Structural Analysis of Embryonic Development of Embryos From the Crossing of Two Species of Siluriform
Abstract
Based on the need for knowledge about the biology of hybrid fish specimens currently being produced by commercial fish farmers, this study aimed to study the initial ontogenesis up to the fifth day after larval hatching of the Hybrid LP produced by crossing Leiarius marmoratus (♀) and Pseudoplatystoma reticulatum (♂), thus producing important data for fish farming, as well as for ichthyoplankton studies. The embryogenesis of the Hybrid LP was typical of teleosts but with differences and similarities in relation to other species and their parents, such as duration of development and moment of mesoderm segmentation, among others. Five stages of embryonic development were defined, from the moment of fertilization until larval hatching: zygote, cleavage, gastrulation, organogenesis, and hatching. The embryogenesis period was 13 h, at an average temperature of 27.98°C. The embryonic development stages established for the hybrid were like those of its parental species L. marmoratus and P. reticulatum. Thus, through this research, it was possible to conclude that the embryonic development of the Hybrid LP originating from interspecific crosses between females of L. marmoratus with males of P. reticulatum occurred in a development period similar to that found in studies of its parental species as well as for the inverse hybrid (PL), originating from females of P. reticulatum with males of L. marmoratus, under similar temperatures, not being possible to morphologically differentiate the embryos and larvae until the fifth day after hatching, neither of the parental species nor the Hybrid PL.
1. Introduction
Fish farming is one of the fastest-growing livestock production activities. Its importance goes far beyond generating income, food security, and jobs. Fish farming provides greater efficiency in the use of water, as after its use in the fish production system, the water can be used for other purposes, such as irrigation, being of great relevance for regions that have water restrictions [1].
Biological information on native neotropical fish species with zootechnical importance remains scarce, and perhaps for this reason, biotechnological packages to produce these fish species and genetically improved specimens have not yet been developed. Thus, what has been common is the production of fish hybrids through the genetic combination of different species to achieve more productive genetic results. With the aim of producing offspring that are more resistant to adverse weather conditions, incubation, more effective larviculture, and often, greater growth than the parental species [2–4].
Among the specimens of hybrid fish produced, those from Siluriformes (catfishes) stand out, and in particular, those produced by crossing the female cachara (Pseudoplatystoma reticulatum ♀) with the male jaguar jundiá (Leiarius marmoratus ♂), owing to the following technical factors: increased efficiency in the incubation of embryos and larvae, improved growth, and good market value [5].
As the escape of fish cultivated in excavated ponds or net tanks is a common event, hybrid specimens are already dispersed in natural bodies of water, and contrary to the biological definition, many of these may be fertile with the potential for reproductive interactions with parental species. However, scientific information on the reproductive biology of these specimens is lacking, as is information on their possible interactions with their parental species [6].
Owing to this lack of information, assessing the effects of hybridization of native fish species from an ecological perspective remains challenging, as studies on the ecological success of these hybrid fish in water bodies are still in their infancy in Brazil [7].
The information obtained from research on the ontogeny of native fish species is fundamental, as it serves as a basis for the management of fishing resources, studies, maintenance, and replacement of natural stocks of fish species aimed at the preservation of aquatic ecosystems. It is also important for the identification of environmental bioindicators, which will assist in evaluating the effects of toxic substances on aquatic fauna. Such studies also generate information for the improvement and effectiveness of the process of breeding fish in captivity and provide fundamental data for the development of biotechnologies that are important for fish farming. Thus, the knowledge of embryogenesis in fish species can be used in complementary multifunctional approaches [8–10].
The study of larvae is also very important; substantial similarity exists between different species, and a scarcity of information on ichthyoplankton of native freshwater species and comparative literature makes the work of taxonomists very difficult [6, 11].
Most studies on fish larvae have focused on systematics and phylogeny without paying particular attention to morphological development in relation to physiology. Studies on fish larvae are necessary to understand the biological role of larval characteristics in survival, especially the functional mechanisms related to vital requirements, such as locomotion, feeding, and breathing [12].
L. marmoratus (Gill, 1870), also known as jundiá, jandiá, or jundiá-onça, belongs to the family Pimelodidae and is distributed throughout the Amazon basin, and occurs most frequently in lotic environments [13]. P. reticulatum, known as surubim or cachara, is also a member of the family Pimelodidae and belongs to the genus Pseudoplatystoma (Linnaeus, 1766) and is found in freshwater. It is demersal and is distributed throughout South America, including the Central Amazon and Paraná River in Argentina, Bolivia, Brazil, Paraguay, and Uruguay [14].
Since information on the reproductive biology of hybrid fish of native Neotropical species is practically nonexistent, and the large-scale exploitation of these animals is a reality, and their escape into the environment can lead to significant environmental impacts on the parental species. This study aimed to obtain information on the initial ontogenesis up to the fifth day after hatching of the larvae of the hybrid popularly known as jundiara (Hybrid LP), produced by the crossing of two economically important Siluriformes species, L. marmoratus (♀) and P. reticulatum (♂). This research provides important biological information for producers and offers technical support for the development of projects aimed at maintaining the ecology of aquatic environments.
2. Materials and Methods
2.1. Species Studied and Collection Location
To obtain the embryos and larvae, five female L. marmoratus and five male adult and mature P. reticulatum were used, belonging to the Piraí fish farm stock (Figure 1), located in the city of Terenos, Mato Grosso do Sul, Brazil. The use of these animals followed the guidelines of the Ethics Committee for the Use of Animals at UNESP, Ilha Solteira Campus (approval no. CEUA-FEIS/UNESP 09/2023). The reproducers were collected with a trawl net in the excavated tank, where they were kept and then transferred to concrete tanks located in the Piscicultura Piraí reproduction laboratory for hormonal induction of reproduction.


The average weight of females was 4.06 ± 0.35 kg, and the average weight of males was 3.28 ± 0.38 kg. The study was conducted during the reproductive period of the parental species starting in November 2022.
2.2. Obtaining Embryos
Hormone induction was carried out by applying a solution of the crude extract of the carp pituitary gland (EBHC) (Cyprinus carpio). Two doses of pituitary extract were applied to the females; the first dose was 5.0 mg/kg body weight, and after 12 h, the second application was carried out at a dosage of 1.0 mg/kg. Males received a dose of 1.0 mg/kg body weight during the second application of the females [15].
Approximately 6 h after applying the last dose of the hormone, oocytes were extruded, and sperm was collected through massaging in the cephalocaudal direction. The oocytes from females and semen from males were then pooled. Fertilization was carried out following the “dry” method, consisting of first mixing the spermatozoa with the oocytes in a dry container, without contact with water, and soon after, adding water for spermatozoa activation, fertilization promotion, and egg hydration. Afterward, the eggs were deposited in four vertical incubators of 200 L each, with ~200 g of eggs placed in each incubator. Incubation took place at an average temperature of 27.98 ± 0.42°C, with constant water renewal. In this study, the hybrid was termed Hybrid LP, a term coming from the initials of the scientific name of the parental species.
2.3. Collection Method
Embryo collection began shortly after fertilization and was carried out at 15 min intervals during the first 2 h of embryogenesis and then at 1-h intervals until the larvae hatched. Subsequently, collections were carried out every 6 h for the first 2 days and at 12 h intervals until the fifth day.
2.4. Processing the Material for Analysis
At each collection, two samples of 50 embryos were collected; one sample was fixed in a fixative solution of 2.5% glutaraldehyde in phosphate buffer (pH 7.2) and the other in a solution of 4% paraformaldehyde and 2% glutaraldehyde in Sorensen’s phosphate buffer (pH at 7.2) (Karnovsky’s fixative). After 24 h, the samples fixed in Karnovsky’s fixative were placed in 70% alcohol for subsequent analysis. The collected material was taken to the Neotropical Ichthyology Laboratory (L.I.NEO.), located at UNESP, Ilha Solteira Campus, São Paulo, Brazil, for further processing and analysis.
2.5. Light Microscopy Analysis
For the histological analysis, 20 prefixed specimens representing each stage of development were individually embedded and oriented in a glycol methacrylate plastic resin for microtomy. Serial transverse and sagittal histological sections of 3 µm thickness were obtained and stained with Harris hematoxylin and eosin, analyzed, and photomicrographed under a Carl Zeiss Scope A1 AX10 microscope equipped with an AxioCam MRc5 ZEISS digital camera and ZEN 3.6 (blue edition) image analysis program. To analyze the material “in toto,” the embryos and larvae were analyzed using a MOTIC SMZ-168 stereomicroscope and photomicrographed using the same image analysis system mentioned above.
2.6. Scanning Electron Microscopy (SEM)
For SEM analysis, the larvae prefixed in 2.5% glutaraldehyde were transferred to a microtube, washed with distilled water, postfixed in 0.5% osmium tetroxide, dehydrated in sequence with increasing ethanol content (7.5%–100%), and dried in critical point equipment (LEICA EM CPD030). Next, the samples were placed on stubs and covered with a 10 µm gold film in a metallizer (BAL-TEC-SCD 050 Sputter Coater), analyzed, and electromicrographed under an SEM (EVO LS15; Carl Zeiss). These procedures were performed at the Electron Microscopy Center of the Biosciences Institute, UNESP, Botucatu Campus, São Paulo, Brazil.
3. Results
3.1. Embryogenesis
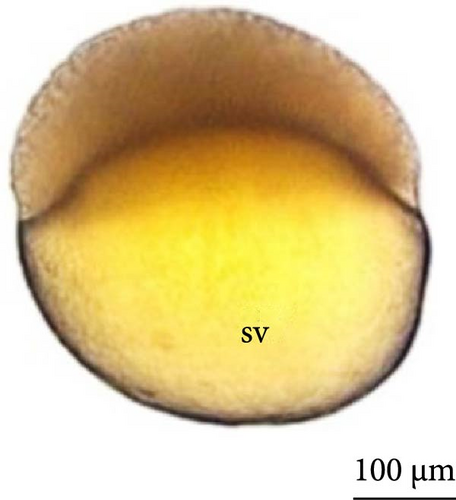
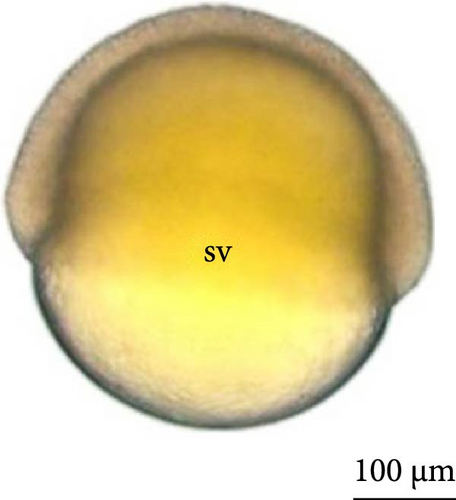
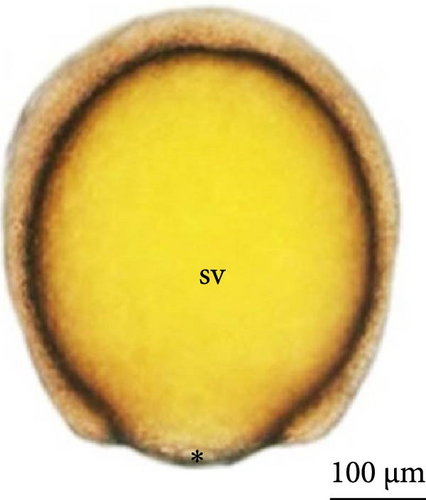
The eggs had a yellowish color, clear chorion, and small perivitelline space after hydration, with no oil drop observed inside the yolk sac during the entire period of embryonic development.
The development period of the hybrid embryos, from the moment of fertilization until the larvae hatched, was 13 h at an average temperature of 27.98°C. After fertilization, the following stages of hybrid embryogenesis were established: zygote formation, cleavage, gastrula formation, organogenesis, and hatching (Table 1).
| Incubation period | Stage | Observations |
|---|---|---|
| 0 | Zigoto | 94.4% zigoto; 5.6% bad eggs |
| 0.25 h | Cleavage | 90.6% 2 blast.; 9.4% bad eggs |
| 0.50 h | Cleavage | 93.1% 4 blast.; 5.4% bad eggs; 1.5% abn. develop. |
| 0.75 h | Cleavage | 1.1% 4 blast; 54.8% 8 blast; 39.1% bad eggs; 5% abn. develop. |
| 1 h | Cleavage | 5.9% 8 blast.; 47.1% 16 blast.44.1% bad eggs; 2.9% abn. develop. |
| 1.25 h | Cleavage | 1.5% 16 blast.; 53.9% 32 blast.; 42.3% bad eggs; 2.3% abn. develop. |
| 1.5 h | Cleavage | 2.2% 32 blast.; 56.2% 64 blast.; 29.9 bad eggs; 11.7% abn. develop. |
| 1.75 h | Cleavage | 54% morula; 40.5% bad eggs; 5.5% abn. develop. |
| 2 h | Cleavage | 62.6% morula advanced; 23.6% bad eggs; 13.8%: abn. develop. |
| 3 h | Gastrula | 70.7%: 25% of epiboly; 14.2%: abn. develop.; 15.1%: bad eggs |
| 4 h | Gastrula | 37.4%: 50% of epiboly; 28.1%: 75% of epiboly; 6.4%: abn. develop.; 28.1%: bad eggs |
| 5 h | Gastrula | 5.1%: 75% of epiboly; 85.3%: 90% of epiboly; 9.6%: bad eggs |
| 6 h | Organogenesis | 91%: 5 somites; 1.1%: abn. develop.; 7.9%: bad eggs |
| 7 h | Organogenesis | 86.6%: 10 somites; 10.4%: bad eggs; 3%: abn. develop. |
| 8 h | Organogenesis | 78.7%: 15 somites; 21.3%: bad eggs |
| 9 h | Organogenesis | 72.2%: 18 somites; 18.3% bad eggs; 9.5%: abn. develop. |
| 10 h | Organogenesis | 92.3:20 somites; 7.7%: bad eggs |
| 11 h | Organogenesis | 90.4%: Larval phase + de 20 somites; 2.4%: bad eggs; 7.2%: abn. develop. |
| 12 h | Organogenesis | 84.2%: Larval phase + de 30 somites; 15.8% bad eggs |
| 13 h | Hatching | 42.8%: hatched larvae; 57.2%: bad eggs |
- Note: blast., blastomeres; bad eggs, spoiled or unfertilized eggs; abn. develop., embryo with abnormal development; larval phase, the embryos already have the morphology of the larva that will hatch.
3.2. Zygote Stage
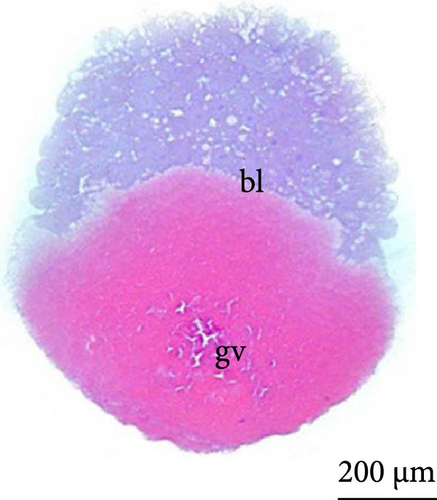
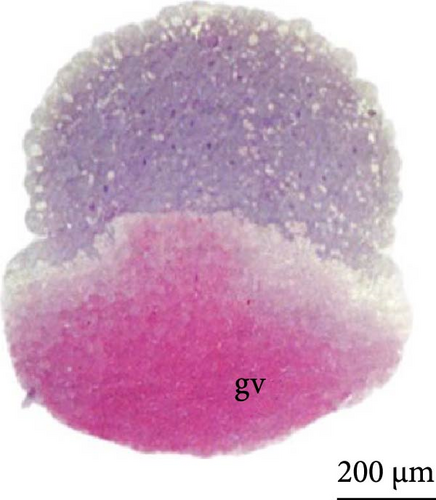
With fertilization, the fusion of the male and female pronuclei, cytoplasmic migration, and animal and vegetative poles (yolk bladder) were established. The animal pole comprises active cytoplasm and a nucleus, with a light and translucent yellow color, whereas the vegetative pole is denser and has a darker yellowish color (Figure 2A, 2A ∗, 2A ∗∗). The average diameter of the yolk bladder was 703.43 ± 35.43 μm at this stage.

3.3. Cleavage Stage
Cleavage was of the discoidal meroblastic type, also known as partial or incomplete; that is, it occurs only at the animal pole, with the following cleavage pattern being observed: the first five cleavages are vertical in relation to the animal pole and perpendicular to each other, sequentially producing 2, 4, 8, 16, and 32 blastomeres (Figure 2B–F) and the sixth cleavage is parallel to the animal pole, giving rise to two layers of cells, totaling 64 blastomeres (Figure 2G).
The first cleavage was observed at 0.25 h after fertilization (HPF), and the embryos presented two blastomeres (Figure 3A,B). The second cleavage was detected at 0.50 HPF, wherein the embryos had four blastomeres. The third cleavage occurred at 0.75 HPF of development, and many small yolk granules could be identified inside the cells (Figure 3C,D). From this moment of incubation, observation of heterogeneity in the development of embryos began, with embryos with four and eight blastomeres being observed (Figure 3A,C).
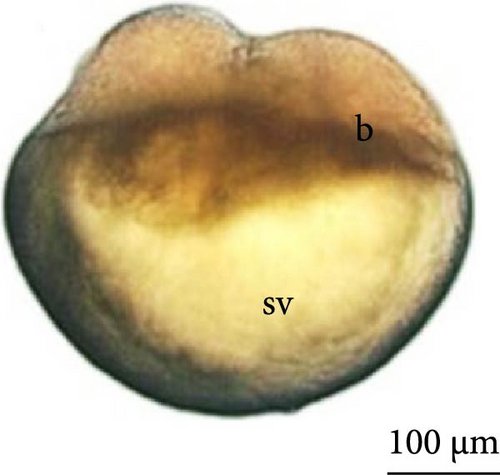
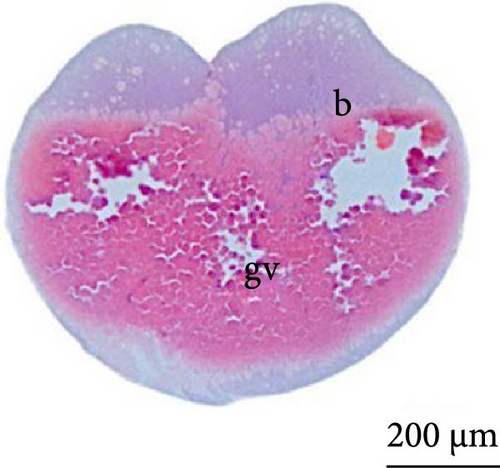
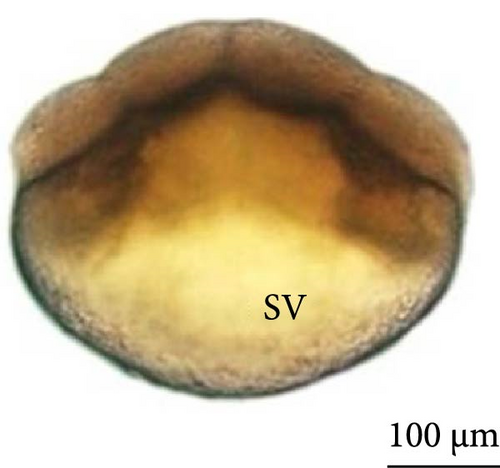
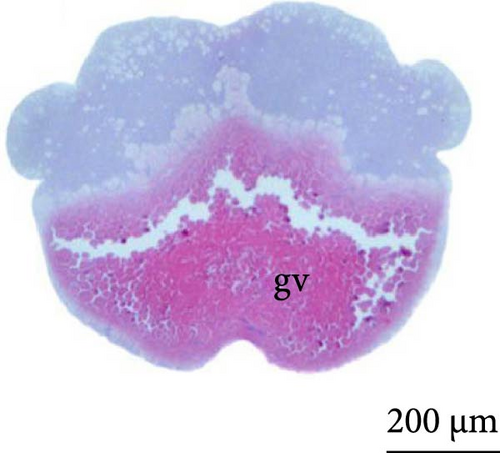
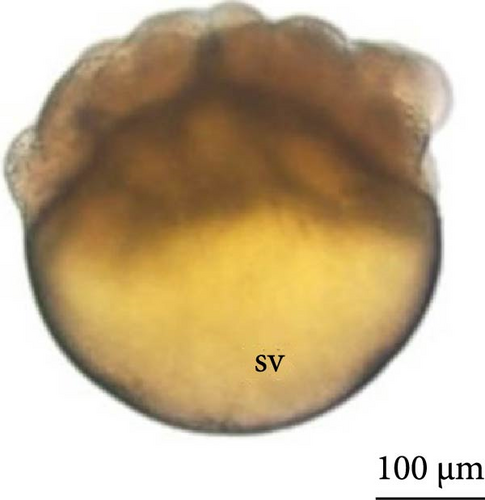

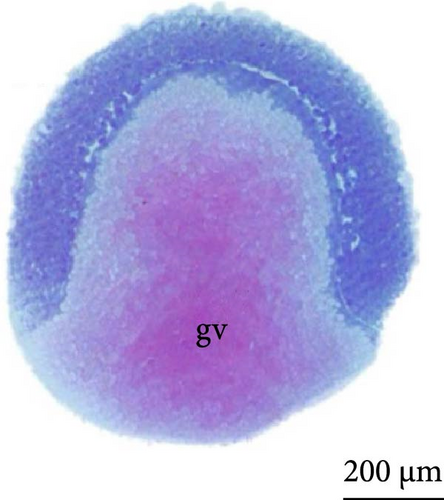
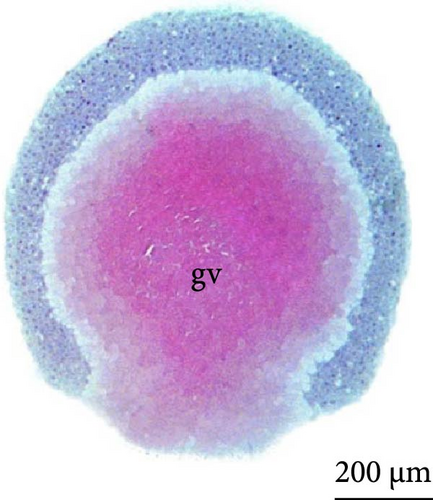
The fourth cleavage was observed at 1 HPF, with embryos containing 16 blastomeres. At this phase, a reduction in the size of the blastomeres became evident, owing to the rapidity of the mitotic processes that occur at this stage. The fifth cleavage was observed at 1.25 HPF (32 blastomeres), a phase in which the yolk bladder presented a more rounded appearance than that in the previous phases (Figure 3E,F).
Until the 4th cleavage, the nucleus could not be observed in the cells, and visualization was possible only after the 5th cleavage. Moreover, until this moment, a structure that separated the yolk from the blastomeres was not observed, and blastomeres were formed by incomplete cleavage, remaining connected to each other and to the cytoplasmic layer that surrounds the yolk (Figure 3B,D, and F).
The sixth cleavage was observed at 1.5 HPF, with the mitotic spindle occurring horizontally, and the blastoderm now had cells arranged in two layers, with the lower layer still in incomplete division (Figure 3G,H). At 1.75 HPF, more than 100 cells were observed (Figure 3I,J).
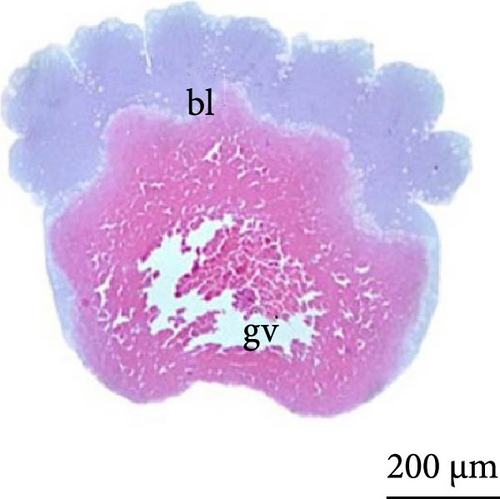
The nuclei in the cells could be identified from the cleavage stage at the 64 blastomere phase (Figure 4A). Until the fifth cleavage, the mitotic spindles are perpendicular to the yolk and promoted incomplete cleavage, as they are unable to cleave the vegetative pole. These cells that were in direct contact with the vegetative pole form the periblast or yolk syncytial layer (Figure 4B), which was better observed in the morula phase.
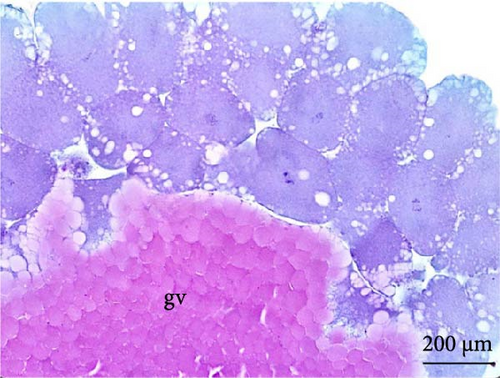
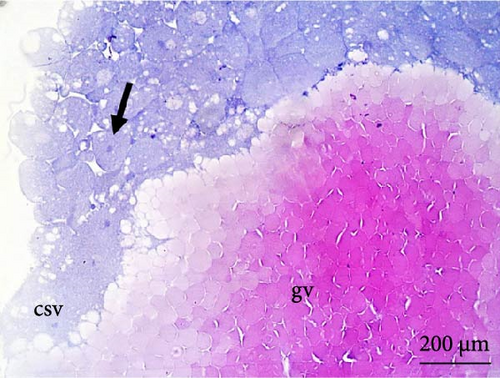
The cleavage stage ended with the beginning of morphogenetic movements, the most visible being epiboly, which promotes the flattening of peripheral blastomeres by shortening their apico-basal axis. The advanced morula could be observed in the cleavage phase after 3 h of incubation. Because the emergence of a space (blastocele) between the blastoderm and periblast was not observed, rather only irregular spaces (Figures 3A,B) were observed between the blastomeres and the existence of a characteristic blastula stage was not considered.
3.4. Gastrula Stage
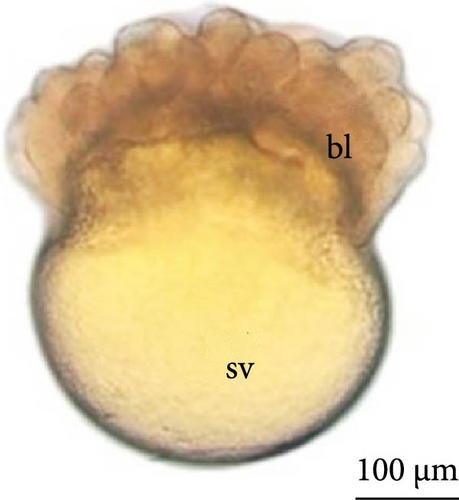
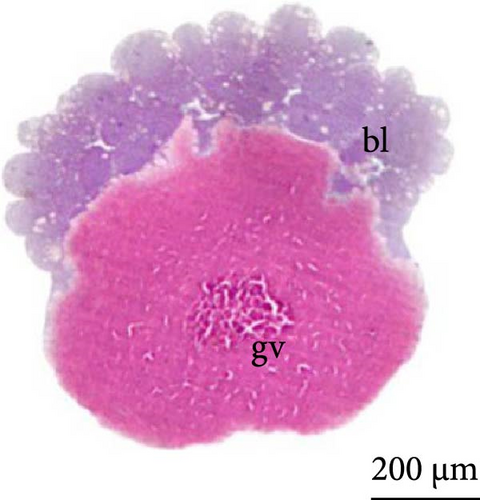
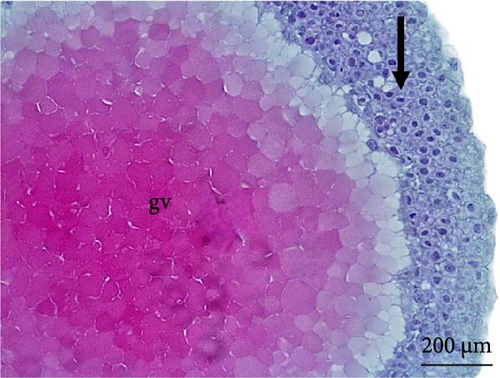
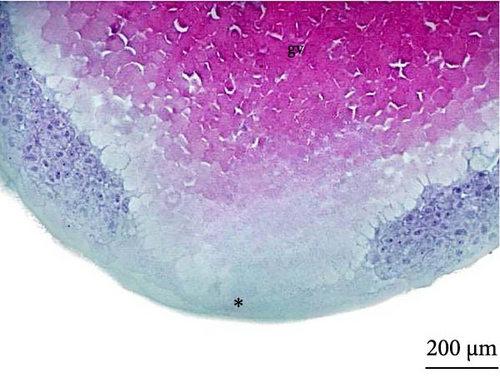
At 3 h of embryogenesis, the diameter of the yolk bladder was 695.96 ± 13.34 μm (Figure 3I,J). The beginning of the flattening of the peripheral cells was observed in preparation for epiboly (Figures 3I and 4C). At 4 h of embryogenesis, the blastoderm had already covered half of the yolk sac with the formation of a germ ring or an embryonic shield from the edge of the blastoderm (Figure 3K,L). However, heterogeneity in embryonic development was observed (Table 1).
After 5 h of incubation, most of the embryos had a blastoderm involving 90% of the yolk bladder, with only a small portion of the vegetative pole exposed, the yolk plug (Figure 3M,N). Involution could also be observed, with part of the blastoderm returning, an action that gives rise to the future endoderm (Figure 4C,D).
3.5. Organogenesis Stage
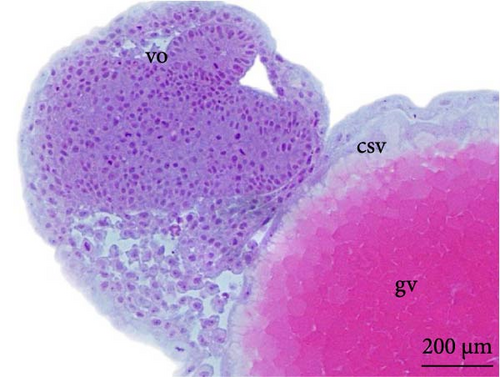
6 h after development, the embryos were in the initial neurula stage (Figure 5A). This stage was marked by cephalocaudal differentiation, the presence of the neural groove, and the segmentation of the mesoderm into more than five somite pairs. After 7 h of development, the following structures were observed: optic vesicles, with a greater distinction between the head and tail (Figure 5B).

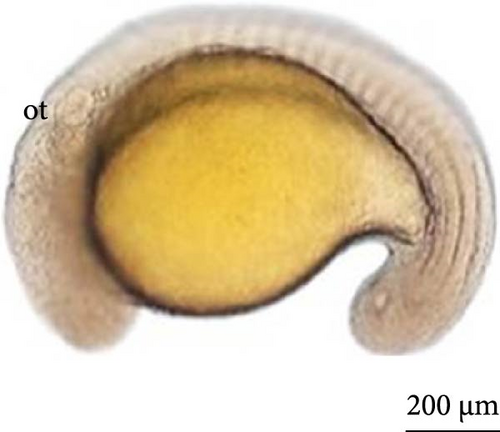
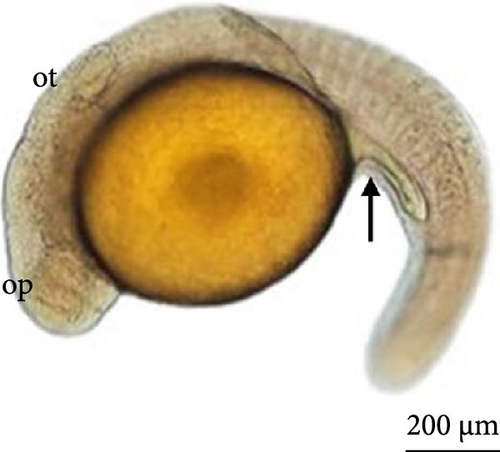

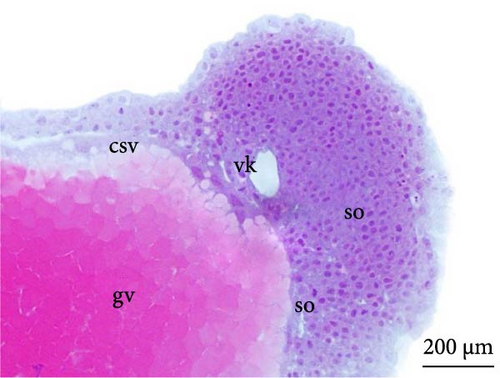
At 8 h, a greater segmentation of the mesoderm could be identified (Figure 5C). Histological analysis revealed the structure of the optic vesicle that gives rise to the animal’s eye, which could be observed in the head region (Figure 4E); in the caudal portion, the structure of the Kupfer vesicle was internally lined by cubic to cylindrical ciliated epithelium cells (Figure 4F). At 9 h, the embryos had a longer tail (Figure 5D).
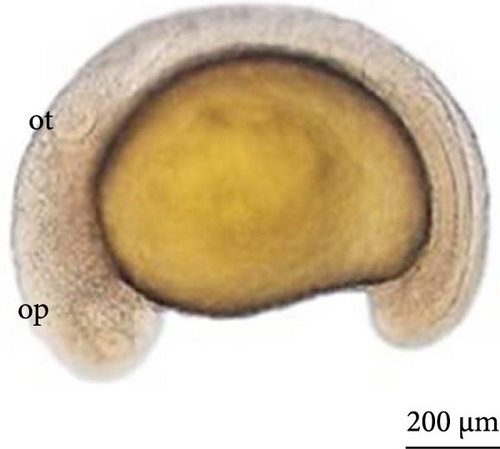
After 10 h, structural differentiation was observed during hindgut formation (Figure 5E). After 12 h, the tail was already very flexible, with many movements observed. The embryo already presents the morphological characteristics of a larva and is ready to hatch. The heartbeat could be observed (Figure 5F).
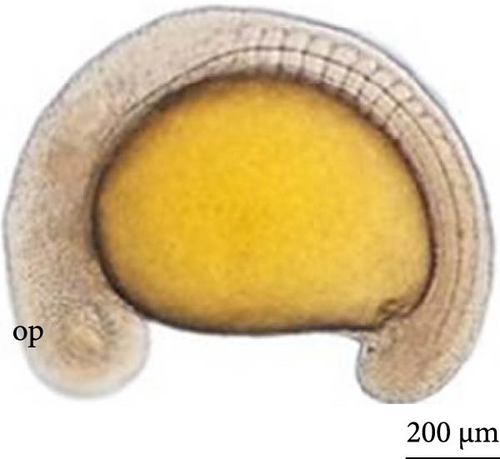
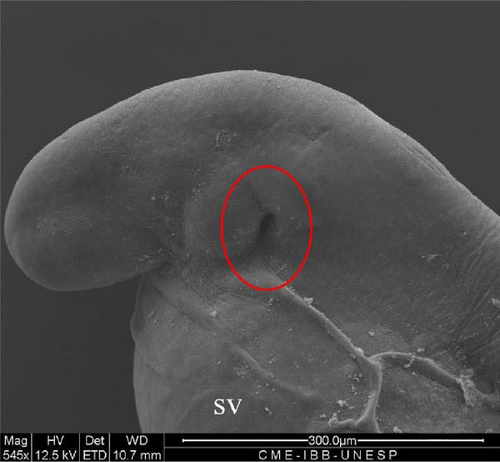
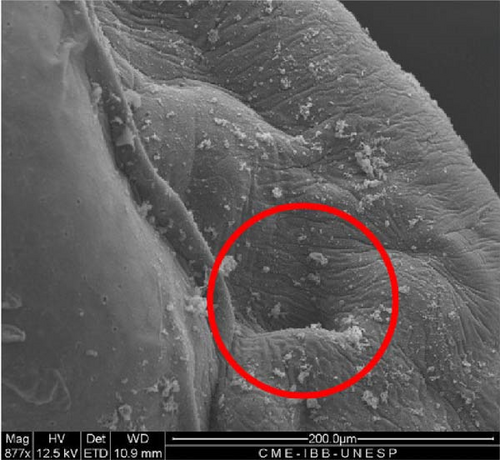
SEM analysis in the prehatch period with 12 h of development showed that the head was more arched, the opening of the future operculum was deeper (Figure 6A), the nasal pit was developing (Figure 6B), the embryonic fin was clearly visible in the tail region (Figure 6C), and the presence of many somites and a well-defined embryonic fin (Figure 6D).




3.6. Hatching of Larvae
After 13 h, the larvae began to hatch and swim, enabling the observation of heartbeats, straight tails, and clearly visible somites (Figure 6E). At this stage, the larvae were 1127.6 μm in length.
3.7. Hybrid Larvae 12 h After Hatching
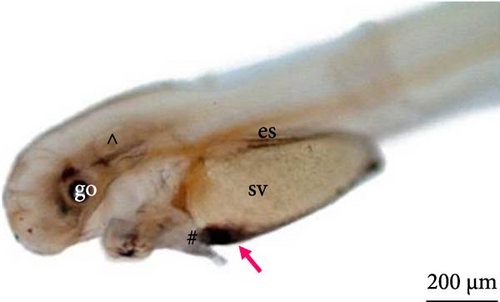
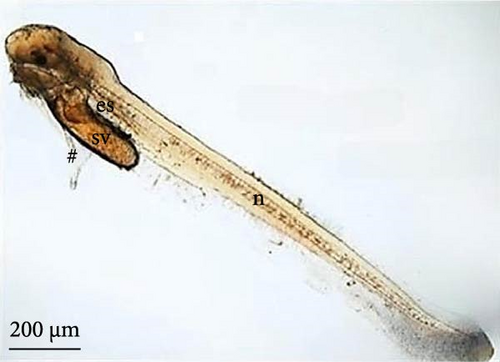
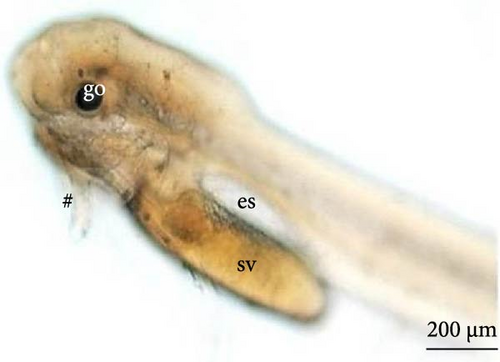
After the larvae hatched, their average length was 3070.9 ± 238.6 μm (Figure 7A). Based on the ultrastructure, the operculum could be seen to have already deepened (Figure 7B), and two pairs of developing barbels were also visible (Figure 7C). Additionally, the anus (Figure 7D) and caudal fin (Figure 7E) were being formed.




3.8. Hybrid Larvae 24 h After Hatching
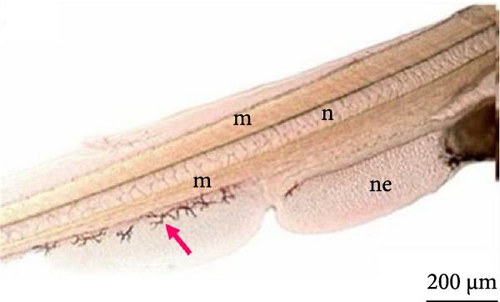
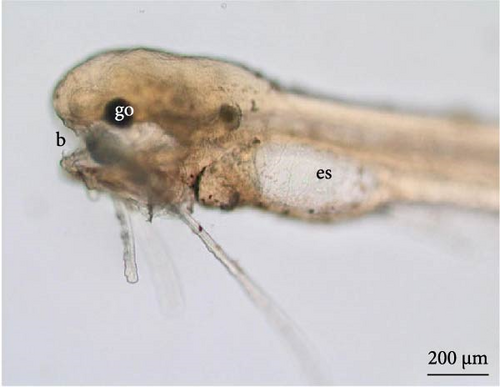
Twenty-four hours after the larvae hatched, the components of the head were further developed, such as a pigmented optic vesicle and a structured mouth. Because of transparency, larval pigmentation could be seen to be more developed owing to the presence of a greater quantity of chromatophores spread across the tail and in the anterior and posterior regions of the yolk sac (Figure 8A). Although the yolk sac remained, the stomach and intestine were already delimited (Figure 8A,B), the latter being already connected to the anus and the notochord extending from the cephalic to the caudal region.
3.9. Hybrid Larvae 30 h After Hatching
At this phase of the organogenesis stage, the larval length has already reached 3754 ± 68 μm (9C). The yolk sac had a very reduced volume with an elongated oval shape. Well-developed and visible wattles were present, and pigmentation was highly evident, with an increased number of chromatophores spread throughout the body. The nervous system was evident with the forebrain, midbrain, and telencephalon visible. The digestive system was well developed, and the oral cavity, esophagus, stomach, and intestine connected to the anal pore could be seen (Figure 8C,D). The eyeball was well developed, with an average diameter of 81.7 ± 10.9 μm, with a well-defined optic cup and a well-pigmented lens (Figure 8D).
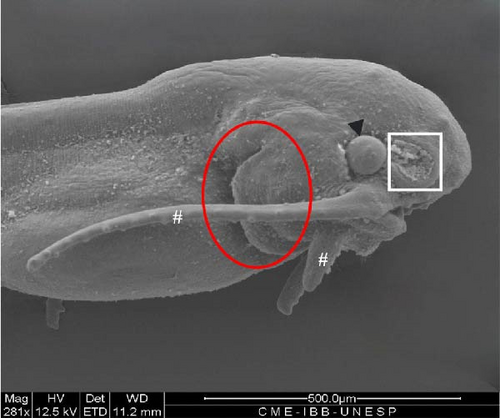
The opercular structure was already formed, the nasal pit remained open, but invagination of the tissue towards closure had begun, and a greater number of olfactory sensors were present (Figure 9A,B). The mouth was open, with several teeth in development (Figure 9D), as well as two pairs of mandibular barbels in clear development and one pair lateral to the more developed mouth opening, with sensors arranged in the form of buttons (Figure 9A,D). The tail began to take on a heterocercal shape, characteristic of the species (Figure 9E).

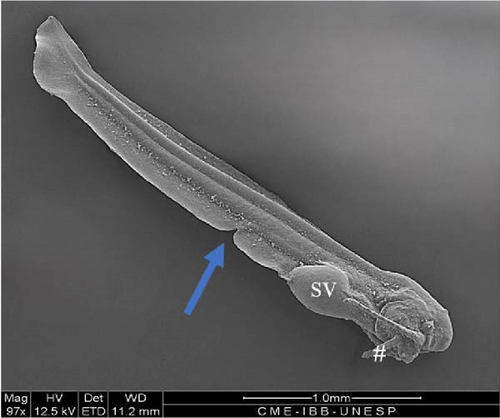
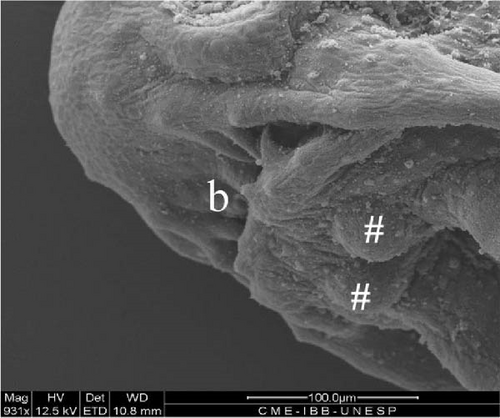

3.10. Hybrid Larvae 60 h After Hatching
At this stage, the stomach was larger (Figure 10D), the yolk was completely absorbed, and the caudal fin had individualized (heterocerca) (Figure 10E) along with the other fins. The two pairs of barbels were larger, and a third pair was visible (Figure 10A). The mouth presented a greater opening, and the teeth were visible under optical microscopy in greater quantities (Figure 10A,B). The anal fin was delimited, and the region where the urogenital pores were positioned was individualized (Figure 10C).
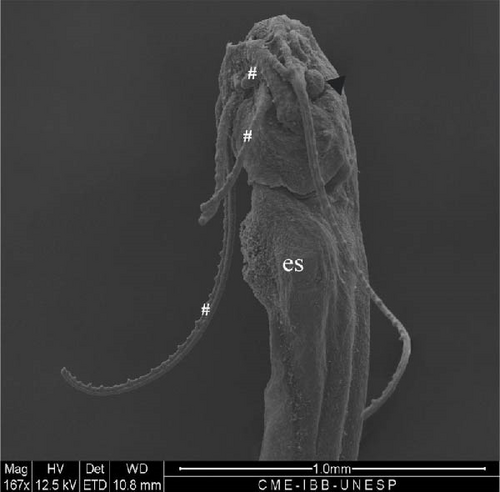



3.11. Hybrid Larvae 108 h After Hatching
At this stage, the region with the nasal pit sensors underwent invagination for the internalization and closure of the nasal pit (Figure 11A). The eyes advanced in development, with an average diameter of 216.3 ± 20.8 μm. The pelvic fin was well-developed (Figure 11B), the operculum was formed (Figure 11C), and gill arches and filaments were observed (Figure 11D).


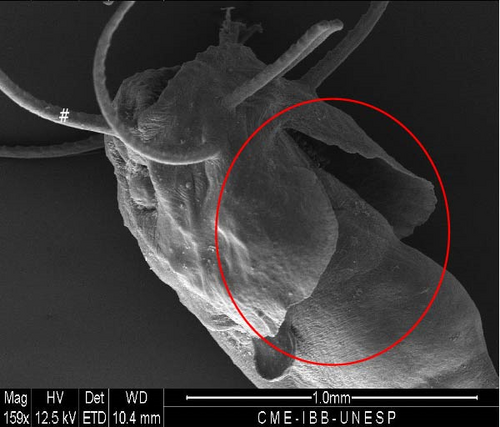

3.12. Hybrid Larvae 120 h After Hatching
On the fifth day, the barbels and eyes were larger (Figure 12A), with an average eye diameter of 231.8 ± 22.8 μm. An increase in the length of the larva was also observed, which is now measured at 7863 ± 171.2 μm (Figure 12B). Histological analysis revealed that the eyes were formed, allowing visualization of the optic cup, retina, and iris; the myomeres had a V shape (Figure 12C) and had begun transforming into myoblasts for the formation of skeletal muscle fibers. The notochord was still present, and in the gill region, the gill filament supports could be seen to be still cartilaginous (Figure 12D).


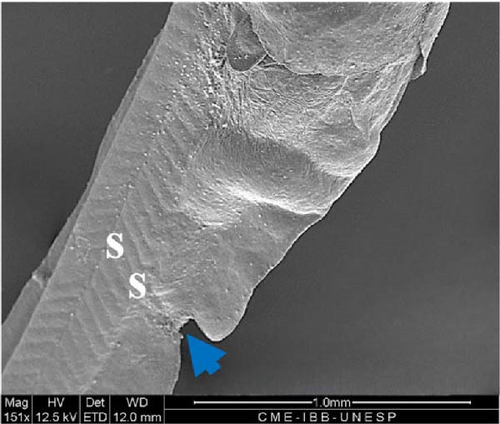
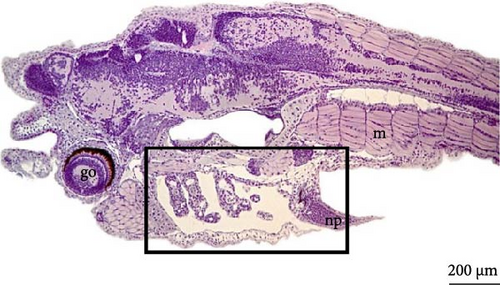
4. Discussion
The sequence of evolution of the structural formation of the embryos of freshwater fish species with external development, reported in scientific articles [16], is similar for most species, with small differences in the formation of some external structures of the larval body, suckers, sharp-pointed projections [17], and barbels [18].
The embryonic developmental stages established for the Hybrid LP were like those of its parental species L. marmoratus, namely, zygote, cleavage, gastrula, organogenesis, and hatching. Furthermore, L. marmoratus eggs are spherical, yellowish, demersal and do not present oil drops in the yolk sac throughout embryo development [19]. Regarding the development of P. reticulatum [20], despite not being described, by analyzing the photographic documentation in the present study, the stages of embryogenesis could be the same, including the nonexistence of the oil drop in the yolk, which was also the case in the LP Hybrid. In other words, the hybrid that is the focus of this study follows the developmental characteristics of its parental species.
In the study carried out with the hybrid from the female P. reticulatum and male L. marmoratus [21], an inverse hybrid (Hybrid PL) to the Hybrid LP, the embryogenesis period was similar to that of the Hybrid LP, 13 h at a similar average temperature (28.16°C), although small differences existed at the beginning of the cleavage and gastrulation stages (two blastomeres occurred after 0.5 h and the gastrula stage was observed after 3.7 h of incubation). Despite the many failed eggs observed during the incubation of the Hybrid LP, based on the analyses of [20, 21], who reported similar information on embryogenesis on the parental species and Hybrid PL, this event was considered to be related to other factors and not only the genetics of the hybrid in question.
In the Hybrid PL, yolk granules were identified at the animal pole in an irregular pattern [21]. A similar pattern was observed in the Hybrid LP, in which the yolk enters the fragmented animal pole and nourishes the embryo throughout development.
Heterogeneity was present at various stages of development of the Hybrid LP; however, this is a common characteristic that has also been identified in the Hybrid PL [21] and in other species, such as L. marmoratus [19], Prochilodus lineatus [10], and Steindachneridion parahybae [22].
The presence, distribution, and shape of chromatophores are characteristics that are related to the color pattern of fish species [6, 23]; that is, their determination could help in the separation of their larvae and, in this case, of the larvae of hybrids and their parents. The chromatophores of the Hybrid LP larvae were grouped in the anterior and posterior regions of the yolk sac, with some dispersed throughout the ventral region of the body. This feature was reported in L. marmoratus, which exhibited a similar distribution pattern. Furthermore, absorption of the yolk sac occurred on the third day after hatching [24], which was also observed for the Hybrid LP; on the third day, the yolk sac could no longer be identified, with only the developed stomach present.
The pattern of initial pigmentation development during embryogenesis and in the first days after hatching was observed in P. reticulatum [6] and for the Hybrid PL [21]. In the period of up to 5 days after hatching, the hybrid larvae could not be separated from the parental larvae based on their color.
The period for yolk absorption and structuring and the beginning of the functionality of the digestive system (exogenous feeding) in the Hybrid LP were like those reported for L. marmoratus [24].
The total length of the Hybrid LP larva was initially at a smaller average (1127.60 µm) than that of the parental species L. marmoratus (2160 µm) [24], but after ~5 days, the hybrid (7863.2 µm) had a slightly longer length than the parental species (7771 µm).
5. Conclusion
Through this research, it was possible to conclude that the embryonic development of the Hybrid LP originating from interspecific crosses between females of L. marmoratus with males of P. reticulatum occurred in a development period similar to that found in studies of its parental species, as well as for the inverse hybrid (PL), originating from females of P. reticulatum with males of L. marmoratus, under similar temperatures, not being possible to morphologically differentiate the embryos and larvae until the fifth day after hatching, neither of the parental species nor the Hybrid PL.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Yasmin Santos dos Reis: conceptualization, data curation, formal analysis, project administration, research, methodology, writing–original draft. Stella Indira Rocha Lobato: research development support. Laicia Carneiro Leite: data analysis, methodology support. Lais Pedroso Borges: conceptualization, data curation. Lorena Pacheco da Silva: formal analysis, research. Rodrigo Yutaka Dichoff Kasai: reproduction fish methodology and research, support. Rosicleire Veríssimo Silveira: morphology research support. Alexandre Ninhaus Silveira: conceptualization, data curation, research methodology, project administration, funding acquisition, writing, validation, and correction.
Funding
This work was supported by the São Paulo State Research Support Foundation (FAPESP) (Proc. 2020/100115-5) and the Coordination for the Improvement of Higher Education Personnel (CAPES).
Acknowledgments
The authors would like to thank Piraí Fish Farm for providing the structure and animals used in the study. To the Neotropical Ichthyology Laboratory (L.I.NEO) and the Electron Microscopy Center of the Biosciences Institute, UNESP, Botucatu, for providing physical space and structure for the development of parts of the research, and to the São Paulo Research Foundation (FAPESP) (Proc. 2020/100115-5) and the Coordination for the Improvement of Higher Education Personnel (CAPES) (Finance Code 001 Brazil), for the financial support for this research.
Open Research
Data Availability Statement
All data produced are available and described in this article; as soon as it is published, it will be available to the community.




