Time and Concentration Effects of Amino Acid Supplements on Stress Responses in the Gilthead Seabream (Sparus aurata)
Abstract
It has been reported that phenylalanine (Phe) or tyrosine (Tyr) supplementations significantly affect fish stress and welfare biomarkers. The objective of this study was to determine the amount of Phe/Tyr in the diet and feeding time necessary to mitigate stress. Seabreams (Sparus aurata) were stocked at 5 kg m−3 in 500 L tanks. The experimental treatments consisted of different types of feeding: control, Phe-enriched (5%, 7.5%, 10% on dry food), and Tyr-enriched (5%, 7.5%, 10%) food for 2, 4, or 8 days each. At the end of each experimental treatment, fish were sampled for blood, and 10 fish from each treatment were previously subjected to stress by exposure to air (3 min) and sampled 30 min and 2 h later. Plasma glucose, lactate, proteins, cortisol, adrenaline, and noradrenaline concentrations were measured. Dietary Tyr supplements led to high mortalities in long treatments (8 days). The best results for attenuating stress biomarkers were found for the 10% Tyr supplements for 4 days since this treatment kept the maximum number of stress biomarkers with no significant changes. The correlations between biomarker concentrations and amino acid (AA) concentration/supply time were not always significant, though it seems that Tyr supplements present more consistent effects, the increase in Tyr concentration or feeding period being related to decreasing biomarker concentrations.
1. Introduction
The gilthead seabream (Sparus aurata) is a typical aquaculture species with a high market value reared in several seafarms from the Mediterranean coast [1]. As gilthead seabream commercial cultures are progressively enhancing, as well as ethic issues on animal welfare, both seafarmers and consumers are increasingly taking care on the importance of keeping fish in a suitable health and welfare status. Therefore, aquaculture studies are aimed at finding new strategies to minimize stress and improve animal welfare in gilthead seabream aquaculture.
There are many factors that active the stress response in fish, such as salinity, transport, stocking density, or temperature [2–6]. For this reason, stress attenuation in fish has been widely studied through several methods, such as applying anesthetics or feeding them with additives as amino acids (AAs) [7–12].
Proteins are key biomolecules in the diet, providing the AAs necessary for energy, growth, and substrates for metabolic pathways. The AA involved in the cellular processes is called functional AA. A deficit of these AAs can affect the body’s metabolism and homeostasis. Hence, the interest in AA is growing in order to improve disease resistance, reproduction, or behavior [13]. In this sense, it has been reported that some AAs could attenuate the stress response in different fish species as meager (Argyrosomus regius), cod (Gadus morhua), and Senegal sole (Solea senegalensis) [9, 14, 15] and affect the immune response [16, 17]. This is due to an increase in the dietary AA concentration that stimulates metabolic pathways related to the stress system.
Besides tryptophan (Trp), other AAs as phenylalanine (Phe) and tyrosine (Tyr), are also precursors of hormones or neurotransmitters related to the stress response. Phe is an essential AA that is metabolized in two ways: oxidation to Tyr and transamination to phenylpyruvate [18]. Tyr is the precursor of catecholamine (adrenaline, noradrenaline, and dopamine) and thyroid (triiodothyronine and thyroxine) hormones, which are released to the bloodstream as stress responses [19].
In mammals, diets enriched with Phe/Tyr have been demonstrated to mitigate the effects of acute stress [20–22]. Only Salamanca et al. [10] and Herrera et al. [15] have reported that Phe/Tyr supplements can mitigate stress in different fish species (G. morhua, A. regius, and S. aurata), where AA supplementation has been shown to reduce physiological markers related to stress. Cotoia et al. [23] have reported that hydroxyphenyl pyruvate, coming from Tyr catabolism, improves cell survival under stress conditions. In addition, both Phe and Tyr decrease cases of bone deformity in white seabream larvae (Diplodus sargus) [24]. Nevertheless, no study has focused on the optimal amount of Phe/Tyr present in the diet or the feeding time period in order to mitigate the stress response and apply the lowest values for both the time period and AA concentration.
The objective of this study was to determine the optimal amount of Phe/Tyr in the diet and feeding time necessary to mitigate stress. In this sense, the methodology was addressed to study the effects of Phe/Tyr concentration and feeding time on stress response in order to determine the most effective treatment for keeping the stress markers stable.
2. Materials and Methods
2.1. Experimental Culture and Sampling
Seabreams (S. aurata) (n = 1080), with a mean body weight of 29.65 ± 1.72 g (mean ± standard error, SE), came from CULMASUR (Isla Cristina, Spain). Fish were stocked at 30 fish/tank in 500 L flat-bottom circular tanks at 2 kg m−3. The culture water was recirculated, and the mean temperature, salinity, and dissolved oxygen levels were 21 ± 1°C, 37 ± 1 g L−1, and above 5 ppm, respectively. Before the experiment began, the fish were fed commercial feed (L2 Alterna Skretting, Burgos, España), with daily biomass rations of 2%. The experimental treatments consisted of different types of feeding (duplicates): control, Phe-enriched (5%, 7.5%, 10%), and Tyr-enriched (5%, 7.5%, 10%) food for 2, 4, or 8 days each. Therefore, 18 treatments (and control) for every AA were applied: 5P2, 5P4, 5P8, 7P2, 7P4, 7P8, 10P2, 10P4, 10P8, 5T2, 5T4, 5T8, 7T2, 7T4, 7T8, 10T2, 10T4, and 10T8; where the first and last numbers indicate the inclusion level (5%, 7.5%, or 10%) and feeding period (2, 4, or 8 days), and letters are for each AA (Phe or Tyr). Fish feed was provided through automatic feeders and the ration was adjusted to 3% tank biomass daily, checking the presence of remaining feed daily to ensure that feed was supplied in excess. The tanks were cleaned and checked daily for possible dead fish [25].
At the end of each experimental treatment, fish were 24 h-fasted and sacrificed with 2-phenoxyethanol overdose (1 mL L−1) for the extraction of blood and tissues. Previously, 10 fish from each treatment were subjected to stress by exposure to air (3 min) and sampled 30 min and 2 h later; 10 fish were also sampled in the basal (nonstressed) state. Blood was collected by puncturing the caudal peduncle with 1 mL heparinized syringes (25,000 units of ammonium heparin/3 mL of 0.6% NaCl saline, Sigma H6279). Plasma was separated from cells by centrifugation of whole blood (3 min; 1200 × g; 4°C) and stored at −80°C until analyses were performed.
The IFAPA facilities are certified and have the necessary authorization for the breeding and husbandry of animals for scientific purposes (REGA Code ES210210000303). All procedures involving the handling and treatment of the fish were approved as far as the care and use of experimental animals are concerned (authorization code 11/05/2021/073), by the European Union (2010/63/EU) and the Spanish Government (Real Decreto 53/2013, de 1 de febrero, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia).
2.2. Experimental Food Making
The procedures described in previous works were followed to make the experimental fish feed [7, 9, 10, 15]. Commercial fish feed (L2 Alterna Skretting, Burgos, Spain) was used as the control diet. L-Phe and L-Tyr (dry powder) were purchased from ThermoFisher (Kandel, Germany). The commercial control diet was finely ground and mixed with AAs and water (300 mL kg−1 dry feed). The amount of Phe and Tyr in each experimental diet was 5%, 7.5%, and 10% (on dry feed), except for the control diet, which did not have any AA added. The mixture was thread pelleted into 2 mm diameter and 20–25 cm length strips, which were dried at 30°C for 48 h. Finally, these were cut to get 2–3 mm size pellets and stored at 4°C.
2.3. Plasma Analysis
According to Salamanca et al. [10], plasma glucose, proteins, lactate, and catecholamines (adrenaline and noradrenaline) levels were measured using commercial kits from Química Analitica Aplicada S.A. (QCA Glucose Liquid Ref. 998,225, QCA Total Proteins Ref. 997,180, Tarragona, Spain), Spinreact (Lactate Ref. 1,001,330, Barcelona, Spain), and LDN (2-CAT (A-N) Research ELISA Ref. BA E-5400, Nordhorn, Germany) adapted to 96-well microplates [10, 26]. All assays were performed with a Varioskan Lux reader, using Skantt software for microplate readers v.6.0 (Thermo Fisher Scientific, Massachusetts, USA). Plasma cortisol levels were quantified by an ELISA kit (EA65, Oxford Biomedical Research, MI, USA) modified and adapted to fish [27]. Cortisol was extracted from 20 μL plasma in 200 μL diethyl ether. The lower limit of detection (88.2% of binding) was 0.005 ng mL−1 plasma. The interassay coefficient of variation was 9.8%, while the mean intraassay coefficient of variation was 4.6%. The mean percentage of recovery was 90%. The main cross-reactivities (>5%; given by the supplier) were detected with prednisolone (66.9%), 11-deoxycortisol (58.1%), cortisone (15.9%), prednisone (13.7%), and 17-hydroxyprogesterone (5.4%).
2.4. Statistical Analysis
- 1.
Detecting every biomarker peak/drop (0.5 or 2 h poststress) according to control feeding treatment.
- 2.
Comparing biomarker values between nonstressed and stressed status in every peak/drop biomarker time (according to the previous step).
- 3.
Studying the relationships (and interactions) between concentration/feeding time and biomarker values.
3. Results
Mortality rates in Tyr treatments for 8 days were significant compared to the rest of the Tyr groups: 86.7 ± 3.3%, 78.3 ± 5%, and 73.3 ± 3.33% for 5Tyr8D, 7Tyr8D, and 10Tyr8D, respectively. No mortalities were registered for the other treatments. Therefore, 8 days and Tyr treatments would not be suitable, though their data have been processed and shown below.
3.1. Detecting Stress Biomarker Peaks/Drops
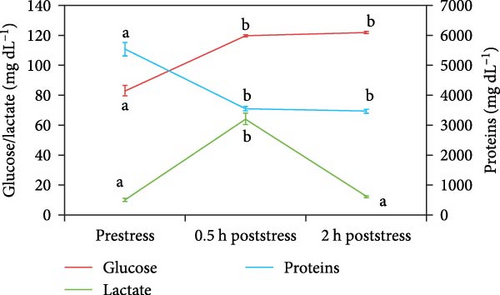
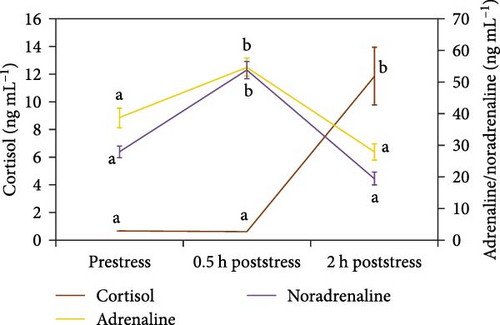
Figures 1 and 2 show plasma glucose, lactate, proteins, cortisol, adrenaline, and noradrenaline concentrations for every sampling under the control feeding treatment. Only plasma proteins decreased significantly after stress submission, being the drop at 0.5 h poststress. The other biomarkers grew significantly after stress at 0.5 h (glucose, lactate, adrenaline, noradrenaline) or 2 h (cortisol).
3.2. Nonstressed and Stressed Status Differences
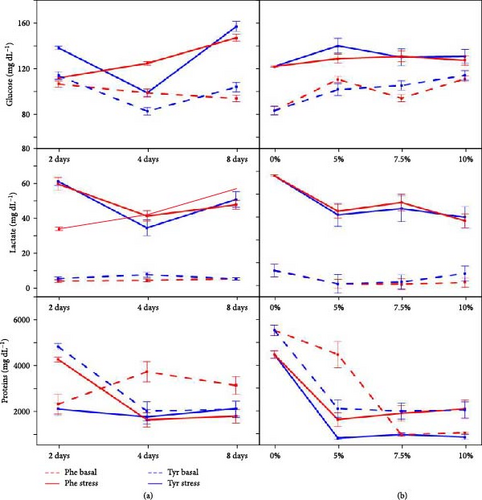
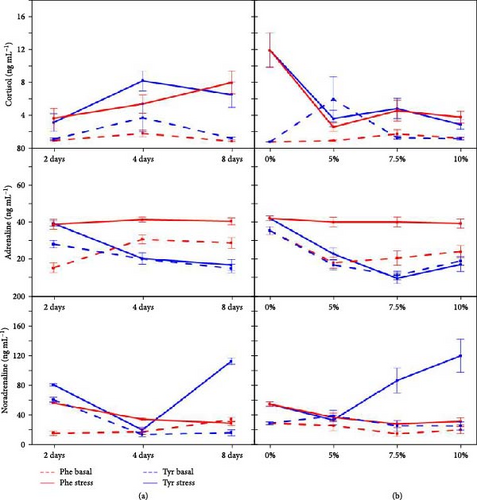
Figures 3 and 4 show biomarker values for stressed and nonstressed (basal) status in every treatment. For glucose, only 10P4, 7T4, and 10T4 treatments did not show significant differences. The variations for lactate were more frequent, 10T4 treatment being the only one with no significant changes. However, proteins in 5T8, 7T4, 7T8, 10T4, and 10T8 did not vary significantly. The effects of experimental diets were more important in plasma hormones. Cortisol did not vary significantly for the 5P4, 7P2, 7P4, 10P2, 10P4, 5T2, 5T4, 5T8, 7T4, 7T8, 10T2, and 10T8 treatments. Adrenaline concentrations were statistically different between basal and stressed fish in 5P4, 5P8, 7P2, 10P2, 5T2, and 10T2 treatments. For noradrenaline, significant differences were detected for 5P2, 5P4, 7P2, 7P4, 10P2, 7T2, 7T8, and 10T8.
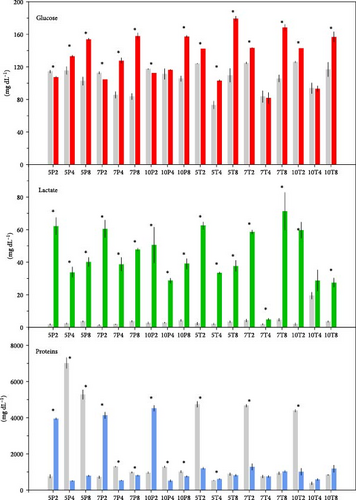
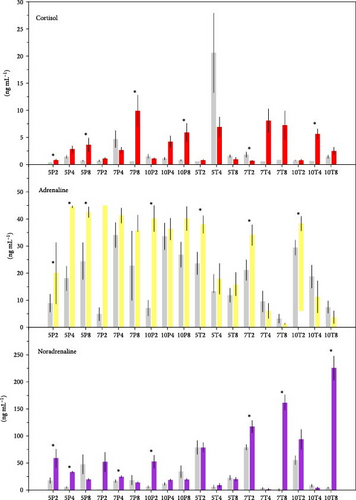
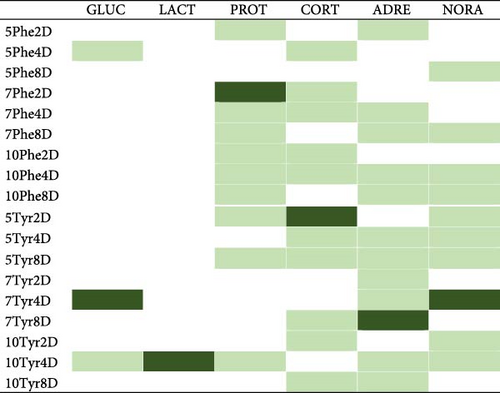
Figure 5 summarizes the detected differences for every biomarker. According to it, the treatment that kept the maximum number of biomarkers unchangeable was 10T4, 10% Tyr added during 4 feeding days. The 10P2 and 5T8 treatments also showed good results, keeping four biomarkers with no changes, though mortalities in the 5T8 treatment were significantly high.
3.3. Concentration and Time Effects
Figures 6 and 7 show the relationships between feeding days/concentration and biomarker concentrations under stress and basal conditions. Similarly, Figure 8 shows the correlations between those factors, indicating also the significant interactions between both factors (concentration and time).
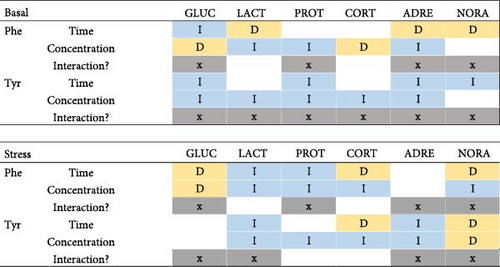
Regards the basal state, most of the stress biomarker concentrations decreased significantly while feed concentration and time grew within the Tyr treatment, the interaction being significant for all cases. However, the Phe treatments showed both inverse and direct correlations between concentration/time factors and biomarker concentrations.
Under a stress state, the Phe/Tyr concentration seems to be inversely correlated to biomarker concentrations, except for a pair of cases. However, the feeding time effects are not so clear since both inverse and direct correlations are detected depending on each stress biomarker.
4. Discussion
It seems clear that Phe and Tyr supplementations significantly affect fish stress and welfare biomarkers, as shown in previous works [10, 15]. However, the effects of increasing (AA) concentrations and delivery time have been studied in this work for the first time in order to find suitable values for both variables, which minimize the nutritional disturbances and stress responses.
In our work, the biomarker peaks/drops were significant at 0.5 h after stress except for cortisol (2 h). It has been reported that the increase in plasma glucose and lactate values can occur after 0.5 h after stress. Cnaani and McLean [28] reported an increase in glucose values after 1 h after the stress event, and Herrera et al. [26] described the same in both metabolites after 3 h of the stress challenge. A similar pattern was followed by catecholamine hormones (adrenaline and noradrenaline). This is due to these hormones, as cortisol, constitute the primary response to stress, although catecholamines have a faster release and clearance than cortisol. That is the reason the concentration of adrenaline and noradrenaline undergoes an increase in a shorter time period than cortisol.
Cortisol peaks usually happen between 0.5 and 2 h poststress as a primary response to stress [26, 28–30]. This has been found in our study, though it may have been delayed by the diet supplemented with AAs or by stressor conditions since an increase after 0.5 h exposed to the stressor and a drop after 3 h could exist [26]. Although the plasma protein peak is related to stress, an AA-supplemented diet can alter the protein metabolism; hence, the response of protein-related biomarkers can be unexpected [7, 10, 15]. In that sense, a significant drop after 0.5 h was registered in our work.
According to our work, the best combination of AA concentration and feeding time for attenuating stress (the maximum number of attenuated biomarkers) was 10Tyr4D, 10% Tyr added for 4 days, where only cortisol varied significantly between stress and basal (Figure 5). For Phe supplements, 10Phe4D was the treatment decreasing and keeping stable the most quantity of biomarkers (5). Therefore, it seems that 4 days of 10% AA-supplemented feeding is a suitable feeding treatment for reducing stress in this species. Additionally, longer feeding periods (8 days) with Tyr can be mortal, according to the survival rates registered (see Section 3). 10Phe4D and 5Tyr8 treatments also kept four biomarkers stable, though the latter was associated with higher mortalities (see above). Therefore, the 10% Phe additives for 4 days could suppose another interesting option for attenuating stress responses.
Although the references on Tyr/Phe dietary supplements are very scarce, Salamanca et al. [10] reported that diets supplemented with 5% Phe and 5% Tyr for 7 days modulated the stress parameters in seabream (S. aurata) and meager (A. regius), though they were significantly reduced only in the latter. In the present study, negative results were obtained for 8 feeding days with Tyr (significant mortality rates); thus, it is possible that by increasing the time and concentration, those diets do not mitigate the stress, and Phe and Tyr accumulate in tissues after 7 days, deriving in physiological disorders and death, mainly for Tyr supplements [10]. High levels of both AAs can be toxic since they would exert antagonistic actions against other AAs, impairing the absorption and utilization of other AAs and resulting in a deficient absorption rate of Phe and/or Tyr [31]. In this sense, both Phe and Tyr are large neutral AAs (LNAAs), which can compete for the same transporter as other LNAAs, such as valine, leucine, isoleucine, and Trp [32].
In the basal state, the effects of Phe supplements on biomarker concentrations were not so clear depending on concentration and feeding time (Figure 8). Both inverse and direct relationships existed between those factors and biomarkers. However, Tyr supplements presented a more consistent pattern; hence, the increase in Tyr concentration or feeding period is related to decreasing biomarker concentrations, except for lactate and cortisol (time) or noradrenaline (concentration). This is in line with the best results for biomarker attenuation were found for 10Tyr4D since the highest Tyr concentration would have decreased biomarkers to the lowest values. However, a longer feeding time at that concentration can be harmful (higher mortality rates). In this sense, it has been reported that high dietary Tyr levels can be toxic for some fish species (Cirrhinus mrigala and Cirrhinus catla), though Ictalurus punctatus showed to be unsensitive to it [33–35]. Therefore, Zehra and Yousif [36] have stated that toxicity of excess Tyr is species-specific.
Xiao et al. [37] studied the effects of several dietary Phe concentrations on hematological parameters in hybrid tilapias (Oreochromis niloticus x Oreochromis aureus). They stated that blood glucose was not affected by Phe content. However, our results showed a direct relationship between dietary Phe and plasma glucose. Besides the species-specific differences, the Phe inclusion in our work was much higher (5%–10% versus 0.43–1.91%); hence, it could be the reason for that difference. Those authors also reported plasma protein differences according to Phe content, the lowest value being associated with the lowest Phe level, though with no clear trend for the rest. Similarly, Sharf and Khan [38] did not report any relationship between these two variables, neither between dietary Tyr and plasma protein levels in Channa punctatus. In our work, plasma proteins were inversely correlated to dietary Phe or Tyr content; hence, the high AA excess could derive in a nutritional unbalance or stress, which would need the transference of proteins from blood to tissues in order to metabolize that AA excess.
In the stress state, AA concentration seems to have a more significant effect than the feeding period since its correlations are inverse in most of the biomarkers (Figure 8). The effects of the feeding time period are not clear, showing both inverse and direct significant correlations. The accumulation of Phe can lead to higher levels of plasma glucose due to its mobilization to tissues in order to get energy for deamination and elimination of AAs that have adverse effects on growth [38, 39]. This fact could happen in the present experiment since Phe concentration was directly correlated to plasma glucose levels.
The correlations between dietary AAs and their derived hormone concentrations have only been studied in Trp and serotonin (Ser). Contradictory results have been published. For instance, Martins et al. [40] did not find significant differences in brain Ser concentration related to different Trp-supplement dietary levels in the Nile tilapia (O. niloticus). And Lepage et al. [41] reported significant differences among brain Ser concentrations from several Trp dietary levels, though they did not state any significant trend or correlation between Ser and Trp in rainbow trout (Oncorhynchus mykiss). In our work, the correlations between AA and derived hormones (adrenaline and noradrenaline) were not consistent and depended on the state (basal or stress).
Therefore, considering both basal and stress status, it seems that AA concentration plays a more relevant role than feeding time for the administration of dietary supplements. Additionally, the biomarker concentrations progressively decrease while AA concentrations grow.
5. Conclusions
According to our results, dietary Tyr supplements could derive in high mortalities if they are supplied for 8 days or more. The best results for attenuating stress biomarkers were found for the 10% Tyr supplements for 4 days since this treatment kept the maximum number of stress biomarkers stable significantly. The correlations between biomarker concentrations and AA concentration/supply time were not absolutely clear, though it seems that Tyr supplements present more consistent effects, the increase in Tyr concentration or feeding period being related to decreasing biomarker concentrations.
Ethics Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board. The experiment complied with the Guidelines of the European Union Council (2010/63/EU) and the Spanish Government (RD1201/2005; RD53/2013 and law 32/2007) for the use of laboratory animals. All experimental protocols were approved by the Ethical Committee of the IFAPA (Andalusian Institute of Researching and Training on Fisheries and Agriculture), sited in Seville (Spain).
Disclosure
The results were presented in the thesis titled “Mejora del Bienestar Animal en Peces de Interés Acuícola Mediante la Suplementación de Aminoácidos en la Dieta” defended in December 2021.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Natalia Salamanca and Marcelino Herrera: conceptualization, methodology, writing–review and editing. Inmaculada Giráldez and Ignacio de La Rosa: software. Inmaculada Giráldez: formal analysis. Ignacio de La Rosa: resources. Natalia Salamanca: writing original draft preparation. Marcelino Herrera: supervision, project administration. Marcelino Herrera and Inmaculada Giráldez: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project INIA RTA2015-00025-C03-01. Marcelino Herrera and Natalia Salamanca belong to the Fish Welfare and Stress Network (AGL2016-81808-REDT), supported by the Agencia Estatal de Investigación (AEI, MICINN, Spanish Government). Natalia Salamanca’s predoctoral contract is cofinanced by the European Social Fund (FSE) through the call “Ayudas para contratos predoctorales para la formación de doctores 2017” from the AEI, currently holds a contract that is part of the PTA2022-022224-I aid, funded by MICIU/AEI/10.13039/501100011033 and by FSE+.
Acknowledgments
This work has been financed by the project INIA RTA2015-00025-C03-01. Marcelino Herrera and Natalia Salamanca belong to the Fish Welfare and Stress Network (AGL2016-81808-REDT), supported by the Agencia Estatal de Investigación (AEI, MICINN, Spanish Government). Natalia Salamanca’s predoctoral contract is cofinanced by the European Social Fund (FSE) through the call “Ayudas para contratos predoctorales para la formación de doctores 2017” from the AEI, currently holds a contract that is part of the PTA2022-022224-I aid, funded by MICIU/AEI/10.13039/501100011033 and by FSE+.
Open Research
Data Availability Statement
Data will be available upon request due to restrictions.




