Comparative Growth and Food Value of Gracilaria verrucosa, Ulva lactuca, and Crassostrea belcheri in Indoor Coculture: Implications for Sustainability
Abstract
Gracilaria verrucosa and Ulva lactuca were cocultured with Crassostrea belcheri for 120 days in captivity while controls were the independent culture of these three species. The study assessed the water quality parameters, growth performance, proximate and biochemical composition of the organisms undergoing the different treatments. Moreover, they were compared with outdoor farmed seaweeds and oysters to assess their nutritional quality. Significant variations were seen in the growth rates of the two seaweeds, U. lactuca exhibiting the highest live weight gain (LWG) and specific growth rate (SGR) in coculture settings. The percentage of surviving oysters varied from 60% to 80% across experiments, with the fastest growth rates observed when cocultured with U. lactuca. The seaweeds’ moisture, ash, protein, carbohydrate, and fiber contents varied significantly, with U. lactuca (indoor) having the highest protein and carbohydrate content (p < 0.05) compared to U. lactuca (outdoor) and G. verrucosa (indoor and outdoor), and also recording the maximum total chlorophyl, carotenoids, and fucoxanthin. Significant variations were found in the fatty acid content of seaweed and oyster samples. Outdoor oyster samples had more omega-3 fatty acids (p < 0.05) than indoor while the indoor seaweed samples had lower saturated fatty acids (SAFA), and higher polyunsaturated fatty acids (PUFA) than outdoor. U. lactuca grown indoors exhibited the highest levels of both essential amino acids (EAAs) and non-essential amino acids (NEAAs). The study emphasizes how culture conditions affect the nutritional and biochemical profiles of seaweeds and oysters, and it offers indoor coculture as a potential substitute for inclement environmental conditions and as a backup strategy to enhance sustainable seaweed mariculture that allow for optimal growth and nutritional quality ensuring the maximum space utilization.
1. Introduction
Bangladesh offers great potential for the cultivation of seaweed and oysters due to its extensive coastline [1]. These aquaculture activities are vital to environmental preservation and food security, in addition to providing viable economic prospects [1]. Although it is still relatively new, seaweed farming in Bangladesh has grown (~97.5 tons in dry weight annually) quite promisingly in recent years [1, 2]. The coastal locations, especially those in the southeast like Saint Martin’s Island and Cox’s Bazar, are perfect for seaweed farming because of their shallow coastal waters and appropriate salinity levels (20–34 ppt) [1, 3]. Bangladesh boasts 335 distinct varieties of natural seaweed species and an exploitable coastline area of approximately 8500 km2 (< 5 m depth) [4]. Coastal communities, especially fishermen who are facing diminishing fish populations, have an alternative source of income in seaweed farming [5]. Additionally, it provides habitats for marine animals and enhances water quality by absorbing excess nutrients [6, 7]. Hence, to counteract coastal pollution, seaweed can also be employed in bioremediation [8].
Seaweed farming, sometimes referred to as seaweed mariculture or aquaculture, is the practice of growing seaweed in marine environment or in coastal waters [1]. However, recent approaches for indoor seaweed farming have explored the opportunities for growing different kinds of seaweeds in confined spaces like tanks, ponds, or containers [9]. Numerous advantages and opportunities are presented by these developing techniques. First off, growing seaweed indoors helps ensure sustainable, eco-friendly supply of the vital nutrients, vitamins, and minerals that seaweed provides when cultured or harvested from outdoors [9, 10]. Seaweed is also abundant in bioactive substances in addition to micronutrients [11]. Among these are antioxidants that help defend cells from oxidative damage, such as carotenoids and polyphenols [12]. Dietary fiber from seaweed, mostly in the form of soluble fiber like alginate, can improve gut health and aid with digestion [13]. Seaweeds possess unique fatty acid profiles, comprising both unsaturated and saturated fatty acids (SAFA), which enhance their nutritional value [14]. However, some species have relatively low total lipid content, making fatty acid extraction challenging [14]. In addition to being a great source of vital amino acids, seaweeds are a helpful dietary addition for protein intake [15]. Growing as a superfood among aquatic plants, seaweed’s nutritional profile supports numerous health benefits, such as enhanced metabolism, cardiovascular health, and possibly even anti-inflammatory and anticancer qualities [16–18]. The production of seaweeds can help ensure food security by offering a wholesome and sustainable food supply for animal feed and human consumption [19]. Furthermore, year-round production which can be made possible for certain species by indoor seaweed farming is independent of the weather or geographical location, providing higher supply consistency and reliability [20–22]. In comparison to open-sea farming, it also reduces the possibility of contamination and pollution, guaranteeing safer and higher-quality goods [9]. Additionally, by using indoor culture techniques, researchers can increase yields and accelerate development rates by optimizing growing parameters including temperature, light intensity, and nutrient levels [9, 23]. This control also makes it easier to cultivate particular seaweed species for use in a variety of industrial purposes, such as the manufacture of edible salts, medicines, cosmetics, and bioplastics [17, 24]. All things considered, indoor seaweed farming is a promising approach to sustainable agriculture that can assist communities all over the world in terms of nutrition, economics, and the environment [25].
Rich in vital vitamins and minerals, oysters are a nutrient-dense shellfish [26]. Raw oysters are a low-calorie, high-protein dietary item; a 100-g portion typically has about 68 calories, 7 g of protein, and 3 g of fat [27]. Their high zinc content, which promotes DNA synthesis, cell division, and immunological function, makes them especially valuable [28, 29]. Up to 605% of the recommended daily intake (RDI) for zinc can be obtained from this portion size [30]. Additionally, oysters are a great source of vitamin B12, providing roughly 324% of the RDI, which is essential for the development of red blood cells and nerve function [31]. The rich nutritional profile of oysters, a prized seafood, is well-known, especially when it comes to fatty acids and amino acids [32]. Shifting conventional oyster production to indoor systems (land-based or tank-based aquaculture) enables more controlled cultivation conditions [33]. Several benefits over conventional ocean farming are provided by the approach of growing oysters in controlled conditions [34]. Oyster growth and health can be optimized in indoor facilities by precisely controlling environmental factors such as salinity, temperature, and water quality [35]. By reducing the chance of illnesses and parasites, this control guarantees a greater yield and better quality of oysters [36]. It also lessens the effects of pollutants and environmental changes, which increases the sustainability and resilience of indoor farming [37]. By enabling effective water usage and waste management, technological developments in filtration and recirculation systems help to lessen the environmental impact of indoor oyster farming [38]. Additionally, being close to markets makes it possible for fresh oysters to be swiftly consumed by customers, improving their quality and marketability [39]. The seasonal constraints of conventional techniques are mitigated by indoor oyster farming, which offers prospects for year-round production [40]. In addition, it encourages creativity in genetic selection and breeding schemes to produce oyster varieties with desired characteristics, therefore augmenting the production and sustainability of the sector [41].
Despite Bangladesh’s considerable potential for oyster and seaweed cultivation, development remains constrained by systemic barriers such as fragmented stakeholder-regulator coordination, insufficient capital investment, undefined maritime zones, and challenging hydrographic conditions [42, 43]. A substantial upfront investment is needed to set up infrastructure, such as nets, racks, rafts, and anchoring systems, for seaweed and oyster farms [44, 45]. It’s possible that many of the local farmers don’t have access to the required funding. Due to environmental risks and a dearth of successful case studies, investors may view mariculture as high-risk and be reluctant to provide money. On the other hand, these species can experience stress from variations in salinity brought on by freshwater intake from rivers or monsoon rains, which can lower their rates of settlement, development and productivity [1, 46, 47]. Excessive monsoon rains alter the turbidity and salinity of seawater, which in turn affects the growth and health of seaweeds and oysters through photosynthesis and filter-feeding [48, 49]. It is important, but difficult, to choose and grow species that can withstand a broad range of salinity levels; this calls for intensive study and adaptation efforts. Rainfall can also bring sediments and contaminants from land runoff into coastal waters, lowering water quality and raising illness risks. Consequently, mass mortality of oyster occurs and seaweed biomass declines during monsoon [47, 50]. Likewise, elevated tidal waves, particularly in the wake of storms and cyclones, have the potential to cause substantial losses by uprooting oysters and seaweed and causing damage to farm structures [51–53]. Farm setup and maintenance costs rise with the construction of sturdy structures that can withstand powerful waves and currents. Cyclones and storm surges are common in Bangladesh’s coastal regions, and they have the potential to completely damage infrastructure, decimate mariculture enterprises, and result in hundreds of deaths [54, 55]. Furthermore, there are long-term risks associated with rising sea levels, warmer seas, and shifting ocean currents that could affect the appropriateness of existing farming zones [56, 57].
Among commercially valuable seaweed species, farmers currently grow Ulva and Gracilaria primarily in outdoor environment [1, 23, 58]. The most commercially cultivable oyster species worldwide, accounting for 28% of global mollusk production, is the cupped oysters (Crassostrea spp.), which are also the most readily available variety in Bangladesh [59, 60]. The cupped oyster, or Crassostrea belcheri, has received the most attention because of its adaptability to the local environment [61]. Given the immediate potential of mariculture for these species, it is critical to figure out a way to cultivate seaweed or oysters year-round or to relocate them to a secure location where fluctuations in the weather will not affect their growth and nutrition. Indoor seaweed cultivation may offer a potential option ensuring the maximum space utilization. It does, however, require a number of research initiatives. Furthermore, if indoor farming proves to be successful, a unique dilemma will emerge because it might necessitate substantial upfront costs. The study aims to establish a baseline for the coculture of Gracilaria verrucosa and Ulva lactuca with C. belcheri in indoor systems, with a focus on these pressing challenges. This will reveal the performance of indoor cultured organisms compared to outdoor grown ones.
2. Materials and Methods
2.1. Experimental Design and Setup
This study was conducted in the Hatchery premises of Coastal Biodiversity, Marine Fisheries and Wildlife Research Centre, Cox’s Bazar. This experiment was set on 1 August 2023 and continued up to the next 120 days. The red seaweed G. verrucosa and the green seaweed U. lactuca were cocultured with the cupped oyster C.. Coculture of G. verrucosa was labeled as T1 and coculture of U. lactuca was labeled as T2 while all the three species were cultured separately as Control (C1– G. verrucosa, C2– U. lactuca, and C3– C. belcheri) (Figure 1). Three replications of all the treatments and control were maintained. Seaweed and live oysters were collected from Nunia Chara (21°28ˈ19.5” N, 91°57ˈ42.7” E) by the local farmers and harvesters for stocking in the experimental unit. Both species were acclimatized for 7 days in captivity prior to the experiment. All the culture tanks (2.0 × 1.5 × 1.3 ft3) contained 100 L of seawater. 8 g of seaweeds were stocked on submerged strings in each treatment and control tanks (Figure 1). On the other hand, 10 individuals of oysters (27.43 ± 1.34 g) were stocked per square feet surface area of culture tanks in all the treatments and controls. Uninterrupted aeration supply and artificial mild wave action were provided to facilitate optimum growth. Led lights were used above the experimental unit that provided 60 µmol.photons.m−2 s−1 light intensity in the culture facility.
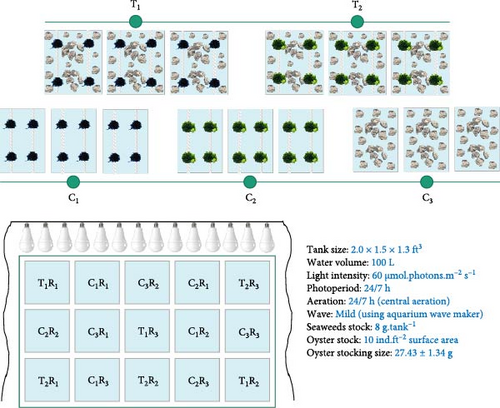
2.2. Routine Maintenance
Oysters were fed once daily with the marine microalgae Chlorella vulgaris and Chaetoceros gracilis. The amount of microalgae was adjusted with the trial on food demand of oysters in each culture tanks. Seawater of the culture tanks were exchanged 30% with natural filtered seawater weekly. Aeration and led lights were available 24/7 h.
2.3. Determination of Water Quality Parameters
Water temperature, pH, salinity, DO (dissolved oxygen) concentration, ORP (oxidation reduction potential), and absolute conductivity (ACon) were determined by using Portable pH/EC/opdo Multiparameter HI98494 (Hanna Instruments Inc., Woonsocket, USA) in every three alternate days. On the other hand, nitrite nitrogen (NO2-N), total ammonium nitrogen (TAN), total alkalinity (TAL), TSS (total suspended solid), and SRP (soluble reactive phosphorus) were measured biweekly. The TSS of seawater was measured using the Nemeth and Nowlis [62] techniques. The alkalinity test kit (HANNA, HI3811) was used to measure the TAL. Using analytical techniques described by Parsons et al. [63], the amounts of SRP, NO2-N, and TAN were measured.
2.4. Growth Parameters and Survival
After 120 days of culture, the seaweeds and oysters were harvested for the estimation of growth parameters and survival rate. They were rigorously washed and spin dried for 1 min before taking the wet weight. Apart from that, the data of mortality rate of oysters were recorded biweekly. Initial and final weight were used to estimate live weight gain (LWG) and specific growth rate (SGR) of both seaweeds and oysters. Besides, shell length, width and thickness data were recorded biweekly to represent the size distribution of oysters across the treatment and control.
Growth of seaweed and oysters were calculated as follows [64, 65]:
Specific Growth Rate:
2.5. Proximate and Biochemical Analysis
At the end of the culture period, all the oyster and seaweeds were collected in the laboratory for further analysis. Besides wild seaweeds and oysters of similar size class were collected from the initial stocking location for comparative analysis. Seaweed and Oyster samples (whole body excluding shell) were hot air oven dried first until the weight became constant for proximate analysis. However, fresh wet samples were freeze dried for fatty acids and amino acids analysis.
2.5.1. Proximate Analysis
First, whole body flesh samples oysters and tissue samples of seaweeds were oven dried for protein, fat, and carbohydrates. Each sample was blended into fine powder. The AOAC [66] standard procedures were used in order to determine the following: moisture, protein, fat, ash, and crude fiber. Samples of wet seaweed and oysters were dried in a freeze-drier until they reached a steady weight. Using a Kjeldahl apparatus and manual titration, the protein content of dry seaweed and oyster samples was measured using the Kjeldahl method (N × 6.25 for oysters and N × 5.85 for seaweed). Diethyl ether was employed as the solvent and the Soxhlet equipment was utilized to measure the amount of lipid at 100°C presented as % DW. The amount of ash was measured using a muffle furnace set at 550°C for 6 h. Using a muffle furnace and fiber extraction equipment, crude fiber was measured. Samples were heated to 100°C for acid and alkali boiling before being filtered through acetone. The residue was then burned in a muffle furnace for 3 h at 550°C. Based on the methodology from [67], a carbohydrate analysis was carried out. Each sample was given 5 mg of dried powder, which was then mixed with 25 mL of distilled water to create a solution. For uniform mixing, a tissue homogenizer was employed. Concentrated sulfuric acid (Merck, Germany) and a 5% phenol solution (Sigma–Aldrich) were prepared prior to the analysis. Samples were subjected to analysis by adding 5 mL of concentrated sulfuric acid and 1 mL of 5% phenolic solution. Glucose was used to prepare the standard. A UV-Vis double beam, Model-T80, HANNA spectrophotometer was used to determine the optical density at 488 nm and presented as (e.g., mg/g).
2.5.2. Pigments
The pigments of seaweeds (Ulva spp. and Gracilaria spp.) were measured according to Arnon [68]. Briefly, in order to get rid of salt, sand, and other debris, samples were first washed with distilled water. Afterwards, 500 mg of seaweed powder and 10 mL of 80% acetone were well-ground in a pestle and mortar, the homogenate was centrifuged for 15 min at 3000 rpm, and the supernatant was collected. After several washes (4–5 times) in 5 mL of 80% acetone until it turned colorless, the particle was extracted again in the same way. After being combined, all of the extracts were used to measure the pigments.
2.5.2.1. Estimation of Chlorophyl
Using Arnon’s [68] approach, the amount of chlorophyl in the algae was estimated. At 645 and 663 nm, absorbance was measured using a UV-Vis double beam spectrophotometer (Model-T80, Hanna). The following formula was used to calculate the chlorophyl content.
2.5.2.2. Estimation of Carotenoid
2.5.3. Estimation of Fucoxanthin
2.5.4. Fatty Acids
The amount of fatty acid was calculated using Prato et al. [71]. Using a Soxhlet device, the lipids were first extracted from the freeze-dried samples. Lipid extraction was carried out using diethyl ether as the solvent. The temperature was maintained at 60°C during the last stage of lipid extraction. Fatty acid methyl esters (FAMEs) were analyzed using gas chromatography mass spectrophotometry with a flame ionization detector utilizing a GCMS-QP2020 (Shimadzu, Japan) apparatus. An internal diameter of 0.25 mm, film thickness of 0.15 μm, phase ratio of 250, and length of 30 m were utilized to separate FAMEs. Around 1.42 milliliters per minute of helium were employed as the carrier gas. Following a 180–280°C gradient at a rate of 5°C per minute, the temperature was maintained at 280°C. Utilizing a standard (FAME mix C8-C24; Sigma–Aldrich, Germany) as a reference, retention periods were compared to identify FAMEs. Amounts were stated in parts per million. The percentage of total fatty acids (TFA) was then obtained.
2.5.5. Amino Acids
A small modification was made to the Moore and Stein [72] approach to detect amino acids. First, 1 g of dried biomass of microalgae was hydrolyzed in 25 mL of previously prepared acidic hydrolysis solution (6 M HCl + 0.1% phenol) for 24 h at 110 ± 2°C. After chilling, the samples were stabilized with a little amount of SDB/Na (sample dilution buffer). After that, the pH of the samples was adjusted between 2.1 and 2.3 using NaOH. The hydrolysates were then added to the injection vials after being filtered (0.45 µm) and diluted with SDB/Na. For the analysis, a SYKAM S 433 amino acid analyzer with a UV detector was utilized. Nitrogen gas was used as the carrier gas, with a consistent flow rate of 0.5 mL/min at 600°C and a reproducibility of 3%. The AA-S-18 standard were obtained from Sigma–Aldrich, Germany to test amino acid concentrations. The total amino acid content is expressed in mg/g of dry samples.
2.6. Statistical Analysis
Mean and standard error of the mean (SE = σ/√n) were calculated by using Microsoft Excel. All the water quality parameters, except TAL, were found non-normally distributed. However, depending on their level of skewness, Box Cox transformation was applied for water temperature, pH, salinity, DO, ORP, ACon, NO2-N, TAN, TSS, and SRP. Principal component analysis (PCA) was performed to assess the water quality parameters.
One-way multivariate ANOVA was performed to test variability in water quality parameters, LWG, SGR, survival rate, proximate compositions, pigments, fatty acids and amino acids of seaweeds and oysters. Two species of seaweeds were considered as two different treatments. Normality and heteroscedasticity of residuals were checked visually and through Shapiro–Wilk test and Levene’s test. When assumptions were met, Tukey’s honestly significant difference test was applied to compare treatments or samples (indoor and outdoor) as well as two seaweed species. The level of significance was set as 0.05. These tests were performed using SPSS (IBM v. 25.0) and R Studio (version 4.3.3) statistical software.
3. Results and Discussion
3.1. Water Quality Variables and Their Relationship
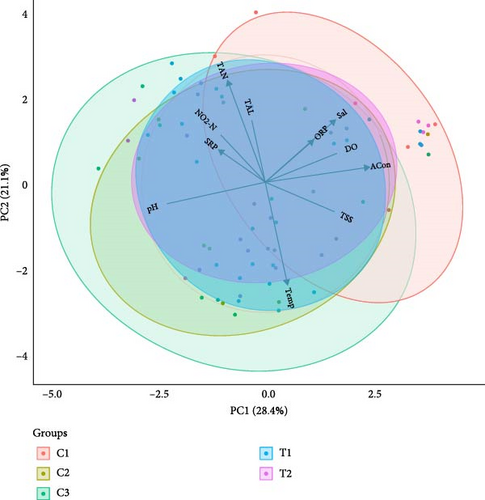
Among the water quality parameters that were recorded in every three alternate days, temperature ranged from 32.36 ± 0.09 – 22.41 ± 0.36°C, pH was 8.55 ± 0.10 – 6.61 ± 0.11, salinity was 32.37 ± 0.19 – 23.25 ± 0.60 g/L, concentration of DO was 7.00 ± 0.10 – 4.24 ± 0.01 mg/L, ORP was 266.67 ± 6.06 – 109.67 ± 8.27 mV, and ACon was 51.64 ± 2.60 – 37.27 ± 0.35 mS/cm across the treatments (Figure 2). On the other hand, among biweekly recorded variables, NO2-N was 0.363 ± 0.203 – 0.031 ± 0.001 mg/L, TAN was 0.040 ± 0.018 – 0.002 ± 0.001 mg/L, TAL was 157.000 ± 4.359 – 116.333 ± 6.741 mg/L, TSS was 39.200 ± 7.201 – 12.333 ± 2.450 g/L, and SRP was 1.632 ± 0.697 – 0.007 ± 0.003 mg/L (Figure S1). However, water quality parameters were not significantly (p > 0.05) different among the experimental tanks. The effect of water quality and their interrelationship across the seaweed coculture settings was represented through PCA biplot (Figure 2). It is easier to evaluate the PCA loads and variability represented in the computed PCA when the PCA’s eigenvalue is bigger than one. PC1 captured 28.44% of the variability with an eigenvalue of 3.13, while PC2 exhibited 21.13% having an eigenvalue of 2.32 (Figure 2, Table S1). PC1 accounted 28.44%, where greater positive loadings temperature, salinity, DO, ORP, ACon, and TSS were observed. PC2 accounted for 21.13%, where Temperature, pH, and TSS indicate negative interactions with all elements. Likewise, previous studies on bivalves and seaweeds also observed similar physio-chemical water quality variables, found in this study [73–76]. Apart from that, salinity ranges slightly deviated from the optimum ranges (25–35 g/L) for seaweed farming. However, each species may tolerate different range [77]. Therefore, culture of U. lactuca and G. verrucosa as two independent treatments may be influenced by the salinity range differently. Although the ranges of values of other variables also represent that the water quality was not stable throughout the experiment, there was no significant difference of water quality among the treatments during the whole experiment. The instability of water variables was the reflection of routine water exchange, while the water was collected from sea at different time during monsoon and post monsoon period.
3.2. Growth Parameters
3.2.1. Growth of Seaweeds
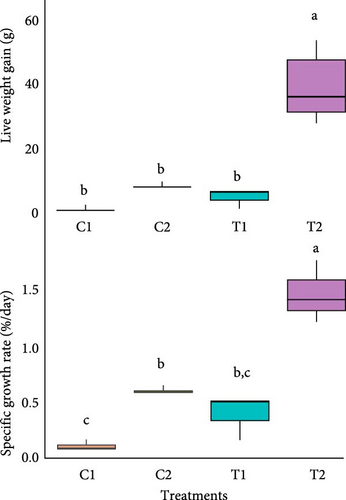
The growth of the two different species of seaweeds under the two treatments (coculture) varied significantly (p < 0.05). The highest LWG and SGR were observed in U. lactuca in T2. However, the LWG did not vary significantly among other treatment and controls (Figure 3). The SGR between C1 and T1 did not vary significantly. Both seaweeds gained weight at least slightly than the initial stocking weight (Figure 3). When the monsoon hits wild coastal areas, most seaweeds – including species like Ulva and Gracilaria – die back. However, they release their spores into the water, ensuring the next generation survives. [78, 79]. Apart from that, growth of U. lactuca was higher than G. verrucosa. However, the weight gain was not significantly different between independent cultures of G. verrucosa and coculture with oyster, even though SGR was significantly different between them. Unlikely, weight gain and SGR was significantly different between U. lactuca independent culture and coculture with oyster. Similarly, higher growth was recorded in previous study for Ulva sp. rather than Gracilaria sp. both indoor and outdoor culture condition [65, 80]. The SGR we observed for both seaweeds were significantly lower than those reported in previous indoor culture studies [81–83]. This difference is likely attributable to key variations in our experimental conditions. Unlike those studies, we added no supplemental nutrients to our tanks, potentially limiting nutrient availability. Furthermore, our cultures experienced lower light intensities and variable salinity levels, whereas other researchers maintained consistent salinity, used natural or optimized lighting, and supplemented their systems with nutrient media [81–83].
3.2.2. Survival and Growth of Oyster
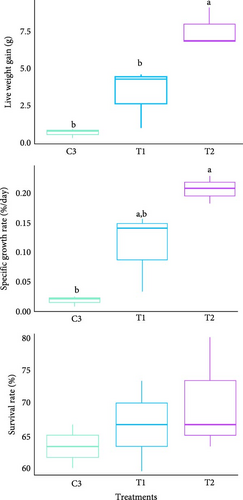
At the end of the experiment, survival rate of oyster varied from 60% to 80% across all treatments but not significantly (p > 0.05, Figure 4A). However, LWG and SGR varied significantly among the treatments (p < 0.05). Both the highest LWG and SGR were recorded in the oysters of T2 (G. verrucosa coculture) while LWG value in T1 (U. lactuca coculture) did not vary from control and SGR value in T1 did not vary significantly from any other treatments (Figure 4A). Apart from that, the size distribution ridge line showed distinct variation of length, width and thickness of oyster across the treatments (Figure 4B). Oysters showed uniform length growth during the initial 90 days, but clear differences in size developed between treatments after 120 days of experiment. Conversely, width and thickness of around 29.17% oysters also decreased after 90 days. Subsequently, the highest number of individuals with the highest length, width and thickness were observed in T2 (Figure 4B). Likewise, Tanyaros et al. [84] found much higher growth rates in juvenile oysters in semi-closed single species culture of C. belcheri. Previous research highlighted the vulnerability of Crassostrea oysters during monsoon, reporting up to 46.04% mortality at our collection site and alarming 100% losses elsewhere [47]. In our coculture system, we observed markedly improved survival: maximum mortality reached just 40% with U. lactuca and dropped to 36.7% with G. verrucosa. LWG and SGR were observed the highest while oysters were cocultured with U. lactuca rather than coculture with G. verrucosa or independent culture. However, the growth rate was not significantly different between independent culture and coculture with G. verrucosa. Our indoor cocultured oysters exhibited lower SGRs than the minimum 2.92% per day observed in their wild source population [64], likely due to limited food particles in our experimental tanks.
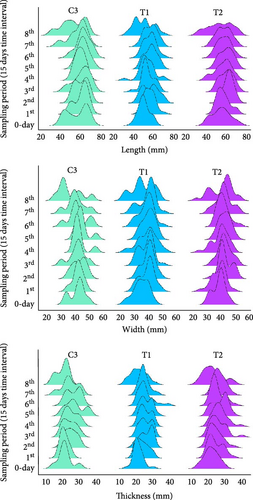
3.3. Proximate Composition of Seaweeds and Oysters
Moisture, ash, protein, carbohydrate and fiber varied significantly among the seaweeds from different sources (p < 0.05, Table 1). The significant variation in moisture, ash, protein, carbohydrate, and fiber content among seaweeds from different sources (p < 0.05) underscores the influence of environmental factors on their biochemical composition. Moisture content did not vary significantly between indoor and outdoor samples of G. verrucosa or U. lactuca (Table 1). Interestingly, our indoor U. lactuca samples had much lower moisture than outdoor G. verrucosa – likely because sunlight exposure drives significant drying in seaweeds, as Flores-Molina et al. [85] observed. The ash content did not vary significantly between indoor and outdoor samples of G. verrucosa and U. lactuca but varied between these two species significantly (Table 1). The observed ash content of G. verrucosa by Cirik et al. [86] and ash content of U. lactuca by Rasyid [87], also align with our findings. Protein content varied between two species but significant lower value was observed for outdoor samples of G. verrucosa. Although the protein content of G. verrucosa aligns with the findings of Cirik et al. [86], the value was much higher than the findings of Rasyid [87] for U. lactuca. Likewise, Marinho-Soriano et al. [88] reported that controlled environments could optimize nutrient availability, leading to enhanced protein and carbohydrate synthesis. Similarly, carbohydrate was the highest for indoor samples of U. lactuca but did not differ between indoor and outdoor samples of G. verrucosa. Unlike other nutrient contents, lipid content did not significantly differ between indoor and outdoor samples in either species, reinforcing findings by Cirik et al. [86] and Rasyid [87]. Besides, Burtin [89] stated that seaweeds are low in fat content (1%–5%) where we report value between 1.90 and 2.69% for U. lactuca and 3.24%–5.35% for G. verrucosa in indoor cocultures. On the other hand, for both species, fiber content was found higher in outdoor samples. Jeong-Ryong et al. [90] found much higher dietary fibers in G. verrucosa and U. lactuca than we observed in this study, while both species had similar fiber content. In contrast, Rasyid [87] found similar fiber content in U. lactuca in previous study. Therefore, highlights of the proximate content of seaweeds suggest that the utilization of indoor farming techniques will not compromise major nutrient benefits.
| Proximate | Gracilaria (indoor) |
Gracilaria (outdoor) |
Ulva (indoor) |
Ulva (outdoor) |
Oyster (indoor) |
Oyster (outdoor) |
|---|---|---|---|---|---|---|
| Moisture (% of wet weight) | 80.09 ± 1.42a, b | 83.60 ± 0.94a | 75.99 ± 0.98b | 79.14 ± 0.82a, b | 70.73 ± 1.05 | 71.66 ± 1.04 |
| Protein (% of dry weight) | 24.54 ± 0.82a | 19.79 ± 0.46b | 25.39 ± 0.72a | 25.72 ± 0.59a | 41.81 ± 1.01 | 46.33 ± 1.27 ∗ |
| Lipid (% of dry weight) | 4.36 ± 0.61 | 4.30 ± 0.34 | 2.18 ± 0.26 | 1.43 ± 0.24 | 18.67 ± 1.23 | 25.29 ± 0.73 ∗ |
| Carbohydrate (% of dry weight) | 38.56 ± 0.62b | 39.29 ± 0.81b | 43.14 ± 0.18a | 39.19 ± 0.63b | 22.26 ± 0.56 | 14.27 ± 0.91 |
| Fiber (% of dry weight) | 17.04 ± 0.81b | 21.45 ± 0.38a | 17.27 ± 0.62b | 23.22 ± 0.77a | 1.31 ± 0.09 | 0.81 ± 0.13 |
| Ash (% of dry weight) | 14.11 ± 0.08a | 12.93 ± 0.36a | 10.81 ± 0.07b | 8.94 ± 0.08b | 12.61 ± 0.63 | 11.62 ± 0.71 |
- Note: Values with different letters and in bold within each row are significantly different (p < 0.05) among indoor and outdoor seaweeds, and with different asterisk ( ∗) within each row are significantly (p < 0.05) different between indoor and outdoor oysters. Values are mean ± SE.
Protein, lipid and carbohydrate content varied between indoor and outdoor samples of oyster. Being a filter feeding organism, oyster filter the microorganisms from the water column which subsequently causes the variation in nutrient composition in oyster [91]. Although, these findings slightly varied with the previous studies on farmed oyster in Bangladesh, this may occur due to the harvesting seasons [92, 93]. However, according to Martino and Cruz [94], proximate composition of C. belcheri from both indoor and outdoor cultures offer a good percentage of carbohydrate, protein and fiber contents which is nutritionally good for human health.
3.4. Biochemical Composition of Seaweeds and Oyster
3.4.1. Pigments of Seaweeds
Chlorophyl a, chlorophyl b, total chlorophyl, carotenoids, and fucoxanthin varied significantly among the different seaweed samples (p < 0.05, Table 2). The highest chlorophyl a, chlorophyl b, total chlorophyl, carotenoids, and fucoxanthin were found in U. lactuca (indoor) sample while the lowest were observed in G. verrucosa (outdoor). Thus, G. verrucosa (indoor) sample also showed significant higher pigments compared to G. verrucosa (outdoor) samples (Table 2). The combined amount of chlorophyl a and b gives a comprehensive picture of the seaweed’s capacity for photosynthetic activity. Our findings support the concept of Ak and Yücesan [95] that indoor culture conditions optimize pigment accumulation in seaweeds. Notwithstanding, Ak and Yücesan [95] reported higher pigments in G. verrucosa than our observation when grown under 50 µmol.photons.m−2 s−1 light intensity. Likewise, Abd El-Baky et al. [96] observed much higher pigments in U. lactuca when cultured in natural seawater, like we did in our study. On the other hand, the considerable differences in fucoxanthin and carotenoid concentrations among the indoor and outdoor seaweeds samples demonstrate the various photosynthetic and defense mechanisms that each variety of seaweed uses [97]. Carotenoids and fucoxanthin were also recorded at the highest content in U. lactuca indoor samples compared to the other three samples (Outdoor U. lactuca, indoor and outdoor G. verrucosa). However, all the pigments were higher in indoor seaweed samples, regardless of the species. Similar to chlorophyl, carotenoids and fucoxanthin were observed lower than the findings of Abd El-Baky et al. [96] for U. lactuca and Ak and Yücesan [95] for G. verrucosa. Notably, Abd El-Baky et al. [96] propose switching to artificial seawater to boost pigments, as it delivers tailored nutrients and avoids the salinity fluctuations of natural seawater. Thus, these findings may offer to apply indoor coculture as an alternative to outdoor mass seaweed farming during monsoon.
| Pigments (μg/g FW) | Gracilaria (indoor) |
Gracilaria (outdoor) |
Ulva (indoor) |
Ulva (outdoor) |
|---|---|---|---|---|
| Chlorophyl a | 662.61 ± 2.97b | 520.13 ± 1.98c | 1067.92 ± 2.32a | 638.56 ± 12.99b |
| Chlorophyl b | 159.93 ± 1.08c | 107.00 ± 1.80d | 865.94 ± 5.53a | 534.19 ± 1.85b |
| Total chlorophyl | 822.35 ± 3.69c | 626.99 ± 2.62d | 1933.28 ± 6.74a | 1172.41 ± 13.40b |
| Carotenoids | 26.21 ± 0.10b | 16.77 ± 0.05d | 47.04 ± 0.16a | 21.65 ± 0.20c |
| Fucoxanthin | 0.17 ± 0.002c | 0.10 ± 0.002d | 0.59 ± 0.001a | 0.29 ± 0.003b |
- Note: Values are mean ± SE. Values with different letters within each row indicate significant difference among indoor and outdoor samples of G. verrucosa and U. lactuca (p < 0.05).
3.4.2. Fatty Acids
Fatty acids content varied significantly among the indoor and outdoor samples of seaweeds and oyster species (Table 3). However, methyl myristate, methyl behenate, methyl cis-11-eicosenoate, methyl nervonate, methyl arachidonate, methyl linolenate, methyl eicosapentaenoate did not vary significantly (p > 0.05) among the indoor and outdoor seaweed samples (Table 3). On the other hand, only methyl laurate, methyl tridecanoate, methyl stearate, methyl palmitoleate, and methyl linolenate varied significantly (p < 0.05) between indoor and outdoor oysters (Table 3).
| Carbon | Fatty acids | Gracilaria (indoor) | Gracilaria (outdoor) | Ulva (indoor) | Ulva (outdoor) | Oyster (indoor) | Oyster (outdoor) |
|---|---|---|---|---|---|---|---|
| C8:0 | Methyl octanoate | 2.39 ± 0.11c | 4.94 ± 0.16a | 3.91 ± 0.18b | 3.54 ± 0.21b | 0.15 ± 0.01 | 0.19 ± 0.02 |
| C10:0 | Methyl decanoate | 3.60 ± 0.27b | 5.16 ± 0.17a | 0.11 ± 0.002c | 4.19 ± 0.20a, b | 0.05 ± 0.003 | 0.05 ± 0.01 |
| C12:0 | Methyl laurate | 0.21 ± 0.09b | 0.10 ± 0.001b | 0.01 ± 0.005b | 11.68 ± 0.47a | 0.09 ± 0.001 | 9.13 ± 0.98 ∗ |
| C13:0 | Methyl tridecanoate | 0.17 ± 0.01b | 0.18 ± 0.08b | 0.08 ± 0.01b | 3.67 ± 0.10a | 0.06 ± 0.1 | 0.17 ± 0.02 ∗ |
| C14:0 | Methyl myristate | 0.14 ± 0.02 | 0.92 ± 0.61 | 0.67 ± 0.02 | 0.12 ± 0.06 | 1.30 ± 0.87 | 1.19 ± 0.06 |
| C16:0 | Methyl palmitate | 5.13 ± 1.03a, b | 2.17 ± 0.62b, c | 0.98 ± 0.09c | 8.27 ± 0.32a | 0.93 ± 0.19 | 2.23 ± 0.65 |
| C18:0 | Methyl stearate | 17.45 ± 1.06a | 20.06 ± 0.26a | 19.79 ± 0.24a | 10.66 ± 0.30b | 2.77 ± 0.01 ∗ | 0.82 ± 0.21 |
| C20:0 | Methyl arachidate | 7.16 ± 0.42b, c | 8.52 ± 0.13a, b | 5.99 ± 0.55c | 9.28 ± 0.18a | 0.72 ± 0.24 | 2.32 ± 0.93 |
| C17:0 | Methyl heptadecanoate | 1.39 ± 0.16c | 0.77 ± 0.08c | 2.39 ± 0.15b | 6.68 ± 0.04a | 2.76 ± 0.05 | 2.03 ± 0.65 |
| C21:0 | Methyl heneicosanoate | 0.49 ± 0.11b | 1.15 ± 0.15b | 0.78 ± 0.26b | 7.25 ± 0.59a | 1.32 ± 0.29 | 0.79 ± 0.10 |
| C22:0 | Methyl behenate | 0.37 ± 0.18 | 0.77 ± 0.08 | 0.13 ± 0.01 | 0.32 ± 0.13 | 0.50 ± 0.11 | 0.15 ± 0.01 |
| C16:1 | Methyl palmitoleate | 0.51 ± 0.11d | 4.82 ± 0.39b | 17.07 ± 0.21a | 2.76 ± 0.11c | 18.35 ± 0.35 ∗ | 11.50 ± 0.60 |
| C18:1 | Methyl oleate | 0.25 ± 0.01c | 0.73 ± 0.004c | 6.15 ± 0.08b | 7.33 ± 0.28a | 8.92 ± 3.47 | 0.94 ± 0.01 |
| C20:1 | Methyl cis-11-eicosenoate | 0.01 ± 0.01 | 0.01 ± 0.004 | 0.01 ± 0.001 | 0.02 ± 0.02 | 25.61 ± 0.46 | 30.90 ± 1.94 |
| C22:1 | Methyl erucate | 12.23 ± 0.32b | 12.03 ± 1.53b | 19.16 ± 0.16a | 3.46 ± 0.11c | 2.45 ± 1.42 | 5.41 ± 0.11 |
| C24 : 1 | Methyl nervonate | 0.04 ± 0.02 | 0.16 ± 0.08 | 0.01 ± 0.003 | 0.01 ± 0.0004 | 0.02 ± 0.002 | 0.02 ± 0.01 |
| C18:2n-6 | Methyl linoleate | 40.50 ± 1.17a | 29.21 ± 2.13b | 10.25 ± 0.87c | 4.34 ± 0.02c | 17.95 ± 1.90 | 18.50 ± 0.63 |
| C20:3n-6 | Methyl 11-14-17-eicosatrienoate | 2.51 ± 0.11b | 2.09 ± 0.14b | 0.28 ± 0.09c | 9.01 ± 0.12a | 14.61 ± 0.97 | 10.05 ± 0.67 |
| C20:4n-6 | Methyl arachidonate | 0.06 ± 0.02 | 0.07 ± 0.003 | 0.26 ± 0.13 | 0.10 ± 0.07 | 0.04 ± 0.03 | 0.02 ± 0.000 |
| C18:3n-3 | Methyl linolenate | 5.21 ± 0.30 | 5.90 ± 0.39 | 6.27 ± 0.01 | 6.01 ± 0.97 | 1.04 ± 0.05 | 3.04 ± 0.25 ∗ |
| C20:5n-3 | Methyl eicosapentaenoate | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.87 ± 0.31 | 0.53 ± 0.28 | 0.003 ± 0.000 | 0.007 ± 0.001 |
| C22:5n-3 | Methyl docosapentaenoate | 0.01 ± 0.01b | 0.04 ± 0.02b | 0.02 ± 0.01b | 0.22 ± 0.01a | 0.01 ± 0.001 | 0.01 ± 0.001 |
| C22:6n-3 | Methyl docosahexanoate | 0.15 ± 0.01c | 0.16 ± 0.02c | 4.82 ± 0.07a | 0.59 ± 0.08b | 0.36 ± 0.01 | 0.56 ± 0.09 |
- Note: Values with different letters within each row are significantly different (p < 0.05) among indoor and outdoor seaweed samples, and with different asterisk ( ∗) within each row are significantly (p < 0.05) different between indoor and outdoor oysters. Significantly different values are in bold. Values are means of duplicates with error bar (standard error; SE = σ/√n).
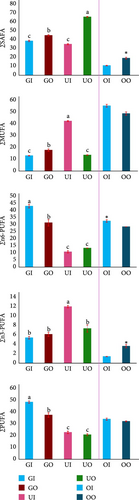
Different groups of fatty acids also varied significantly among different seaweed species (p < 0.05, Figure 5A). The highest SAFA was observed in Ulva (outdoor) while the lowest was in U. lactuca (indoor) but significantly indifferent (p > 0.05) from G. verrucosa (indoor) samples. The highest monounsaturated fatty acid (MUFA), n6 (omega 6 fatty acid)- polyunsaturated fatty acid (PUFA), n3 (omega 3 fatty acid)-PUFA and PUFA were recorded in U. lactuca (indoor), G. verrucosa (indoor), U. lactuca (indoor), and G. verrucosa (indoor), respectively. Consequently, the fatty acid ratios also varied significantly among the seaweed samples (p < 0.05, Figure 5B). The highest n3/n6, n6/n3, docosahexaenoic acid (DHA)/eicosapentaenoic acid (EPA), SAFA/TUFA (total unsaturated fatty acids), SAFA/TFA, and TUFA/TFA were recorded in U. lactuca (indoor), G. verrucosa (indoor), G. verrucosa (outdoor), U. lactuca (outdoor), U. lactuca (outdoor), and U. lactuca (indoor), respectively.
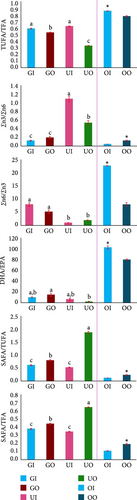
Conversely, only SAFA, n3 and n6 fatty acid groups varied significantly between indoor and outdoor oyster samples (p < 0.05, Figure 5A). The highest SAFA and n3 fatty acids were observed in outdoor oyster samples while n6 fatty acid was the highest in indoor oyster sample. All the ratios of fatty acids were significantly different between indoor and outdoor oyster samples (p < 0.05, Figure 5B). The highest n6/n3, DHA/EPA, and TUFA/TFA were observed in indoor oyster sample while the highest n3/n6, SAFA/TUFA, and SAFA/TFA were observed in outdoor oyster sample.
The findings of this study deviated from the findings of Khotimchenko [98] for G. verrucosa, and Ortiz et al. [99] and Yaich et al. [100] for U. lactuca. In this study, the most abundant fatty acid in G. verrucosa was linoleic acid while Khotimchenko [98] reported arachidonic acid as the most abundant fatty acids for G. verrucosa. On the other hand, the most abundant fatty acid in U. lactuca was stearic acid for indoor sample while Ortiz et al. [99] found oleic acid as the most abundant fatty acid in their study for U. lactuca. Low grade SAFA can cause significant cardiovascular diseases [101]. Crucially, lower saturated fats in our indoor-grown seaweeds suggest these cultivation systems may offer greater health advantages than traditional sources (Figure 5A). Conversely, our indoor cocultured G. verrucosa and U. lactuca accumulated higher levels of unsaturated fatty acids – compounds linked by Kapoor et al. [102] to critical health benefits like improved brain function, cardiovascular protection, and immune support. This nutritional edge highlights a key advantage of controlled indoor farming over traditional outdoor methods. Indoor-cultured U. lactuca showed the most favorable n3/n6 ratio in our study – aligning with the reported values of Ortiz et al. [99] – indicating a nutritionally optimized profile linked to cardiovascular protection and reduced inflammation [103]. On the other hand, indoor sample of G. verrucosa showed the greatest n6/n3 ratio that also aligns with the findings of Khotimchenko [98] (Figure 5B). Although outdoor G. verrucosa had the greatest DHA/EPA ratio, it was not significantly different from both indoor G. verrucosa and U. lactuca. The ratio of DHA to EPA can affect several physiological processes (e.g., niacin-induced cutaneous flushing, antioxidant pathway, cardiac ischemia-reperfusion, anti-inflammatory signaling pathway), and it is essential for brain health [104, 105]. The samples from U. lactuca (indoor) exhibited the greatest TUFA/TFA ratio, suggesting a greater content of unsaturated fats, which are generally regarded as healthier [104]. For enhanced nutritional value, indoor coculture systems with these seaweeds might be worth exploring further as an alternative to traditional outdoor approaches.
Octanoic acid, decanoic acid, myristic acid, palmitic acid, arachidic acid, heptadecanoic acid, heneicosanoic acid, behenic acid, oleic acid, eicosenoic acid, erucic acid, nervonic acid, linoleic acid, eicosatrienoic acid, arachidonic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, and DHA did not vary between indoor and outdoor samples of oyster during the experiment (Table 3). Notably, we detected higher levels of six key fatty acids in our oysters – heptadecanoic, heneicosanoic, oleic, eicosenoic, erucic, and eicosatrienoic acid – compared to the lower values reported by Minhaz et al. [64] in wild oysters from our same outdoor collection site. On the other hand, lauric acid, tridecanoic acid, stearic acid, palmitoleic acid, and linolenic acid varied significantly between indoor and outdoor samples, while stearic acid and palmitoleic acid was higher in indoor samples of oyster. Interestingly, Minhaz et al. [64] reported at least 10 times higher lauric acid and tridecanoic acid in outdoor oysters from our same collection site. Since we harvested oysters in late monsoon while Minhaz et al. [64] collected theirs in late winter, seasonal differences likely explain these variations – a pattern also noted by Linehan et al. [106]. Apart from that, saturated fatty acid was higher in outdoor oyster samples, while mono and PUFA did not vary between the two samples. Long-chain omega-3 PUFAs must be taken through diet by humans as they cannot synthesize those fatty acids [107]. However, we found higher omega-6 PUFAs than omega-3 PUFAs, with omega-6 PUFAs abundant indoor oysters, and omega-3 PUFAs abundant outdoor oysters. Notwithstanding, Martino and Cruz [94] and Prato et al. [108] reported higher omega-3 fatty acids than omega-6 fatty acids, resulting in varied n-3/n-6 PUFA and DHA/EPA ratios compared to our findings. Interestingly, Minhaz et al. [64] also detected elevated omega-6 fatty acids at the same coastal site – aligning with our results despite seasonal differences in harvest timing. Likewise, all the fatty acid ratios reported in our study were similar to the findings of Minhaz et al. [64] except DHA/EPA. Oysters mostly consume phytoplankton, which is available in coastal locations in varying quantities depending on the season and geography [109]. Bachok et al. [110] found that the content of energetically significant fatty acids in the water rose as a result of the increased phytoplankton in the water. Dalsgaard et al. [111] found distinct fatty acid patterns in the tissue of marine primary producers that are immutably transferable to higher trophic level species through metabolic processes when consumed. The presence of herbivore zooplankton, algae, and fungi as a dietary source is indicated by the availability of 20:1 and C18:2n-6 in marine bivalves [112–114]. Furthermore, a higher amount of C22:6n-3 in tissues indicates that dinoflagellates are a primary source of food [115, 116]. Conversely, the presence of C22:6n-3, C20:1, and C14:0 indicates the abundance of dinoflagellates, herbivorous zooplankton, and diatoms, while the presence of C20:5n-3 and C16:1 suggests the dominance of diatoms [112, 115–117]. The concentrations of C20:4n-6, C18:2n-6, and C17:0 in marine bivalves are indicative of the abundance of bacteria, algae, fungus, and diatoms [113, 114, 118, 119]. As we fed only Chlorella vulgaris and Chaetoceros gracilis for feeding indoor oysters, this is likely to vary their fatty acid contents compared to outdoor oyster where they can get a variety of food sources. However, the lack of significant lipid variation in these cases suggests indoor oyster farming in coculture setting can be explored during monsoon seasons – where natural outdoor culture systems typically fluctuate.
3.4.3. Amino Acids
Around nine essential amino acids (EAAs) and eight non-essential amino acids (NEAAs) were assessed in samples of seaweeds and oysters. The highest total EAAs were reported (113.14 ± 1.29 mg/g) in indoor U. lactuca and the lowest (56.36 ± 2.41 mg/g) in indoor G. verrucosa. Likewise, the highest total NEAAs were observed (179.60 ± 1.15 mg/g) in indoor U. lactuca and the lowest (72.45 ± 0.15 mg/g) in indoor G. verrucosa. Among all the amino acids, cysteine did not vary significantly (p > 0.05) and was the lowest across indoor and outdoor seaweeds (Figure 6). Out of other NEAAs, alanine, aspartic acids, glutamine, serine, and tyrosine were the highest in indoor U. lactuca and lowest in indoor G. verrucosa (Figure 6). Although glycine and proline were the highest in indoor U. lactuca, the lowest were recorded in outdoor G. verrucosa and U. lactuca, respectively. However, arginine was the lowest in outdoor U. lactuca, histidine was the lowest in outdoor G. verrucosa, and the rest other amino acids were the lowest in indoor G. verrucosa. Because the human body cannot synthesis EAAs and must receive them through diet, they are essential for many physiological processes [120]. The highest EAAs concentration in indoor cocultured U. lactuca suggests that U. lactuca is very amenable to the buildup of EAAs when grown indoors, maybe because of ideal growth circumstances (nutrients, temperature, light intensity, photoperiod, pH, DO, etc.) that encourage protein synthesis [121]. The enhanced EAA profile found in U. lactuca is consistent with earlier studies showing that regulated conditions can improve seaweed’s nutritional value [122]. NEAAs, which help protein synthesis and metabolic functions, are also important for good health [123]. Indoor Ulva samples had the highest non-essential amino acid (NEAA) content, while indoor G. verrucosa samples had the lowest. Increased NEAA levels of indoor U. lactuca imply that controlled surroundings promote amino acid production, which raises the nutritional value of the food. This finding is in line with Harnedy and FitzGeralds’ [124] research demonstrating that environmental influences have a major impact on the amino acid composition of seaweed. Contrarily, values of amino acids deviated from the findings of Shiomi et al. [125] for G. verrucosa that reported higher values of serine, glycine, alanine, and isoleucine than this study. Notably, all the amino acids except Valine were observed higher in this study than Yaich et al. [100] reported for U. lactuca. However, the values of total EAAs and total NEAAs were consistent with Shiomi et al. [125] for G. verrucosa and Yaich et al. [100] for U. lactuca. Apart from that, only aspartic acid was significantly higher in outdoor G. verrucosa compared to other seaweed samples (indoor G. verrucosa, indoor and outdoor U. lactuca), resulting in higher NEAAs as well. Conversely, both total EAAs and total NEAAs were significantly higher in U. lactuca indoor sample compared to other seaweed samples (indoor and outdoor G. verrucosa, outdoor U. lactuca). Therefore, a significantly higher concentration of essential and NEAAs can be obtained by indoor growing, particularly U. lactuca, which seems to benefit from this practice.
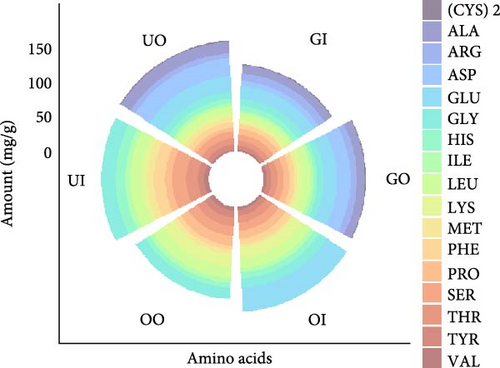
Conversely, alanine, glutamine, proline, serine, tyrosine, arginine, histidine, lysine, methionine, phenylalanine, and threonine were significantly different between two oyster samples (p < 0.05, Figure 6). Aspartic acid, glycine, cysteine, isoleucine, leucine, and valine were not significantly different between indoor and outdoor oyster samples (p > 0.05, Figure 6). Although essential amino acid (EAA) content did not different between indoor and outdoor oyster samples, NEAAs varied significantly between them. The highest non-essential amino acid (NEAAs) was observed 140.03 ± 1.22 mg/g in outdoor oyster. Since they must be received from nutrition, EAAs such as lysine, methionine, phenylalanine, and threonine are vital for human health [120]. The fact that there is no discernible change suggests that oysters, whether grown indoors or outdoors, are a reliable source of vital amino acids, which makes them useful for nutritional purposes. Conversely, the amount of NEAAs differed considerably between oyster samples collected indoors and outdoors, with outdoor oysters having the greatest NEAAs concentration. The amino acids alanine, glutamine, proline, and serine, or NEAAs, are essential for tissue healing, immunological response, and metabolic processes [123]. Because of parameters such as water movement, natural food availability, and environmental stressors, outdoor settings may encourage higher synthesis or storage of these amino acids, as seen by the substantial fluctuation in NEAAs levels [123]. This result supports research demonstrating that environmental factors have a major impact on the metabolic makeup of marine organisms [126]. Additionally, it is also proved that the variation and availability of food may result in significant changes in amino acid profile of oysters [127]. The restricted microalgal diet in this study (Chlorella vulgaris and Chaetoceros gracilis) led to distinct amino acid signatures compared to outdoor oysters– likely reflecting diet-driven metabolic adaptations. Thus, indoor coculture could offer C. belcheri safe monsoon sheltering and later returning them to the wild may preserve their nutritional quality for human consumption.
4. Conclusions
The study investigated how indoor and outdoor cultivation conditions affected U. lactuca, G. verrucosa, and C. belcheri growth and biochemical composition. No significant variations of water quality parameters were observed among the treatments. U. lactuca showed increased growth and buildup of pigment under coculture setting, suggesting that regulated conditions improve its nutritional value. On the other hand, G. verrucosa displayed fewer notable growth variations among culture conditions. The growth and survival rates of oysters varied, with indoor circumstances producing superior results in terms of growth rate and survival during coculture with U. lactuca. Further research efforts can explore the potentiality of artificial seawater for improving the survival rate of oyster in coculture setting. Apart from that, only the lipid content did not vary among indoor and outdoor seaweeds samples. On the other hand, particularly protein and lipid contents varied significantly between indoor and outdoor oysters. Increased nutrient accumulation was encouraged by indoor circumstances, especially in U. lactuca, which had increased amounts of both essential and NEAAs, indicating an enhanced nutritional profile. Seaweeds grown indoors were shown to have higher levels of unsaturated fatty acids and lower levels of SAFA, which are associated with greater health advantages. The nutritional reliability of oysters was confirmed by the study, which did not find any significant changes in the necessary amino acid content between biomass grown indoors and outdoors. Nonetheless, outdoor oysters have larger levels of NEAAs, possibly as a result of natural environmental variables. In summary, growing U. lactuca and G. verrucosa in indoor coculture setting may provide significant nutritional advantages without sacrificing important nutrient benefits, especially in the event of a bad monsoon, ensuring maximum space utilization. Additionally, oysters retain their nutritional quality in a variety of settings, indicating the potential benefits of indoor production as a backup strategy in inclement weather. The growth and nutritional value of aquaculture species can be controlled by using controlled settings, as this study emphasizes.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Sadia Afrin: writing – original draft, conceptualization, methodology, execution, data curation, formal analysis. Mohammad Ekramul Haque: writing – review and editing, data collection, data curation. Mahima Ranjan Acharjee: writing – review and editing, data collection. Sifatun Nur: writing – review and editing, data collection. Trina Das: writing – review and editing, data curation. Subeda Newase: writing – review and editing, data curation. Tashrif Mahmud Minhaz: validation, statistical analysis, supervision. Helena Khatoon: writing – critical review and editing, supervision.
Funding
This research was partially funded by the Ministry of Science and Technology Fellowship for MS in 2023–24, Bangladesh, through the National Science and Technology Fellowship. It was also partially funded by the University Grants Commission, Bangladesh, through the UGC Fellowship Program in 2023–24.
Acknowledgments
The authors express their gratitude to the Director, Coastal Biodiversity and Wildlife Research Centre, Chattogram Veterinary and Animal Sciences University, Cox’s Bazar to avail all of their facilities for conducting field experiments.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Data will be made available upon request.




