Impact of Acute and Long-Term Hypoxia and Hyperoxia on Antioxidant Metabolism and Gene Expression in Juvenile Rainbow Trout (Oncorhynchus mykiss)
Abstract
Aquatic organisms experience oxidative stress due to environmental stressors that promote oxygen exchange, such as eutrophication, algal photosynthetic activity, or changes in water temperature. This study aimed to clarify the effects of oxidative stress on the liver of juvenile rainbow trout (Oncorhynchus mykiss) exposed to hypoxia and hyperoxia by examining the physiological roles of hydrogen peroxide, glutathione (GSH) redox status, malondialdehyde (MDA) concentration, antioxidant enzyme activity, and antioxidant gene expressions. Dissolved oxygen levels were maintained at 4.0 ± 0.5 mg/L for hypoxia, 7.5 ± 0.5 mg/L for normoxia, and 12 ± 1.2 mg/L for hyperoxia. Liver samples were collected from each experimental group following exposure (6, 12, 24, 48, and 72 h) and chronic exposure (28 days). Under both hypoxia and hyperoxia conditions, reduced GSH levels and the oxidative stress index (OSI) decreased compared to normoxia (control group), whereas oxidized glutathione (GSSG) levels, the GSH/GSSG ratio, and MDA concentrations increased. Hydrogen peroxide levels were unstable. Superoxide dismutase (SOD) activity remained consistently lower than that of the control group. Catalase (CAT) activity decreased with chronic exposure to hyperoxia. Glutathione peroxidase (GPx) activity fluctuated with varying oxygen treatments. Glutathione-S-transferase (GST) activity increased after 12 h of hypoxia exposure but decreased with acute hyperoxia; however, chronic hyperoxia exposure eventually increased GST activity. Glutathione reductase (GR) activity is generally reduced under hypoxia. The expressions of SOD and CAT increased at 24 h but decreased at other times. GPx expression increased under chronic hypoxia but decreased under hyperoxia, while GST expression decreased with chronic treatments. Hypoxia and hyperoxia influence the antioxidant defense system in the fish liver through different pathways. While a coordinated relationship between gene expression and enzyme activities is observed under acute exposures, this coordination diminishes during chronic exposures, leading to the depletion of defense mechanisms. This suggests that the capacity of aquatic organisms to adapt to oxidative stress is limited and that post-transcriptional mechanisms play a significant role in regulating antioxidant responses.
1. Introduction
In aquaculture, sufficient concentrations of dissolved oxygen are required for glycolysis in the cytoplasm, the continual functionality of the mitochondrial electron transport chain, and energy production via oxidative phosphorylation [1]. These metabolic processes involve cytochrome oxidase, which catalyzes the reduction of molecular oxygen to water. More than 90% of the oxygen consumed by the organism is used in these reactions, which also involve the formation of various by-products [2]. These by-products are reactive oxygen species (ROS), such as the superoxide radical (), hydroxyl radical (˙OH), hydrogen peroxide (H2O2), single oxygen, lipid peroxides, peroxynitrite (ONOO−), and nitric oxide (NO). ROS are generated due to electrons leaking from oxidase reactions and the electron transport chain [3]. Low levels of ROS are related to signal transduction [4], and the regulation of various cellular activities such as cell cycle checkpoint, energy metabolism, redox balance [5], apoptosis, and regulation of protein and gene expression [6]. However, high levels of ROS cannot be tolerated by the organism and can lead to oxidative stress. Consequently, macromolecules such as proteins, lipids, and DNA are damaged, leading to the formation of malondialdehyde (MDA) and lipid peroxidation (LPO) [7].
The removal of radical and non-radical oxygen-derived species, as well as the mitigation of oxidative stress, is facilitated by enzymatic defense mechanisms involving superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-S-transferase (GST), and glutathione reductase (GR). Non-enzymatic ROS cleavages include glutathione (GSH), vitamin E, and ascorbate. These cleavages work together to remove ROS in cells [7]. SODs are metalloenzymes that catalyze the dismutation of the superoxide anion into hydrogen peroxide (H2O2) and molecular oxygen (O2) [8]. H2O2 is reduced to water by GPx in the cytosol and by CAT in peroxisomes utilizing reduced GSH as a cofactor. GST is a crucial member of of xenobiotic detoxification enzymes that function in pro-oxidant environments to facilitate the detoxification of hazardous compounds created by oxidative stress. GR utilizes nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) to convert oxidized glutathione (GSSG), into its reduced form GSH, maintaining cellular redox homeostasis t. Oxidative stress induces the production of SOD, CAT, GPx, GST, and GR. The levels of these enzymes are directly related to the amount of oxidative stress in the cells. GSH and glutathione disulfide (GSSG) are crucial intracellular thiols for biological processes [7, 9]. MDA is an oxidative product generated by the oxidation of polyunsaturated fatty acids in the cell membrane structure and by free radicals-induced LPO [10]. The activity levels of these antioxidant enzymes and the amounts of components directly or indirectly linked to antioxidant capacity are important for the antioxidant defense system and the detoxification mechanism. Impairment or inhibition of these enzymes, which are crucial for antioxidant defense, may lead to mass fish mortality [11].
Organisms in aquatic environments are temporarily or continuously subject to variations in oxygen levels, on both daily and seasonal scales, due to factors such as temperature, water flow, atmospheric conditions, eutrophication, algal photosynthetic activity, organismal respiratory, and increased biomass and production in aquaculture [12–14]. For this reason, oxygen concentration is one of the most important environmental parameters for aquatic organisms. Aquatic hypoxia in freshwater environments is defined as dissolved O2 concentrations below 5–6 mg O2/L [15]. Hyperoxia is commonly defined as a higher than normal O2 tension in water. An average of 12 mg O2/L is defined as a hyperoxic condition [16, 17]. Chronic exposure to hypoxia and hyperoxia can cause oxidative stress in fish [18]. Rainbow trout (Oncorhynchus mykiss) are recognized as a fish species particularly sensitive to hypoxia, especially when dissolved oxygen levels fall below 7 mg/L [19]. Hypoxia has been associated with increased respiratory rate and amplitude, as well as elevated aortic blood pressure, ultimately resulting in bradycardia [20] and a reduction in ATP concentration [21]. In addition, hypoxia reduces weight gain, specific growth rate (SGR), and survival rate (SR), thereby negatively affecting overall growth parameters. It also triggers stress responses and suppresses certain physiological processes [18]. In the literature, many studies related to hypoxia or hyperoxia can be found [22–24]. However, these studies often focus on protein levels or enzymatic activity, which we consider insufficient for understanding physiological responses in fish. To better understand both the acute and chronic effects of mild hypoxia and hyperoxia on metabolism, this study aimed to investigate the effects of these exposures on gene expression, enzyme activities, and antioxidant-related metabolites in the liver, a metabolically active organ.
2. Materials and Methods
2.1. Fish Material and Experimental Design
A total of 360 juvenile rainbow trout (O. mykiss), weighing 8.79 ± 1.8 g and aged 3 months, were obtained from a commercial company in Erzincan, Türkiye. The experimental application was carried out in an ARS-1500-RAS (Recirculation Aquaculture System) provided by Akuamaks, Türkiye. After a 2-week acclimatization phase, the fish were subjected to hypoxia, normoxia, and hyperoxia treatments for 28 days in 18 fiberglass tanks (6 tanks per group, 20 fish per tank) with a volume of 60 L each. During the experiment, a 12-hour light and 12-hour dark photoperiod was applied. The animals were fed commercial trout pellets at 1.5% of their body weight per day, divided into four feedings (at 08:00, 12:00, 16:00, and 20:00) [18]. Dissolved oxygen levels were maintained at 4.0 ± 0.5 mg/L for hypoxia, 7.5 ± 0.5 mg/L for normoxia, and 12 ± 1.2 mg/L for hyperoxia. The water temperature for the experimental groups was kept between 15.5 and 16.5°C. The pH levels were 7.2 ± 0.1 for hypoxia, and 7.4 ± 0.1 for both normoxia and hyperoxia. The temperature, pH, oxygen saturation level, and dissolved oxygen amount of the water were automatically monitored every minute using a Smartoxy Oximeter (Technos Company, Italy). Hypoxia was created by adding 30 mmHg of N2 to the water [25] and hyperoxia was provided by adding pure oxygen. All fish were starved 24 h prior to sampling and one fish was randomly selected from each tank for dissection at the end of the 28-day trial, yielding a total of six fish per treatment (e.g., for the hypoxia treatment: 6 tanks × 1 fish per tank = 6 fish). Each fish was stunned by a blow to the head and dissected. The liver samples were evaluated individually; thus, the resulting data represent the biochemical response of each fish at the individual level. All animal handling and trial protocols were approved by the Chair of the Local Ethics Committee of Animal Experiments at Atatürk University, Türkiye (Number: 36643897-119), and all treatments and trout sampling were conducted in accordance with these guidelines. Liver tissue samples were collected after 0, 6, 12, 24, 48, and 72 h to determine the acute effect, and after the 28th day to determine the chronic effect, for molecular studies (in RNAlater solution) and enzyme studies (by freezing in liquid nitrogen) from each experimental group. Tissues were stored at −80°C until analysis.
2.2. Determination of Glutathione, Malondialdehyde, and Hydrogen Peroxide Contents
Quantitative protein amounts were measured by spectrophotometer according to the Bradford Method [26]. The amounts of total glutathione (tGSH) and total glutathione disulfide (tGSSG) were determined using a µdrop spectrophotometer (Thermo, Multiskan GO) by modifying the method described by Griffith [27]. Based on these results, GSSG was calculated using the formula (GSSG = tGSSG/2), GSH was calculated using the formula (GSH = tGSH−GSSG), and the oxidative stress index (OSI) was calculated using the formula (OSI = (2 ∗ GSSG)/tGSH ∗ 100) [28].
The concentration of MDA, an LPO product, was measured colorimetrically using the Lipid Peroxidation (MDA) Assay Kit (Sigma-Aldrich) in accordance with the manufacturer’s recommendations.
Hydrogen peroxide concentration was determined colorimetrically using the Hydrogen Peroxide Assay Kit (Abcam, ab102500) following the manufacturer’s instructions.
2.3. RNA Isolation and cDNA Synthesis
Fifty milligrams of tissue was homogenized with 1 mL of QIAzol Lysis Reagent in a homogenizer. Then, 200 µL of chloroform was added and vortexed for 15 s. After a 2-minute incubation, the mixture was centrifuged at 12,000g at 4°C for 15 min. After taking 500 μL of the supernatant, RNA was isolated using a robot (Qiagen, Qiacube) and the RNeasy Mini Kit (Qiagen). Qualitative and quantitative assays of RNA were determined by measuring the concentration and purity using RNA gel electrophoresis and a μdrop spectrophotometer (Thermo, Multiskan GO), respectively. cDNA synthesis was performed with the SuperScript First-Strand Synthesis System (Invitrogen) Kit using 1 μg of total RNA. Samples were stored at −20°C until analysis.
2.4. TaqMan Probe and Primer Design
Specific primers and probes for β-actin (as a housekeeping gene), SOD, CAT, GPx, GST, and GR genes, as given in Table 1, were designed using Primer3web version 4.1.0 (https://bioinfo.ut.ee/primer3/). The oligonucleotide sequences obtained were verified using BLAST.
| Gene | Primer and probe | Sequence (5′→3′) | Product length (bp) | Genbank accession number | References |
|---|---|---|---|---|---|
| SOD | Forward | TGCTTATGGAGACAACACCAA | 156 | AF469663.1 | [11] |
| Reverse | TGGATGTTGATCTTAGCCACA | ||||
| Probe | FAM- CCACTGATGCTGTTCGGCACGT -TAMRA | ||||
| CAT | Forward | TCCTTTATCCACTCTCAGAAGCG | 125 | NM_001140302.1 | Primers and probe were designed |
| Reverse | CCACGGTCACTGAACAGGAA | ||||
| Probe | FAM- TGGTGTGGGACTTCTGGAGCCTGC -TAMRA | ||||
| GPx | Forward | GTGCCCTGCAACCAGTTT | 134 | AF281338.1 | [11] |
| Reverse | TTCCCATTCACATCCATCTTC | ||||
| Probe | FAM- CCGTCCCGGAAATGGCTTTGA -TAMRA | ||||
| GR | Forward | ATCACGCCATCACCACCAG | 117 | HF969248.1 | Primers and probe were designed |
| Reverse | CTTGCAACATCTCATCACAGCC | ||||
| Probe | FAM- GGCAAGGAGGAGAAGGTGGTTGGCC -TAMRA | ||||
| GST | Forward | TGGCTGACGTTATTGTCTTCC | 112 | NM_001160559.1 | [11] |
| Reverse | CTGGGTCTGTCCTTCACCATA | ||||
| Probe | FAM- CGGCGCGTTACCCCAAACTG -TAMRA | ||||
| β-Actin | Forward | TGGCCGTACCACCGGTAT | 79 | AF254414 | [29] |
| Reverse | GCAGAGCGTAGTCCTCGTAGATG | ||||
| Probe | Cy5- CTCCGGTGACGGCGTGACCC -BQ2 | ||||
- Abbreviations: CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; SOD, superoxide dismutase.
2.5. Gene Expression Analysis
cDNA concentrations were equalized to 200 ng/µL for use in quantitative real-time PCR. The expression levels of the genes studied were investigated using the Qiagen Rotor-Gene Q (Qiagen, Germany). The reaction tubes for each sample were prepared using FastStart TaqMan Probe Master (Applied Biosystems), following the manufacturer’s instructions. The reaction cycle consisted of an initial denaturation at 95°C for 10 minutes, followed by 45 cycles at 95°C for 15 s, and 60°C for 1 min. Real-time PCR data were analyzed using the mathematical modeling method of Pfaffl [30].
2.6. Determination of Specific Activities
During the preparation of homogenates for enzyme activity analysis, 60 ± 10 mg tissue samples were diluted in 50 mM KH2PO4 buffer solution (pH 7.5) at a 1:5 ratio and homogenized. The homogenization process was performed at 40 hertz using a Benchmark Bead Blaster 24 homogenizer. The resulting homogenate was then centrifuged at 13,000g for 1 h, and the supernatant was collected and transferred into tubes for further analysis. The specific activities of the enzymes were measured using a µdrop Spectrophotometer (Thermo, Multiskan Go). The specific activity of CAT was determined using the Beers and Sizer [31] method, SOD activity was assessed according to Sun, Oberley, and Li [32], GPx activity was measured using the Wendel [33] method, GST activity was analyzed following the Habig, Pabst, and Jakoby [34] method, and the specific activity of GR was determined using a modified version of the Carlberg and Mannervik [35] method.
2.7. Statistical Analysis
Statistical analyses on levels of antioxidant-dependent metabolites, enzyme activities, and mRNA expressions were performed by the SPSS 22.0 software package. Relative gene expression was calculated according to the efficiency-corrected 2−ΔΔCt method [30, 36]. The normality of the data was assessed using the Shapiro–Wilk test. One-way analysis of variance (ANOVA) was applied with Duncan’s multiple comparisons test to analyze differences among the gene expression of the experimental groups. Initial samples were excluded from statistical analyses to ensure accuracy and consistency The correlation of the parameters was calculated using the Pearson test (two-tailed) with bivariate correlation analysis. The correlation coefficient value is indicated as “r”. Statistical significance was set at p < 0.05. All values are shown as Mean ± SEM (n = 5).
3. Results
3.1. Glutathione Dependent Parameters
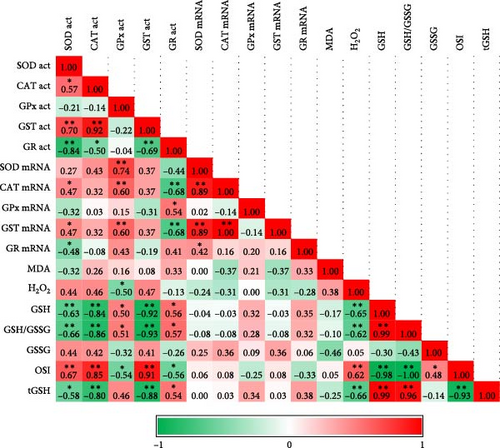
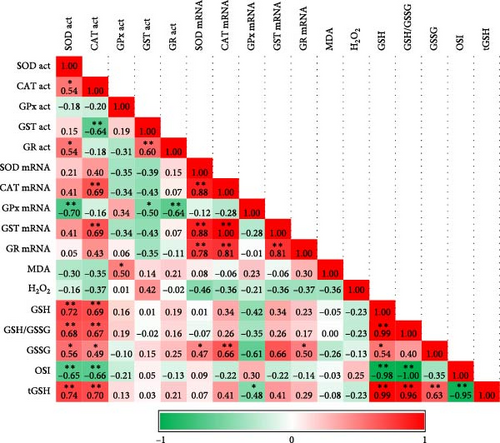
Acute hypoxia and hyperoxia treatments significantly increased the tGSH level compared to the control (normoxia) (p < 0.05). Conversely, chronic exposures significantly decreased it (p < 0.05). The GSH level was initially high with hypoxia treatment, but the chronic effect decreased the level (p < 0.05). GSSG levels increased inversely to GSH in acute exposures, but chronic exposure did not change the GSSG level. The level of the GSH/GSSG ratio, an oxidative stress indicator, was affected by hypoxia and hyperoxia and its level generally decreased. It was expected that GSH was significantly correlated with GSH/GSSG levels (Figure 1, p < 0.01). The OSI increased in comparison to control groups after 48 h, 72 h, and 28 days following exposure. Hyperoxia generally increased OSI compared to control groups. Interestingly, hypoxia affected the stress index after 48 h. Acute hypoxia and hyperoxia exposures did not change the MDA and H2O2 levels, and no correlation was found between them (Table 2, Figure 1).
Initial level |
Exposure | Acute exposures | Chronic exposure |
|||||
|---|---|---|---|---|---|---|---|---|
| 6 hr | 12 hr | 24 hr | 48 hr | 72 hr | 28 days | |||
|
0.021 | Normoxia | 0.028 ±0.0005aB | 0.023 ±0.0008aCD | 0.022 ±0.0009aD | 0.032 ±0.0003aA | 0.023±0.0010bCD | 0.024±0.0009bC |
| Hypoxia | 0.021 ±0.0005bC | 0.024 ±0.0007aB | 0.025 ±0.0010aB | 0.016 ±0.0008bD | 0.026±0.0012abB | 0.028±0.0008aA | ||
| Hyperoxia | 0.025 ±0.0019aA | 0.024 ±0.0032aA | 0.024 ±0.0030aA | 0.011 ±0.0018cB | 0.027±0.0006aA | 0.030±0.0010aA | ||
| H2O2 (pmole/µL) | 0.023 | Normoxia | 0.33 ± 0.01aC | 0.19 ± 0.01bD | 0.20 ± 0.01abD | 0.30 ± 0.01aC | 0.44 ± 0.01aB | 0.68 ± 0.01aA |
| Hypoxia | 0.11 ± 0.01bC | 0.46 ± 0.00aAB | 0.28 ± 0.03aBC | 0.41 ± 0.13aABC | 0.41 ± 0.15aABC | 0.61 ± 0.12aA | ||
| Hyperoxia | 0.30 ± 0.05aB | 0.31 ± 0.09abB | 0.14 ± 0.02bB | 0.92 ± 0.28aA | 0.41 ± 0.15aB | 0.60 ± 0.15aAB | ||
| tGSH (ng/mL) | 1.42 | Normoxia | 1.42 ± 0.02cB | 1.41 ± 0.02cB | 1.44 ± 0.02bB | 1.47 ± 0.02abAB | 1.45 ± 0.01bB | 1.52 ± 0.01aA |
| Hypoxia | 1.94 ± 0.02aaA | 1.50 ± 0.01bBC | 1.57 ± 0.04aB | 1.52 ± 0.0aBC | 1.55 ± 0.02aBC | 1.47 ± 0.02bC | ||
| Hyperoxia | 1.49 ± 0.01bB | 2.01 ± 0.02aA | 1.49 ± 0.01abB | 1.45 ± 0.0bB | 1.50 ± 0.03abB | 1.45 ± 0.02bB | ||
| GSH (ng/mL) | 1.04 | Normoxia | 0.74 ± 0.02bA | 0.73 ± 0.02bA | 0.74 ± 0.02abA | 0.74 ± 0.02aA | 0.75 ± 0.02aA | 0.76 ± 0.0aA |
| Hypoxia | 1.17 ± 0.02aA | 0.76 ± 0.01bBC | 0.79 ± 0.05aB | 0.69 ± 0.0bC | 0.75 ± 0.02aBC | 0.69 ± 0.0bC | ||
| Hyperoxia | 0.74 ± 0.0bB | 1.18 ± 0.03aA | 0.67 ± 0.01bCD | 0.65 ± 0.0bD | 0.72 ± 0.03aBC | 0.67 ± 0.02bCD | ||
| GSSG (ng/mL) | 1.51 | Normoxia | 0.68 ± 0.0cD | 0.68 ± 0.0cD | 0.70 ± 0.0cC | 0.73 ± 0.0cB | 0.70 ± 0.01cC | 0.76 ± 0.0aA |
| Hypoxia | 0.77 ± 0.0aC | 0.74 ± 0.0bD | 0.78 ± 0.0bC | 0.83 ± 0.0aA | 0.81 ± 0.01aB | 0.78 ± 0.01aC | ||
| Hyperoxia | 0.75 ± 0.01bD | 0.83 ± 0.01aA | 0.81 ± 0.0aB | 0.79 ± 0.0b | 0.78 ± 0.01b | 0.78 ± 0.0a | ||
| GSH/GSSG | 1.38 | Normoxia | 1.09 ± 0.03bA | 1.07 ± 0.03bA | 1.05 ± 0.04aA | 1.02 ± 0.02aA | 1.07 ± 0.03aA | 1.00 ± 0.01Aa |
| Hypoxia | 1.52 ± 0.02aA | 1.02 ± 0.02bB | 1.02 ± 0.06aB | 0.83 ± 0.0bC | 0.92 ± 0.02bC | 0.90 ± 0.01bC | ||
| Hyperoxia | 0.99 ± 0.01cB | 1.41 ± 0.05aA | 0.83 ± 0.01bCD | 0.82 ± 0.02bD | 0.93 ± 0.04bBC | 0.85 ± 0.02bCD | ||
| OSI | 84.21 | Normoxia | 95.65 ± 1.36bA | 96.48 ± 1.60aA | 97.39 ± 1.68bA | 99.24 ± 1.08bA | 96.74 ± 1.48bA | 100.04 ± 0.40bA |
| Hypoxia | 79.39 ± 0.51cD | 99.05 ± 0.92aC | 99.38 ± 3.08bC | 109.36 ± 0.23aA | 104.38 ± 1.24aB | 105.45 ± 0.50aAB | ||
| Hyperoxia | 100.40 ± 0.64aB | 82.94 ± 1.84bC | 109.20 ± 0.74aA | 109.64 ± 0.97aA | 103.74 ± 2.22aB | 108.21 ± 1.47aA | ||
- Note: Different letters denote significant differences from each other at p < 0.05 by one-way ANOVA with Duncan’s post hoc test. The statistically significant difference is indicated by different lowercase letters at each period and by different uppercase letters in each treatment from each other.
- Abbreviations: GSH, glutathione; GSSG, glutathione disulfide; MDA, malondialdehyde; OSI, oxidative stress index; tGSH, total glutathione.
3.2. Gene Expression Parameters
Exposures reduced SOD mRNA levels. Initially, CAT, GST, and GR mRNA levels were significantly reduced due to the exposures, they later increased and responded. However, these levels decreased significantly with chronic exposures. This was also consistent with the correlation analysis, which showed a significant positive correlation between SOD CAT and GST mRNA levels at hypoxia and hyperoxia (Figure 1). As a result of hyperoxia, GR expression was significantly positively correlated with SOD, CAT, and GST expression levels (Figure 1). We cannot emphasize that the level of GPx mRNA showed a steady increase or decrease, as it did not correlate with the level of activity. The only mRNA level that correlated with its activity was that of CAT under hyperoxia. Following exposure to hyperoxia, GR mRNA levels showed a positive correlation with SOD and CAT mRNA levels (Table 3, Figure 1).
| Gene | Initial mRNA level | Exposure | Acute exposures | Chronic exposure |
||||
|---|---|---|---|---|---|---|---|---|
| 6 hr | 12 hr | 24 hr | 48 hr | 72 hr | 28 days | |||
| SOD mRNA | 2.06 | Normoxia | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00cA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA |
| Hypoxia | 0.73 ± 0.04bCD | 0.49 ± 0.07bE | 1.84 ± 0.05bA | 0.93 ± 0.02aB | 0.82 ± 0.03bBC | 0.65 ± 0.02bD | ||
| Hyperoxia | 0.53 ± 0.01cC | 0.93 ± 0.06aB | 2.08 ± 0.12aA | 0.50 ± 0.03bC | 0.46 ± 0.02cC | 0.61 ± 0.03bC | ||
| CAT mRNA | 0.93 | Normoxia | 1.00 ± 0.00bA | 1.00 ± 0.00aA | 1.00 ± 0.00bA | 1.00 ± 0.00bA | 1.00 ± 0.00aA | 1.00 ± 0.00cA |
| Hypoxia | 1.49 ± 0.12aAB | 0.77 ± 0.06bC | 1.57 ± 0.05aA | 1.43 ± 0.11aAB | 0.82 ± 0.03bC | 2.87 ± 0.12aB | ||
| Hyperoxia | 0.88 ± 0.05bB | 0.94 ± 0.06aB | 1.60 ± 0.04aA | 0.91 ± 0.02bB | 0.70 ± 0.02cC | 1.79 ± 0.05bB | ||
| GPx mRNA | 1.50 | Normoxia | 1.00 ± 0.00bA | 1.00 ± 0.00aA | 1.00 ± 0.00abA | 1.00 ± 0.00aA | 1.00 ± 0.00bA | 1.00 ± 0.00bA |
| Hypoxia | 1.37 ± 0.08aB | 0.30 ± 0.04cD | 0.93 ± 0.03bC | 0.87 ± 0.15aC | 0.90 ± 0.04bC | 1.62 ± 0.08aA | ||
| Hyperoxia | 1.33 ± 0.04aA | 0.63 ± 0.04bC | 1.05 ± 0.03aB | 0.85 ± 0.15aBC | 1.48 ± 0.11aA | 0.78 ± 0.04cC | ||
| GST mRNA | 0.93 | Normoxia | 1.00 ± 0.00aA | 1.00 ± 0.00bA | 1.00 ± 0.00cA | 1.00 ± 0.00bA | 1.00 ± 0.00aA | 1.00 ± 0.00aA |
| Hypoxia | 0.82 ± 0.05bC | 0.54 ± 0.08cD | 2.04 ± 0.05bA | 1.43 ± 0.10aB | 0.54 ± 0.03bD | 0.42 ± 0.03bD | ||
| Hyperoxia | 0.60 ± 0.03cD | 1.75 ± 0.09aB | 2.33 ± 0.11aA | 0.82 ± 0.08bC | 0.50 ± 0.03bD | 0.39 ± 0.01bD | ||
| GR mRNA | 1.40 | Normoxia | 1.00 ± 0.00aA | 1.00 ± 0.00bA | 1.00 ± 0.00bA | 1.00 ± 0.00aA | 1.00 ± 0.00bA | 1.00 ± 0.00aA |
| Hypoxia | 0.94 ± 0.04aA | 0.61 ± 0.03cB | 1.00 ± 0.05bA | 0.59 ± 0.04cB | 1.08 ± 0.08abA | 0.71 ± 0.02bB | ||
| Hyperoxia | 0.90 ± 0.03aC | 1.31 ± 0.10aB | 1.65 ± 0.11aA | 0.85 ± 0.05bC | 1.19 ± 0.05aB | 0.96 ± 0.06aC | ||
- 1Note: Different letters denote significant differences from each other at p < 0.05 by one-way ANOVA with Duncan’s post hoc test. The statistically significant difference is indicated by different lowercase letters at each period and by different uppercase letters in each treatment from each other.
- Abbreviations: CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; mRNA, messenger ribonucleic acid; SOD, superoxide dismutase.
3.3. Biochemical Parameters
SOD activity decreased as a result of acute exposures, although the chronic exposure did not change the activity compared to the control. Following hypoxia exposure, CAT activity, which was lower at 6 h and 12 h, then increased compared to the control. The acute hyperoxia effect increased CAT activity, while the chronic effect decreased it compared to the control. A significant positive correlation was found between SOD and CAT activities as a result of both exposures (Figure 1, p < 0.05). GPx activity was unstable during acute exposures compared to the control. While Chronic Hypoxia did not change GPx activity, hyperoxia increased it compared to the control. Although a positive correlation was expected between GPx and CAT activity due to their functions, no significant correlation was observed. GST activity increased with hypoxia and decreased with acute hyperoxia exposure compared to the control, while chronic hyperoxia increased GST activity. GST activity showed a positive correlation with CAT under hypoxia and a negative correlation under hyperoxia (Figure 1). GR activity was low as a result of exposure to hypoxia compared to the control. GR activity was negatively correlated with SOD and positively correlated with GST under hypoxia (Figure 1). The opposite was observed following exposure to hyperoxia (Table 4).
| Enzyme | Initial activity level | Exposure | Acute exposures | Chronic exposure |
||||
|---|---|---|---|---|---|---|---|---|
| 6 hr | 12 hr | 24 hr | 48 hr | 72 hr | 28 days | |||
| SOD (EU/mg) | 0.18 | Normoxia | 0.18 ± 0.0aAB | 0.19 ± 0.01aAB | 0.17 ± 0.01aB | 0.21 ± 0.01aA | 0.21 ± 0.01aA | 0.13 ± 0.02aC |
| Hypoxia | 0.07 ± 0.01cC | 0.13 ± 0.0bB | 0.15 ± 0.02aB | 0.21 ± 0.02aA | 0.11 ± 0.01bBC | 0.13 ± 0.02aB | ||
| Hyperoxia | 0.13 ± 0.01bB | 0.20 ± 0.0aA | 0.13 ± 0.01aB | 0.13 ± 0.01bB | 0.08 ± 0.0bC | 0.13 ± 0.02aB | ||
| CAT (EU/mg) | 71.1 | Normoxia | 68.26 ± 0.27bB | 70.18 ± 1.15bB | 69.41 ± 1.24cB | 67.67 ± 1.45cB | 65.00 ± 2.65bB | 78.33 ± 1.76bA |
| Hypoxia | 12.27 ± 0.82cD | 43.01 ± 1.86cC | 82.56 ± 1.38bA | 74.06 ± 1.21bB | 70.42 ± 1.53bB | 84.13 ± 1.78aA | ||
| Hyperoxia | 110.90 ± 1.59aC | 151.97 ± 2.53aA | 120.37 ± 1.67cB | 94.37 ± 0.86aD | 84.52 ± 1.27aE | 39.78 ± 0.91cF | ||
| GPx (EU/mg) | 0.34 | Normoxia | 0.34 ± 0.02aA | 0.33 ± 0.01aA | 0.33 ± 0.02bAB | 0.28 ± 0.01aB | 0.33 ± 0.01bAB | 0.16 ± 0.01bC |
| Hypoxia | 0.32 ± 0.03aB | 0.14 ± 0.01bC | 0.48 ± 0.01aA | 0.07 ± 0.0cE | 0.10 ± 0.01cDE | 0.12 ± 0.01bCD | ||
| Hyperoxia | 0.23 ± 0.12bC | 0.36 ± 0.01aB | 0.18 ± 0.01cD | 0.20 ± 0.01bCD | 0.61 ± 0.01aA | 0.35 ± 0.01aB | ||
| GST (EU/mg) | 15.75 | Normoxia | 16.54 ± 0.27aAB | 16.87 ± 0.19bA | 16.17 ± 0.17bABC | 15.83 ± 0.44bBC | 15.68 ± 0.16bC | 12.38 ± 0.20cD |
| Hypoxia | 2.66 ± 0.30bD | 18.36 ± 0.19aC | 25.16 ± 0.19aA | 25.56 ± 0.29aA | 21.83 ± 0.44aB | 21.50 ± 0.29bB | ||
| Hyperoxia | 1.82 ± 0.07cD | 14.17 ± 0.09cB | 2.30 ± 0.01cD | 14.27 ± 0.22cB | 9.65 ± 0.10cC | 29.55 ± 0.30aA | ||
| GR (EU/mg) | 2.70 | Normoxia | 2.69 ± 0.01aB | 2.76 ± 0.04bB | 2.35 ± 0.02aBC | 2.38 ± 0.01aBC | 2.19 ± 0.01aC | 3.52 ± 0.34aA |
| Hypoxia | 2.28 ± 0.06bA | 1.84 ± 0.02cC | 1.66 ± 0.03bD | 1.56 ± 0.04cD | 2.14 ± 0.05aAB | 2.07 ± 0.08bB | ||
| Hyperoxia | 2.37 ± 0.05bC | 2.87 ± 0.04aB | 2.45 ± 0.11aC | 2.10 ± 0.06bD | 1.39 ± 0.14bE | 3.53 ± 0.03aA | ||
- Note: Different letters denote significant differences from each other at p < 0.05 by one-way ANOVA with Duncan’s post hoc test. The statistically significant difference is indicated by different lowercase letters at each period and by different uppercase letters in each treatment from each other.
- Abbreviations: CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; SOD, superoxide dismutase.
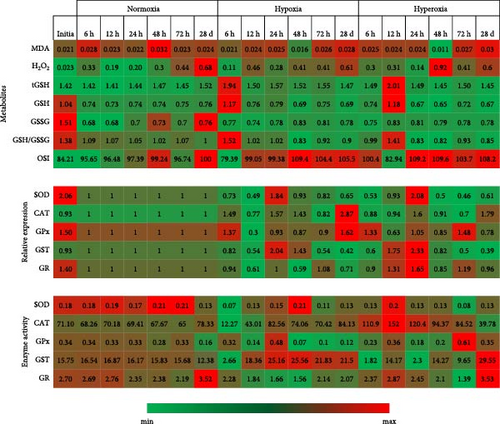
3.4. Correlation Analysis and Heat Map Indicators
The significant increase, decrease, or insignificant changes of all parameters in our study are presented in Table 5. Providing all the parameters clearly in this table helps us see the whole picture at once. Correlation analysis and Heat Map indicators showing the correlations and changes in the antioxidant-dependent metabolites, enzyme activities, and gene expressions of antioxidants are shown in Figures 1 and 2, respectively.
| Time | 6 h | 12 h | 24 h | 48 h | 72 h | 28 d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypoxia | Hyperoxia | Hypoxia | Hyperoxia | Hypoxia | Hyperoxia | Hypoxia | Hyperoxia | Hypoxia | Hyperoxia | Hypoxia | Hyperoxia | |
| tGSH | ↑ | ↑ | ↑ | ↑ | ↑ | ns | ns | ns | ↑ | ns | ↓ | ↓ |
| GSH | ↑ | ns | ns | ↑ | ns | ns | ↓ | ↓ | ns | ns | ↓ | ↓ |
| GSSG | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ns | ↓ |
| GSH/GSSG | ↑ | ↓ | ns | ↑ | ns | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ns |
| OSI | ↓ | ↑ | ns | ↓ | ns | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| MDA | ↓ | ns | ns | ns | ns | ns | ↓ | ↓ | ns | ↑ | ↑ | ↑ |
| H2O2 | ↓ | ns | ↑ | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| SOD exp. | ↓ | ↓ | ↓ | ns | ↑ | ↑ | ns | ↓ | ↓ | ↓ | ↓ | ↓ |
| CAT exp. | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ns | ↓ | ↓ | ↓ | ↓ |
| GPx exp. | ↑ | ↑ | ↓ | ↓ | ns | ns | ns | ns | ns | ↑ | ↑ | ↓ |
| GST exp. | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ns | ↓ | ↓ | ↓ | ↓ |
| GR exp. | ns | ns | ↓ | ↑ | ns | ↑ | ↓ | ↓ | ns | ↑ | ↓ | ns |
| SOD act. | ↓ | ↓ | ↓ | ns | ns | ns | ns | ↓ | ↓ | ↓ | ns | ns |
| CAT act. | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ns | ↑ | ↑ | ↓ |
| GPx act. | ns | ↓ | ↓ | ns | ↑ | ↓ | ↓ | ↓ | ↓ | ↑ | ns | ↑ |
| GST act. | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↑ |
| GR act. | ↓ | ↓ | ↓ | ↑ | ↓ | ns | ↓ | ↓ | ns | ↓ | ↓ | ns |
- Note: (↑) symbols with red color indicates the increase, (↓) symbols with green color the decrease, and (ns) not significant indicates that the levels have not changed. The increase and decrease are statistically significant (p < 0.05).
- Abbreviations: act., activity; CAT, catalase; exp., expression; GR, glutathione reductase; GSH, glutathione; GSSG, glutathione disulfide; GST, glutathione-S-transferase; MDA, malondialdehyde; OSI, oxidative stress index; SOD, superoxide dismutase; tGSH, total glutathione.
4. Discussion
The examination of the genomic composition of rainbow trout (O. mykiss) and its susceptibility to hypoxic and hyperoxic conditions has become a critical area of focus in recent aquaculture research. This highlights its significance in understanding environmental stress adaptation and the management of fish health. The potential implications of such research, including the enhancement of fish health management, and the promotion of environmentally sustainable and economically viable aquaculture practices, are of considerable importance [18, 22, 37]. The effects of hypoxia and hyperoxia conditions on the antioxidant defense system of rainbow trout are closely linked to the organism’s biological adaptation mechanisms for maintaining oxygen homeostasis [19]. The initial increase in GSH levels under hypoxia likely reflects an acute adaptive response to oxidative stress, as GSH is a crucial non-enzymatic antioxidant that neutralizes
ROS [38]. However, the subsequent decline in GSH levels after prolonged exposure suggests that the antioxidant defense system becomes overwhelmed, leading to redox imbalance and oxidative damage [22]. In our study, GSH levels initially increased under hypoxia but gradually decreased after 48 h, especially following chronic exposure. Similarly, the reduced to oxidized glutathione (GSH/GSSG), which was elevated in the early stages, showed a progressive decrease in both acute and chronic hypoxia and hyperoxia conditions. These findings suggest that prolonged oxidative stress impairs the cellular redox balance, leading to the depletion of antioxidant reserves.
GSH level, initially high due to hypoxia treatment, gradually decreased after 48 h, including as a result of chronic exposure. Although the ratio of reduced to oxidized glutathione (GSH/GSSG) increased at the beginning, a decrease in progressive acute and chronic exposure occurred in both hypoxia and hyperoxia in our study. Therefore, GSH and GSH/GSSG levels are expected to show a positive correlation with each other in both hypoxia (p < 0.01, r = 0.999) and hyperoxia (p < 0.01, r = 0.999). Decreased GSH/GSSG ratio and GSH concentration, as well as increased OSI and GSSG levels, are also good biomarkers of oxidative stress and evidence of redox imbalance [7]. Although OSI and GSSG were correlated under hypoxia (p < 0.05, r = 0.48), no correlation was found under hyperoxia. In this context, it is not surprising that OSI showed a significant negative correlation to GSH and GSH/GSSG in both exposures. GSH is required for GPX to reduce hydrogen peroxide. Moreover, in animal organisms, GSH can also scavenge ROS directly and, therefore, GSH depletion contributes to oxidative stress [38].
The observed increase in MDA levels after 24 h, especially under chronic exposure, suggests intensified LPO, a key indicator of oxidative stress. MDA is a byproduct of polyunsaturated fatty acid oxidation, and its accumulation is indicative of oxidative membrane damage [39]. The fact that MDA levels remained stable during early exposure suggests that initial antioxidant responses, including increased GSH activity and enzymatic defenses, were sufficient to mitigate LPO. Increased hepatic LPO following the reduction of GSH levels has also been observed by Jia et al. [39] in black rockfish under hypoxia stress [39]. Another study on rainbow trout reported that sufficient antioxidant defenses limited LPO [28]. This suggests that chronic oxidative stress disrupts membrane stability, potentially impairing cellular functions.
The major reducer of hydrogen peroxide in antioxidant defense is GPx ([40]). However, GPx protects against low levels of oxidative stress by detoxifying hydrogen peroxide, while CAT protects against severe levels of oxidative stress [7]. We found that hydrogen peroxide and GPx activity were negatively correlated in hypoxia (p < 0.05, r = 0.501), but hydrogen peroxide was not correlated with CAT enzyme activity levels. The change in hydrogen peroxide concentration indicates the presence of oxidative stress [7]. In our study, hydrogen peroxide levels in the liver exhibited considerable variability during the early stages of acute exposure but later returned to levels comparable to those in the control group. This fluctuation was primarily attributed to the high standard error of the mean. A stable hydrogen peroxide level may suggest that antioxidant enzymes, such as CAT and/or GPx, effectively mitigate oxidative stress by neutralizing this peroxide.
How different oxygen levels regulate or alter antioxidant responses can be discovered by examining enzyme activities and gene expression simultaneously. Generally, an increase in intracellular ROS levels leads to an upregulation of antioxidant enzymes in zebrafish [41]. This might result from an increase in ROS caused by the loss of mitochondrial electron transport chain cytochromes and the subsequent leaking of these cytochromes to leftover oxygen molecules [7]. In our study, SOD activity was lower in both treatments compared to the control. A decrease in SOD activity against oxidative stress in mice [42] and expression against environmental stress conditions in rainbow trout [43] was also observed in other studies. These low levels of SOD may not have caused a significant increase in hydrogen peroxide.
Acute hyperoxia enhanced CAT activity in the liver of rainbow trout during all exposure periods. It was reported that CAT activity decreased in chronic hyperoxia, matching data obtained from 48-hour hyperoxia exposure in trout [24]. Hyperoxia increased CAT activity first, which then gradually inhibited. This pattern partly paralleled the gene expression results. There was a significant positive correlation between CAT gene expression and activity levels as a result of hyperoxia exposure (p < 0.01, r = 0.69).
Enzymes involved in antioxidant defenses exist as a coordinated system and include GSH-dependent enzymes such as GPx which neutralizes H2O2, and GR which catalyzes the transformation of GSSG to reduced GSH using NADPH [7]. We determined that the pattern of GPx activity does not correlate with gene expression, a situation observed in other enzymes as well, except for CAT. The incompatibility between activity and gene expression of antioxidant enzymes has been reported in mice and rats [44, 45]. This discrepancy arises because these enzymes are largely controlled post-transcriptionally in zebrafish embryos [46]. Post-transcriptional modifications, the presence of pre-formed antioxidant enzymes or conformational changes can alter the activity levels of this enzyme [47]. MDA and GPx activity showed a significant correlation (p < 0.05, p = 0.498) in hyperoxia. GPx activity is directly related to LPO [7]. We estimate that the GPX enzyme functions exclusively, rather than the CAT enzyme, to eliminate the peroxides produced under stress conditions. Considering the levels of hydrogen peroxide and LPO, although CAT activity increased, GPX activity was inconsistent. This finding is consistent with those reported by Izawa, Inoue, and Kimura [48] and was also observed in a study conducted on Saccharomyces cerevisiae, indicating that the affinity of GPx for H2O2 is higher than that of CAT.
One of the main antioxidant enzymes and the first-line markers of the antioxidant status is GST, which detoxifies various substances by conjugating them with GSH [49]. This antioxidant and detoxification mechanism was shown to be related to the increase in MDA in the freshwater fish Channa punctata [50]. In addition, an oxidative state linked to hypoxia can increase GST activity. Studies conducted on rats [51] and rainbow trout [52] indicate that GST activity decreases in acute exposure to hyperoxia [51, 52]. Zhao et al. [37] found in Schizothorax prenanti that the SOD, CAT, and GPx activity data are compatible with our study after 12 and 24 h of hypoxia [37]. GST gene expression correlated positively with CAT and SOD gene expressions (p < 0.01). We found that GST expression decreased over time with chronic effects. A similar situation was observed at the transcriptome levels in other enzymes. Jiang et al. [53] also noted that antioxidant genes were expressed more often on Cyprinus carpio under oxidative stress [53]. However, gene expression levels decreased below normal levels in our study. This suggests that the fish can tolerate the oxidative stress effects caused by chronic hypoxia and hyperoxia at the gene expression level.
The regulation of antioxidant defense systems under different oxygen conditions is largely controlled at the molecular level through transcriptional and post-translational modifications [38]. One of the primary regulators of oxidative stress responses is the Nrf2-Keap1-ARE pathway, which modulates the expression of antioxidant enzymes, such as SOD, CAT, GPx, and GST [54]. This mechanism could explain the initial upregulation of SOD and CAT gene expression in our study, followed by their subsequent downregulation as prolonged oxidative stress may lead to feedback inhibition [55]. The interaction between HIF-1 α and Nrf2 plays a key role in hypoxic stress adaptation. Under low oxygen conditions, HIF-1 α enhances glycolysis and antioxidant defenses to reduce ROS accumulation [56]. However, prolonged hypoxia can induce Nrf2 degradation, leading to decreased antioxidant enzyme transcription [46]. This aligns with our findings, where antioxidant enzyme activity and gene expression initially increased but later declined under chronic hypoxia.
The decrease in GPx activity after 48 h of the same treatment was notable. GPx activity induced by hypoxic conditions positively correlated with GSH levels (p < 0.05, r = 0.50). In addition, GR activity also showed a positive correlation with GSH activity (p < 0.05, r = 0.56) indicating that these GSH-dependent enzymes act together in the conversion of oxidized and reduced GSH. Under stressful conditions, various enzymatic antioxidants work together, sometimes partially replacing one another [57]. The activities of GSH-dependent enzymes changed as a result of the in vivo inhibition of CAT in the goldfish brain [58, 59]. High levels of oxidized GSH may result from the low activity levels of the GR in hypoxia. GR activity, like other examined parameters, was not correlated with gene expression. A study conducted on zebrafish embryos demonstrated that antioxidant enzymes are regulated by multiple factors and that transcription and translation play a crucial role in the temporary adaptation to oxidative stress [46]. The increase in SOD, CAT, and GST expressions at the 24th h after both exposures indicates a stress-dependent response. This suggests that 24 h may be the maximum defense time at the transcriptomic level for the rainbow trout.
5. Conclusion
This study determined that hypoxia and hyperoxia exposures, compared to normoxia conditions, significantly affected various parameters, including gene expression levels. OSI, GSH, GSSG, and GSH/GSSG levels revealed that hypoxia and hyperoxia caused liver damage and negatively impacted the components of the antioxidant defense system. Acute and chronic exposure to low and high levels of dissolved oxygen resulted in various changes in enzyme activity and gene expression in the liver. A large number of genes undergo expression changes under various stimuli, with some genes being downregulated, others upregulated, some modulated in the early stages of adaptation, and other genes’ expression impacted later on. The roles of hydrogen peroxide and post-transcriptional modifications in this process are inevitable. Changes in enzyme activity, GSH metabolism, hydrogen peroxide, and MDA observed after 6 h of exposure to hypoxia or hyperoxia conditions may represent the organism’s initial response to oxygen-induced ROS. The continued high enzyme activity after chronic exposures indicates that rainbow trout are in constant defense against hypoxia and hyperoxia.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was funded by the Scientific and Technological Research Projects Funding Program as a part of Türkiye Bilimsel ve Teknolojik Araştırma Kurumu (TUBITAK) (project no. 114O366).
Acknowledgments
I would like to express my deepest gratitude and utmost respect to my PhD advisor, the late Ercüment Aksakal, whose invaluable contributions and intellectual guidance were instrumental in writing this study. As one of the authors of this study, his profound insights, unwavering dedication, and scholarly wisdom played a pivotal role in the development of the research. His mentorship extended beyond academic boundaries and left a legacy of wisdom, curiosity, and integrity. I am profoundly honored to have the privilege of working under his guidance. May his memory will inspire future generations of scholars.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




