CAT activity increased with the increase of choline chloride supplementation, and choline chloride-supplemented was at 6 g/kg, CAT activity was significantly higher than the control group (p < 0.05). The control group decreased SOD value in serum compared with the choline chloride-supplemented groups, and the highest value occurred in the 6 g/kg group (p < 0.05) (Table 4).
3.5. Digestive Enzyme Activity
Lipase activity was the highest and significantly greater than the other groups when choline chloride supplementation was 8 g/kg (p < 0.05). When choline chloride supplementation was 6 g/kg, amylase and trypsin had the highest vigor, which was significantly higher than the other groups (p < 0.05) (Table 5).
| Parameters | Dietary choline chloride added levels (g/kg) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 2.00 | 4.00 | 6.00 | 8.00 | 10.00 | |
| Lipase (U/g prot) | 9.94 ± 3.97a | 13.16 ± 5.07a | 16.88 ± 5.29a | 12.17 ± 1.38a | 34.67 ± 6.34b | 19.06 ± 3.41a |
| Amylase (U/mg prot) | 0.19 ± 0.02a | 0.18 ± 0.00a | 0.20 ± 0.01a | 0.37 ± 0.11b | 0.15 ± 0.01a | 0.10 ± 0.05a |
| Trypsin (U/mg prot) | 3887.09 ± 1265.48a | 1476.55 ± 567.65a | 2817.43 ± 1576.40a | 15107.96 ± 1199.76b | 1543.34 ± 820.68a | 1543.34 ± 312.62a |
- Note: Means in the same row with different superscripts are significantly different (p < 0.05).
3.6. Intestinal Microbiota
Sequencing analysis of intestinal 16S rDNA yielded a total of 694,342 sequences, with an average length of 409 bp. The sequencing results were clustered into operational taxonomic units (OTUs), and a total of 1224 OTUs were identified, excluding those specific to each group, and 176 OTUs common to the control group and the five choline chloride-added groups. (Figure 2A). Analysis of the Alpha diversity of bighead carp gut microorganisms. The ACE, Chao, Shannon, and Simpson’s indices were not significantly affected in the groups supplemented with different levels of choline chloride compared to the control group (p > 0.05) (Table 6).
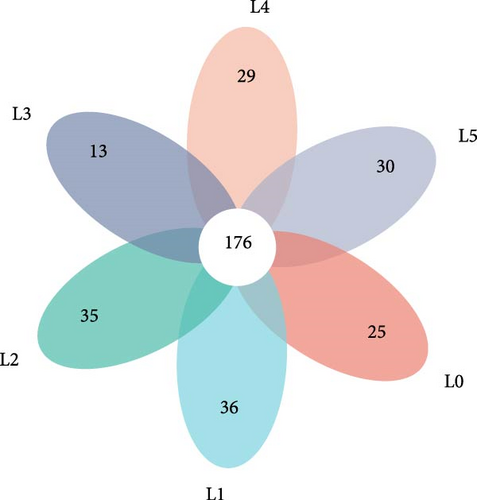
| Parameters | ACE | Chao | Shannon | Simpson |
|---|---|---|---|---|
| L0 | 302.62 ± 40.95 | 304.02 ± 44 | 2.48 ± 0.43 | 0.22 ± 0.04 |
| L1 | 325.38 ± 65.3 | 324.48 ± 63.89 | 2.28 ± 0.07 | 0.31 ± 0.04 |
| L2 | 312.45 ± 48.05 | 315.99 ± 44.7 | 2.68 ± 0.12 | 0.20 ± 0.08 |
| L3 | 265.5 ± 10.21 | 269.97 ± 0.27 | 2.33 ± 0.34 | 0.25 ± 0.04 |
| L4 | 295.86 ± 83.18 | 290.23 ± 93.42 | 2.61 ± 0.33 | 0.20 ± 0.05 |
| L5 | 324.58 ± 38.09 | 329.68 ± 58.59 | 2.46 ± 0.19 | 0.24 ± 0.08 |
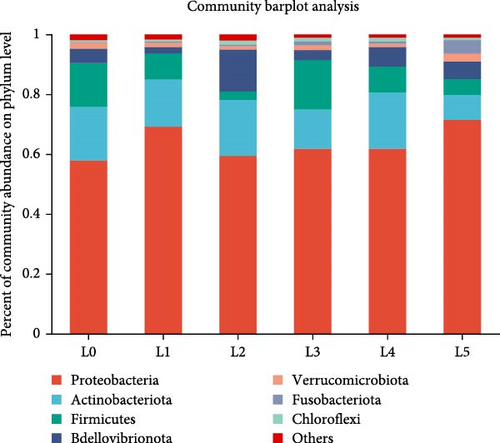
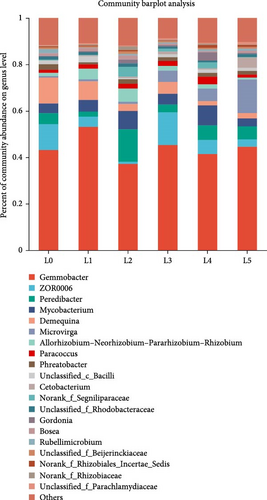
At the phylum level, Similar composition of dominant intestinal phyla. Among them, Proteobacteria, Actinobacteria, Firmicutes, Vibrio vermicularis, Bdellovibrionota, and Verrucomicrobia, with abundances of 69.12%, 15.79%, 8.68%, 2.12%, and 1.45%, respectively, and these five phyla accounted for 97% of the gut microbes in juvenile bighead carp, with minor contributions from Fusobacteria and Chloroflexi. Proteobacteria were more abundant in the supplemented group than that in the control group. Firmicutes were more abundant in the 6 g/kg group than in control group (Figure 2B). At the genus level, the most abundant bighead carcass microbes were Gemmobacter, then ZOR0006 and Peredibacter as the main dominant genera. Next came Mycobacterium, Demequina, Microvirga, Allorhizobium–Neorhizobium–Pararhizobium–Rhizobium, Paracoccus, Cetobacterium. Gemmobacter and ZOR0006 were more abundant in the 6 g/kg group than in the control group (Figure 2C).
4. Discussion
To investigate the effect of choline on growth, antioxidant function, and intestinal function of bighead carp, we added different levels of choline chloride to diets and found that the appropriate choline chloride supplementation improves the growth performance, antioxidant capacity, and intestinal digestive capacity of bighead carp. Broken-line regression analysis of WGR and SGR yielded choline chloride requirements of 6.62 and 6.51 g/kg, respectively, for juvenile bighead carp to obtain optimal growth. Similarly, the following species have been identified as requiring choline for optimal growth and development: lake trout lake sockeye salmon [20], juvenile hybrid striped bass [21], junco [11], juvenile yellowtail (Seriola lalandi) [7] and heterozygous silver carp [22] exhibited feed choline requirements of 1, 0.5, 0.696, 0.75, and 2.02 g/kg, respectively. Indicates that there are differences in the feed choline chloride requirements of different fish. The reason for this difference may be that the feeds make the fish differently sensitive to dietary choline chloride. Furthermore, it was also shown that choline supplementation did not have a significant effect on juvenile Oplegnathus fasciatus [23] and juvenile Epinephelus lanceolatus [24]. The different results may be due to variations in dietary composition, feeding conditions, size, and species of cultured fish.
Dietary addition of 6 g/kg choline chloride significantly reduced serum TG, T-CHO, and GLU levels. Feeding choline-rich diets reduced TG and CHO levels in Atlantic salmon [25]. There is a study that also showed that the choline supplementation lowered serum CHO and TG levels, although the reduction was not statistically different between treatments [24]. However, others found that choline supplementation caused an increase in blood TG and CHO levels [4, 26]. Additionally, the studies conducted [27] on the pomfrets and [28] on the goshawk sturgeon demonstrated that the choline supplementation increased TG level, which is a result of choline’s affinity for TG level, which promotes the transport of fats through the bloodstream, but choline may also be capable of facilitating the metabolism of fat in the blood, which has a hypolipidemic effect.
In normal physiological conditions, ROS are generated during metabolism in the animal body [29], and oxidative stress occurs when the body produces an excess of ROS, resulting in a disruption of equilibrium [30]. SOD is the first line of defense against antioxidant defense and can accelerate the rate of O denaturation by 2-to H2O2. MDA is a stable end product of lipid peroxidation that can induce high toxicity in cells and can destroy the function of oxidative capacity within cells [31]. CAT can remove harmful ROS and participate in the protective mechanism of tissue damage after the oxidation process [32]. Typically, there is a strong correlation between the antioxidant system and nutrients [33]. Choline deficiency often leads to oxidative stress and diminished antioxidant capacity of the body; on the contrary, the addition of choline to the diet may also prevent or treat hepatic steatosis and oxidative stress caused by other factors [34]. The present study demonstrated that serum MDA level was reduced when choline supplementation was at 6 g/kg, and significantly increased SOD and CAT activities and increased the antioxidant capacity of bighead carp. The same results have also been reported, such as choline supplemented reduced serum MDA level of largemouth bass [31], and enhanced SOD activity in juvenile largemouth bass [35].
The growth performance of fish is closely related to their digestive and absorptive capacity of fish [36, 37]. The results of the present study indicate that moderate amounts of choline chloride can improve the lipase, amylase, and trypsin and optimize the digestive capacity of juvenile bighead carp. This finding is similar to that reported for eels [38]. Furthermore, the gut is home to a vast and diverse microbial population [39]. Evidence indicates that fish gut microbiota is intricately and harmoniously linked to the metabolic processes, growth and development, immune system, and nutritional status of fish [40, 41]. The dominant gate between the addition groups are the same, mainly Proteobacteria, Actinobacteria, and Firmicutes. The genus Gemmobacter and Peredibacter are classified within the phylum Proteobacteria, which is the dominant bacteria in mariculture and freshwater pond culture systems [42]. Mycobacterium is a member of the phylum Actinobacteria. The Actinobacteria phylum is capable of degrading a range of compounds, including starch and proteins, is a prolific producer of antibiotics, vitamins, and enzymes, and inhibits the activity of pathogenic bacteria [43]. An increase in Mycobacterium by 2–8 g/kg choline chloride as compared to the control group may have enhanced the digestive capacity of bighead carp larvae to some extent. The Firmicutes phylum is the dominant intestinal microbiota in most freshwater fish species. It can inhibit the growth of multiple pathogenic bacteria by proliferating in the intestinal tract as well as producing a variety of digestive enzymes, which promote the digestive and absorptive functions of the intestinal tract of fishes [44]. The addition of 6 g/kg increased the abundance of the phylum Firmicutes and the relative abundance of the genus ZOR0006 compared to the control. It suggests that the addition of choline chloride may be able to regulate the intestinal flora of bighead carp larvae and improve digestion to some extent.
5. Conclusion
Moderate choline chloride supplementation could enhance the antioxidant capacity and the vigor of the intestinal digestive enzymes of juvenile bighead carp. Furthermore, choline chloride supplementation had no significant impact on the abundance of intestinal microbes of juvenile bighead carp, and the dominant phylum and the dominant genus remained largely consistent across the groups. The broken-line regression analysis, which considered the WGR and the SGR, respectively, led to the conclusion that the optimal growth of juvenile bighead carp was achieved when choline chloride was supplemented at concentrations of 6.62 and 6.51 g/kg. In general, the addition of 6 g/kg choline chloride supplementation of bighead carp promoted antioxidant activity and intestinal digestion, thus improving growth performance. However, it was not possible to calculate the intake of bighead carp due to filter feeding. In addition, liver antioxidant function and lipid metabolism were not taken into account, which makes the overall assessment of the effect of choline chloride on bighead carp health incomplete. And the fish were relatively small, it is questionable whether the requirement of choline is the same amount as the larger fish. If subsequent trials are conducted, considerating raising fish with larger initial body weights, and attempt to assess food intake and to consider indicators of liver health.
Ethics Statement
All authors followed all applicable international, national, and/or institutional guidelines for the care and use of animals.
Disclosure
The title and abstract in the link tohttps://csfish.org.cn/upFilesCenter/upload/file/20241114/1731573560716022451.pdf are exactly from this paper, because when Lili Chen attended the 2024 Chinese Society of Aquaculture’s at that time, when Lili Chen attended the 2024 Annual Meeting of the Chinese Society of Aquaculture (2024.11.11-11.13), the organizers asked me to submit an abstract of the article, so Lili Chen submitted the abstract of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Lili Chen, Chengjie Wang, and Liming Zhang are equally the first authors.
Funding
The funds were provided by the COFCO Feed Co., Ltd. and the Hubei Key Laboratory of Animal Nutrition and Feed Science (Grant 202319).
Acknowledgments
The authors are grateful to provide funds by COFCO Feed Co., Ltd. and Hubei Key Laboratory of Animal Nutrition and Feed Science (Grant 202319).
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




