Genome-Wide Association Studies of Polysaccharides, Collagen, and Saponin Traits in the Sea Cucumber Apostichopus japonicus
Abstract
Apostichopus japonicus is a commercially important marine species known for its high nutritional value and a range of bioactive compounds, including polysaccharides, collagen, and saponins. In this study, a genome-wide association study (GWAS) was carried out on 143 A. japonicus individuals. A total of 72 single nucleotide polymorphisms (SNPs) and 36 candidate genes were identified through the analysis. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were then conducted, leading to the identification of 10 candidate genes related to polysaccharide, collagen, and saponin traits. These candidate genes were further validated through subsequent analyses. Five candidate genes, ALDH5A1, FAT1, KDR, ORC2, and PIGK, were identified to affect polysaccharides synthesis, among which the SNPs of ALDH5A1 had nonsynonymous substitution (S12G). BMI1 and UBR4 were identified as key candidate genes for collagen synthesis. A nonsynonymous SNP at Chr7_28028124, although not annotated to a known gene, was located within an exon and resulted in a serine-to-cysteine (S→C) substitution, which may influence collagen synthesis by affecting the function of an uncharacterized protein. CUBN and structural maintenance of Chromosomes 1B (SMC1B) were identified as candidate genes associated with saponin synthesis. The SNPs and candidate genes identified in this study serve as valuable genetic resources for selective breeding in A. japonicus and contribute to a better understanding of the genetic basis of these traits.
1. Introduction
Apostichopus japonicus is a commercially valuable marine species mainly distributed in the northwestern Pacific, especially along the coasts of China, Japan, and South Korea [1]. There are approximately 140 species of sea cucumber in China, with over 20 of these edible. Apostichopus japonicus has high nutritional value and is mainly produced in Shandong and Liaoning, China [2].
Their structural features—such as molecular weight, sulfation pattern, and sulfate content—are closely linked to a range of biological and pharmacological activities, including antitumor [3], antioxidant [4], anticoagulant [5], and antiparkinson effects [6].
In recent years, both domestic and international researchers have conducted extensive studies on the structure, function, and applications of A. japonicus polysaccharides. For example, studies on their anticoagulant activity have shown that they function by inhibiting the activity of intrinsic factor Xase [7].
Sea cucumber polysaccharides are generally classified into two types. The first type is sea cucumber glycosaminoglycan (GAG), commonly referred to as fucosylated chondroitin sulfate (FCS) [8]. While chondroitin sulfate is typically derived from animal cartilage and connective tissue, sea cucumbers exhibit a unique fucosylation pattern. The second type is sea cucumber fucoidan sulfate (SC-FUC), commonly known as fucoidan [9]. Fucose is an essential glycosyl component of glycoproteins located on the surfaces of intestinal epithelial cells and gut microorganisms. In addition, both fucose and fucoidan sulfate serve as carbon and energy sources for gut microbes, contributing to microbial–host symbiosis and supporting human health [10]. Sea cucumber polysaccharides help regulate intestinal health by interacting with a variety of gut microorganisms [11].
A genomic study of the tropical sea cucumber (Stichopus monotuberculatus) revealed evolutionary features of key gene families involved in the biosynthesis of FCS [12]. The biosynthesis of sea cucumber polysaccharides is mediated by several essential genes. Notably, 13 CHST11, 10 CHST13, and 22 CHST15 genes—encoding enzymes of the carbohydrate sulfotransferase (CHST) family—were found to be significantly expanded in the genome. Kyoto Encyclopedia of Genes and Genomes (KEGG)–based functional enrichment analysis showed that these expanded genes were involved in 62 pathways, with significant enrichment in GAG pathways.
Pathways related to sugar biosynthesis—including chondroitin sulfate production, pentose and glucuronic acid metabolism, glycan biosynthesis, and GAG-binding protein functions—are closely linked to FCS biosynthesis. The genome of the tropical sea cucumber uniquely contains multiple FUT genes, including eight copies of FUT4, nine copies of FUT7, and one copy of FUT3, all of which encode enzymes in the fucosyltransferase (FUT) family. Gene Ontology (GO) enrichment analysis indicated that these genes are associated with polysaccharide binding, proteoglycan binding, FUT activity, and α-1,3-FUT activity, highlighting their role in the specific biosynthesis of FCS.
Collagen, a major structural protein in animals, plays an essential role in maintaining tissue stability and functional integrity. In sea cucumbers, collagen accounts for roughly 70% of the body wall and contributes significantly to both their physiological function and commercial value [13]. Sea cucumber collagen is mainly composed of Type I collagen and has a characteristic triple-helix structure. It is rich in glycine (Gly), proline (Pro), and hydroxyproline (Hyp), which are typical of collagen proteins. These features give sea cucumber collagen excellent biocompatibility and mechanical strength. In addition, its high water solubility and thermal stability set it apart from collagen derived from terrestrial animals.
Collagen synthesis involves a complex series of posttranslational modifications. For example, prolyl hydroxylase (P4H) catalyzes the hydroxylation of collagen, which improves its thermal stability and resistance to enzymatic degradation [14]. Lysyl oxidase (LOX) promotes cross-linking of collagen fibers, thereby reinforcing the collagen network [15]. In addition, glycosyltransferases from the UDP-glycosyltransferase (UGT) family are involved in collagen glycosylation, which affects its water solubility and interactions with the extracellular matrix (ECM) [16].
Collagen synthesis is controlled by multiple signaling pathways, among which the transforming growth factor β (TGF-β)/Smad pathway serves as a central regulatory mechanism [17]. It promotes the transcription of collagen genes (e.g., COL1A1) by activating Smad2/3 proteins and simultaneously inhibits collagen-degrading enzymes (e.g., MMPs), thus, maintaining collagen homeostasis [18]. The Wnt and Hippo pathways also influence collagen synthesis by regulating genes involved in ECM remodeling [19]. Sea cucumber collagen releases various bioactive peptides with notable antioxidant properties, which help neutralize free radicals and reduce cellular aging [20]. Studies have shown that sea cucumber collagen and its bioactive peptides promote wound healing and tissue regeneration by enhancing fibroblast proliferation and migration, thereby accelerating tissue repair [21].
Saponins are glycosides composed of a sugar moiety linked to an aglycone, which is typically a triterpenoid or spirostane compound [22]. They are widely found in plants such as ginseng, platycodon, polygala, licorice, and bupleurum [23], but are rarely present in animals. However, saponins have been detected in some marine species, including sea cucumbers and starfish [24]. Sea cucumber saponins represent a unique type that functions in defense against predators and parasites [25]. These compounds are distributed throughout the body wall, digestive glands, gonads, respiratory trees, and Cuvierian tubules of sea cucumbers and exhibit a range of biological activities [26–29].
The biosynthesis of sea cucumber saponins involves multiple key genes, several of which have been successfully identified. Oxidosqualene cyclase (OSC) has been identified in the A. japonicus genome as a key enzyme in saponin biosynthesis [30]. Similar to its role in plants, OSC catalyzes the formation of parkeol, a precursor in the saponin biosynthetic pathway. OSC primarily catalyzes the cyclization of 2,3-epoxysqualene to produce sterols and triterpenoids. Parkeol, a naturally occurring terpenoid, is formed via OSC-mediated cyclization of 2,3-epoxysqualene.
Yang et al. [31] analyzed genes involved in triterpenoid saponin biosynthesis and their expression across various developmental stages, identifying 30 enzymes associated with this pathway in the sea cucumber genome. Acetyl-CoA C-acetyltransferases (AACTs) initiate the conversion of acetyl-CoA and isoprene units, while farnesyl pyrophosphate synthases (FPSs) polymerize isoprene to form preterpenoid diphosphate, leading to the production of various triterpenoid compounds. 3-Hydroxy-3-methylglutaryl-CoA synthases (HMGSs) catalyze the formation of mevalonate from 3-hydroxy-3-methylglutaric acid monoaldehyde in the mevalonate pathway. OSCs catalyze the formation of specific triterpenoid saponin skeletons from precursor compounds, while glycosyltransferases (SSs) add sugar moieties to the triterpene core to produce glycosidic compounds during biosynthesis. In addition, UGTs further modify saponins, enhancing their structural diversity. These genes show distinct expression patterns across developmental stages—fertilized eggs, blastula, gastrula, multiporus, and adults—highlighting the dynamic regulation of triterpenoid saponin biosynthesis.
Studies have shown that the biosynthetic pathway of saponin-based defense compounds in sea cucumbers differs from the typical steroid biosynthesis pathways found in other animals. In most animals, steroid biosynthesis involves the cyclization of 2,3-oxidosqualene to cholesterol, catalyzed by the OSC enzyme lanosterol synthase (LSS). However, sea cucumbers lack LSS and instead possess two distinct OSC enzymes that produce triterpenoid saponins. These enzymes are thought to have evolved from an ancestral LSS via gene duplication and neofunctionalization. In addition, sea cucumbers are believed to produce alternative steroidal compounds to counteract the toxicity of their own saponins, allowing them to synthesize both saponins and antisaponin steroids [32].
Jiang et al. [33] investigated the cloning and functional characterization of the phosphomevalonate kinase (PMK) gene involved in saponin biosynthesis in A. japonicus. The full-length PMK cDNA was obtained using rapid amplification of cDNA ends (RACE). The enzymatic activity of the recombinant PMK protein (rAjPMK) was assessed using enzyme-linked immunosorbent assay (ELISA) and its interacting proteins were identified through liquid chromatography-tandem mass spectrometry (LC-MS/MS). The results showed that rAjPMK interacts directly or indirectly with key pattern recognition receptors (PRRs) and is influenced by immune-related processes such as antioxidant activity, stress response, and enzymatic hydrolysis. Furthermore, its interaction with PMK suggests that rAjPMK may have a direct role in the saponin biosynthetic pathway.
Genome-wide association studies (GWASs) examine the relationship between genetic markers and specific traits based on genome-wide variation data [34]. This method has been widely used to study complex traits in both plants and animals [35, 36]. In aquaculture, GWAS is mainly applied to improve traits and enhance disease resistance, with research covering species such as fish, mollusks, crustaceans, and echinoderms. For example, a GWAS conducted on 289 giant groupers (Epinephelus lanceolatus) identified 36 single nucleotide polymorphisms (SNPs) associated with growth [37]. Similarly, GWAS on six growth traits in scallops identified two growth-related SNPs [38], and a study on 200 Pacific white shrimp (Litopenaeus vannamei) detected four SNPs linked to body weight [39]. To date, GWAS in A. japonicus has mainly focused on growth-related traits. A total of 39 candidate genes have been identified in A. japonicus through GWAS, with two of them significantly associated with growth-related metrics [40]. However, no GWAS studies have yet been reported on polysaccharide, collagen, or saponin traits in A. japonicus. The content of these bioactive compounds directly influences the economic value and potential applications of A. japonicus. Therefore, identifying genetic markers related to these traits is essential for improving the quality and commercial potential of this species.
In this study, genotypic data from 143 A. japonicus individuals were obtained through whole-genome resequencing. Genotypic data were integrated with phenotypic trait data for genome-wide association analysis. Candidate genes were screened based on GO and KEGG analyses and the identification of nonsynonymous SNPs, providing a scientific foundation for the future selection of high-quality A. japonicus.
2. Materials and Methods
2.1. Sample Collection and Trait Measurement
Samples were collected from seven A. japonicus populations in the Yellow Sea and Bohai Sea regions: Bangchui Island (BC), Changhai County (CH), Beihuangcheng Island (HC), Jiming Island (JM), Jinzhou (JZ), Lvshun (PJ), and Wafangdian (WF) (Figure 1). A total of 320 A. japonicus individuals were collected between May and July 2023 and were cultured under uniform environmental conditions after being transferred to the Key Laboratory of Northern Seawater Aquaculture, Ministry of Agriculture. For each specimen, a 0.5 cm × 0.5 cm section of the inner body wall was excised, immediately snap-frozen in liquid nitrogen, placed in a 1.5 mL centrifuge tube, and stored in liquid nitrogen for preservation.
Polysaccharide content in A. japonicus was determined using the phenol–sulfuric acid method [41]. Absorbance was measured at 490 nm using a microplate reader (SpectraMax i3, Shanghai Kehua Experimental Systems Co., Ltd.), and polysaccharide content in the body wall was calculated from a standard curve, with results expressed as percentage (%). Collagen content in A. japonicus was measured using the double-antibody sandwich ELISA method [42]. Microtiter plates were coated with collagen-specific antibodies to serve as the solid-phase capture antibodies. Collagen in the sample acted as the antigen and bound to the coated antibodies, forming an antigen–antibody complex with an enzyme-labeled secondary antibody. After washing, TMB substrate was added for color development. The TMB substrate, catalyzed by HRP, changed from blue to yellow upon acidification, and the color intensity was proportional to the collagen concentration. Absorbance was measured at 450 nm, and collagen content was calculated from a standard curve, with results expressed in micrograms per gram (µg/g). Saponin content in A. japonicus was quantified using high-performance LC (HPLC, Agilent 1260, Agilent Technologies, USA), with results reported in milligrams per kilogram (mg/kg) [43].
2.2. Bioactive Ingredient Content
Data on bioactive ingredient content from 143 samples were processed in R software (v4.3.3) using quality control procedures, including the scale and Shapiro.test functions, as well as the dplyr (v1.1.4) and outliers (v0.15.2) packages. Scale normalization and outlier detection were conducted to remove phenotypic extremes, following the 3δ rule (values beyond the mean ± three standard deviations were considered outliers). As linear models are highly sensitive to extreme values, the presence of outliers can introduce bias into the analysis. A normality test was also performed to evaluate whether the data met the assumptions required for parametric statistical analysis.
2.3. DNA Extraction and Sequencing
Genomic DNA was extracted from the inner body wall tissue of A. japonicus using the TIANGEN Marine Animals DNA Kit (TIANGEN, Beijing, China). Agarose gel electrophoresis was used to assess DNA quality, detect nonspecific bands, and check for RNA or protein contamination as well as to evaluate DNA degradation. DNA concentration was accurately measured using a Qubit 2.0 fluorometer. Resequencing libraries were then prepared using the GenoBaits DNA Library Prep Kit for Illumina (ILM). Library concentration was quantified with the Qubit 2.0 fluorometer and quality was evaluated by determining the effective concentration using qPCR. High-throughput sequencing was performed using the PE150 template on the UW MGI-2000/MGI-T7 platform. The sequencing data have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number (PRJNA1182943; The dataset is uploaded in sequential batches and certain entries may not be fully rendered during the process, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1182943).
2.4. Sequence Alignment and Mutation Detection
To ensure the quality of the analysis, fastp (version 0.20.0, parameters: -n 10 -q 20 -u 40) [44] was used to filter the raw reads. High-quality clean reads were obtained for further analysis. BWA software [45] (version 2.1) was used to align the clean reads to the reference genome following quality control.
Functional annotation of the detected gene variants was performed using NOVAR [46] (November 25, 2023). This annotation was combined with positional and gene localization data in the reference genome to identify synonymous and nonsynonymous mutations resulting from variants in multiple genes, including those located in intergenic, intronic, and coding sequence (CDS) regions. Population SNPs detection was performed using GATK software [47] (v4.4.0.0), followed by filtering to obtain reliable SNPs, which were saved as VCF files.
2.5. GWAS and Gene Annotation
GWAS was performed using both general linear model (GLM) and mixed linear model (LMM), with a significance threshold set at −log10 (p) > 6.
- 1.
Genes corresponding to significant SNPs (adjusted for false discovery rate (FDR) using the Benjamini–Hochberg method) were annotated and subjected to GO and KEGG pathway analyses. Candidate genes were then screened based on functional annotation.
- 2.
Genes annotated to nonsynonymous SNPs were further examined to assess their potential functional roles.
2.6. Quantitative Real-Time PCR (qRT-PCR) for Gene Expression Analysis
Gene expression analysis was performed using qRT-PCR. Primers were designed using Primer 6.0, targeting genes related to polysaccharide, collagen, and saponin traits. For gene validation, three samples representing high, medium, and low levels of each trait were selected, with three biological replicates per group. Total RNA was extracted using the Trizol method and assessed for purity (A260/280 ratio) and integrity via denaturing gel electrophoresis. RNA was reverse transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Tiangen). Relative gene expression levels were calculated using the 2−ΔΔCt method.
3. Results
3.1. Bioactive Ingredient Data Results
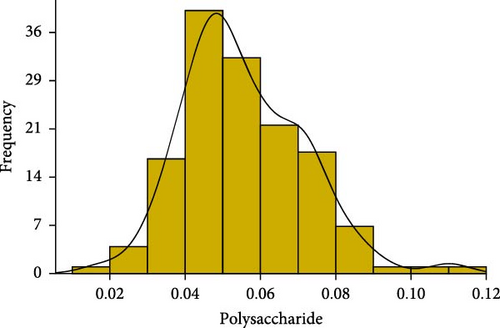
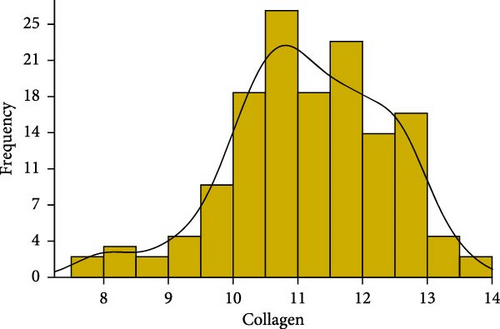
We conducted statistical analyses of polysaccharide, collagen, and saponin contents in A. japonicus, including normality testing, data normalization, outlier detection and removal, and calculations of skewness and kurtosis (Figure 2). The results indicate that all three traits—polysaccharides, collagen, and saponin—exhibit characteristics of near-normal distributions. For polysaccharides, the Shapiro–Wilk W statistic was 0.97, with a skewness of 0.237 and kurtosis of −0.368, indicating a slight right skew and a relatively flat distribution (Table 1). Similarly, collagen showed a Shapiro–Wilk W statistic of 0.98, skewness of −0.439, and kurtosis of 0.075, suggesting a slight left skew and near-symmetric distribution (Figure 2). Saponin content exhibited a more pronounced positive skew, with a Shapiro–Wilk W statistic of 0.89, skewness of 0.816, and kurtosis of −0.368, indicating a flatter distribution. Overall, these statistical results suggest that the distributions of polysaccharides, collagen, and saponin contents approximate normality.
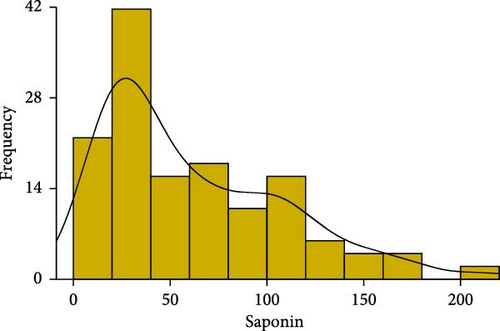
| Trait | Mean | SD | Median | Min | Max | Skew | Kurtosis | W value |
|---|---|---|---|---|---|---|---|---|
| Polysaccharides | 0.055 | 0.015 | 0.052 | 0.016 | 0.093 | 0.237 | −0.368 | 0.97 |
| Collagen | 11.146 | 1.205 | 11.12 | 7.669 | 13.567 | −0.439 | 0.075 | 0.98 |
| Saponin | 60.514 | 43.949 | 47.42 | 2.938 | 174.86 | 0.816 | −0.368 | 0.89 |
3.2. Genome-Wide Resequencing and Variant Analysis
Whole-genome resequencing was performed on 143 A. japonicus individuals, generating a total of 2125.58 Gb of raw data. As shown in Table 2, the ratio of clean data to raw data after filtering ranged from 92.73% to 99.38%. The proportion of bases with a Phred score ≥20 ranged from 97.82% to 98.94%, while those with a score ≥30 ranged from 93.89% to 96.11%. After quality control, clean reads were aligned to the reference genome, yielding an average sequencing depth of 13.59x and an average coverage of 93.29%. Detailed sequencing information for each sample is provided in Table S1. These results confirmed that the sequencing quality was sufficient for downstream analyses.
| Measurement parameters | Mean | SD | Max | Min |
|---|---|---|---|---|
| Effective rate (%) | 96.11 | 0.98 | 99.38 | 92.73 |
| Q20 (%) | 98.10 | 0.19 | 98.94 | 97.82 |
| Q30 (%) | 94.67 | 0.37 | 96.11 | 93.89 |
| Average depth (X) | 13.59 | 11.60 | 54.48 | 7.01 |
| Coverage (%) | 93.29 | 1.57 | 98.24 | 90.5 |
3.3. GWASs
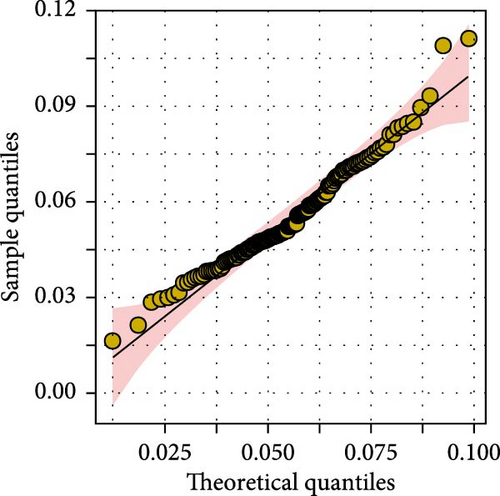
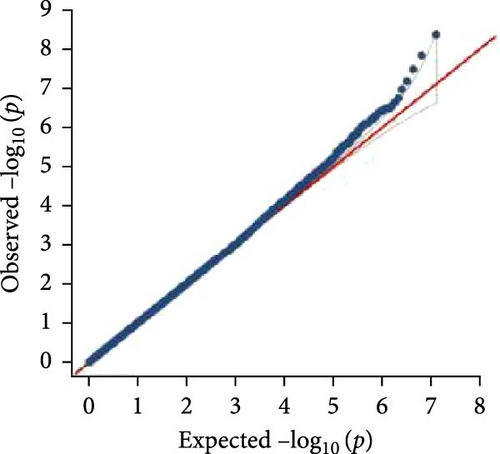
In this study, genome-wide association analysis (GWAS) of A. japonicus was performed using both GLM and LMM, with a significance threshold set at −log10 (p) > 6. A total of 72 significant SNPs were identified across the genome. The QQ plot supported the reliability of the model and the robustness of the GWAS results (Figure 3).
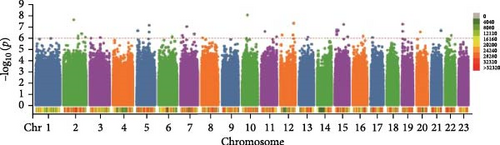
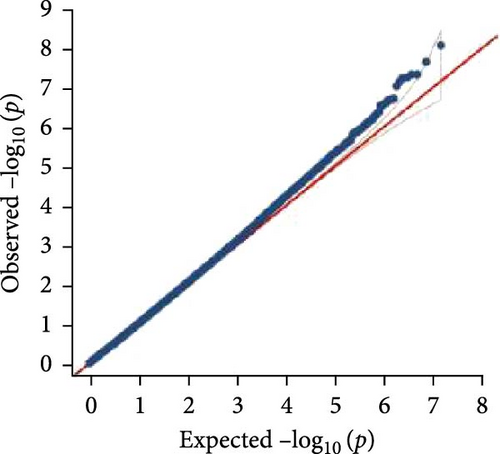
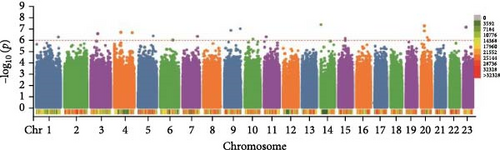
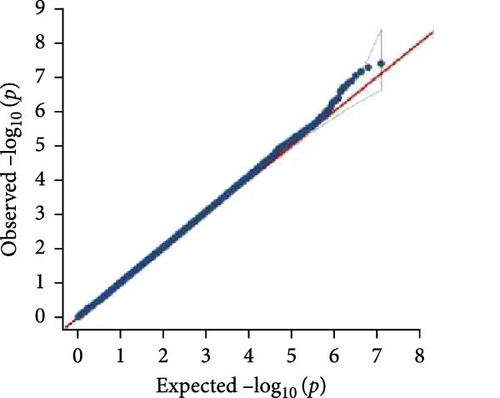
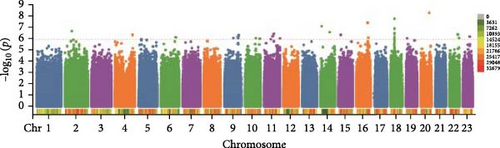
3.4. Gene Annotation
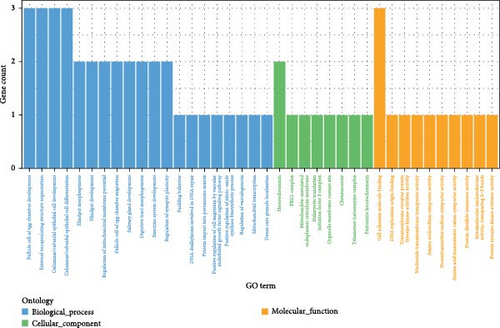
Polysaccharide traits in A. japonicus were analyzed using GWAS based on the GLM (Figure 3a,b). After applying FDR correction, 37 significant SNPs were identified. The distribution of these SNPs is shown in Table 3, with detailed information provided in Tables S2–S4 of the Supporting Information. A nonsynonymous SNP, Chr5_2691446, was identified within an exon region on Chromosome 5. This variant involves a T-to-C base substitution, leading to an A-to-G change at the 34th nucleotide of the cDNA sequence and a corresponding amino acid change from serine (S) to Gly (G) at position 12 of the protein. GO enrichment analysis (Figure 4a) mapped 17 genes to various GO terms, with most enriched in biological processes. Enriched biological processes included follicular cell development in the egg chamber, differentiation and development of columnar/cuboidal epithelial cells, and organization of peripheral structures. KEGG pathway analysis revealed significant enrichment in the yeast cell cycle, HIF-1 signaling pathway, Rap1 signaling pathway, glycosylphosphatidylinositol (GPI)-anchor biosynthesis, and endocytosis (Table 4).
| Trait | Chr | Position (bp) | SNPs number |
|---|---|---|---|
| Polysaccharides | 2 | 19339577–41484825 | 5 |
| Polysaccharides | 3 | 19663694 | 1 |
| Polysaccharides | 5 | 2691446–24071413 | 3 |
| Polysaccharides | 6 | 25384154–25444823 | 2 |
| Polysaccharides | 7 | 3127281–25902643 | 4 |
| Polysaccharides | 8 | 2296426 | 1 |
| Polysaccharides | 10 | 10138633 | 1 |
| Polysaccharides | 11 | 6790624–28739868 | 2 |
| Polysaccharides | 12 | 3001868–25010053 | 4 |
| Polysaccharides | 13 | 16768309 | 1 |
| Polysaccharides | 15 | 3123651–23681825 | 6 |
| Polysaccharides | 16 | 19138691 | 1 |
| Polysaccharides | 17 | 4072850 | 1 |
| Polysaccharides | 19 | 1757793–1757835 | 2 |
| Polysaccharides | 20 | 6964976 | 1 |
| Polysaccharides | 21 | 18817455 | 1 |
| Polysaccharides | 22 | 11695188 | 1 |
| Trait | Pathway ID | Description | Gene ID |
|---|---|---|---|
| Polysaccharides | ko04111 | Cell cycle-yeast | evm.TU.Chr10.306/evm.TU.Chr2.619 |
| Polysaccharides | ko04066 | HIF-1 signaling pathway | evm.TU.Chr15.192/evm.TU.Chr13.448 |
| Polysaccharides | ko04015 | Rap1 signaling pathway | evm.TU.Chr2.619/evm.TU.Chr13.448 |
| Polysaccharides | ko00563 | Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | evm.TU.Chr15.512 |
| Polysaccharides | ko04144 | Endocytosis | evm.TU.Chr5.770/evm.TU.Chr13.448 |
| Collagen | ko04540 | Gap junction | evm.TU.Chr10.441 |
| Collagen | ko05202 | Transcriptional misregulation in cancer | evm.TU.Chr20.343 |
| Collagen | ko04530 | Tight junction | evm.TU.Chr10.441 |
| Saponin | ko00514 | Other types of O-glycan biosynthesis | evm.TU.Chr10.828 |
| Saponin | ko04512 | ECM–receptor interaction | evm.TU.Chr5.167 |
| Saponin | ko04977 | Vitamin digestion and absorption | evm.TU.Chr10.614 |
GWAS was performed using the GLM to analyze collagen traits in A. japonicus (Figures 3c, d). After FDR correction, 17 significant SNPs were identified. The distribution of these SNPs is shown in Table 5, with detailed information available in Tables S2–S4 of the Supporting Information. A nonsynonymous SNP, Chr7_28028124, was located within an exon on Chromosome 7. This mutation involves a C-to-G base substitution at position 1067 of the cDNA sequence, leading to a serine (S) to cysteine (C) change at the 356th amino acid in the protein.
| Trait | Chr | Position (bp) | SNPs number |
|---|---|---|---|
| Collagen | 1 | 41637966 | 1 |
| Collagen | 3 | 12874796 | 1 |
| Collagen | 4 | 11986718–33221664 | 2 |
| Collagen | 5 | 28920036 | 1 |
| Collagen | 6 | 23347864 | 1 |
| Collagen | 7 | 28028124 | 1 |
| Collagen | 9 | 12134333–29847337 | 2 |
| Collagen | 10 | 15601918 | 1 |
| Collagen | 11 | 4077160 | 1 |
| Collagen | 14 | 837743 | 1 |
| Collagen | 15 | 12587508 | 1 |
| Collagen | 20 | 6845053–12181190 | 3 |
| Collagen | 23 | 5321767 | 1 |
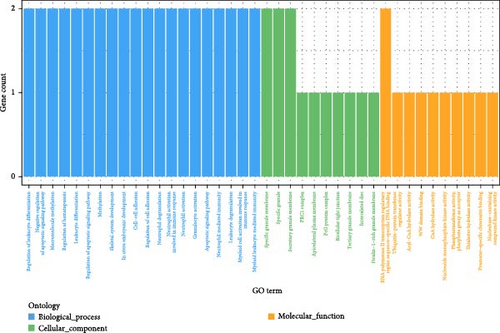
GO enrichment analysis (Figure 4b) mapped six genes to GO terms, with most enriched in biological processes. Enriched biological processes included regulation of leukocyte differentiation, negative regulation of apoptotic signaling, macromolecular methylation, and regulation of hematopoiesis. Enriched cellular components included the specific granule membrane, specific granules, and secretory granule membrane. Enriched molecular functions involved sequence-specific DNA binding within transcriptional regulatory regions of RNA polymerase II. KEGG pathway analysis identified significant enrichment in gap junction, transcriptional misregulation in cancer, and tight junction pathways (Table 4).
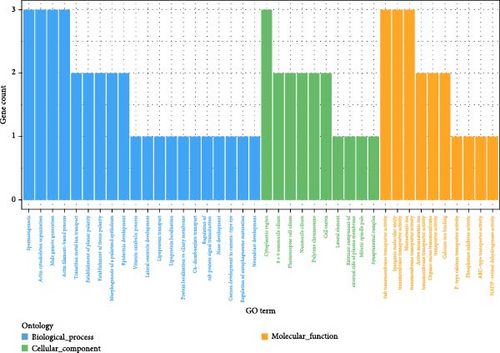
In the GWAS using the LMM to analyze saponin traits in A. japonicus (Figures 3e, f), 18 significant SNPs were identified following FDR correction. The distribution of these SNPs is shown in Table 6, with detailed information available in Tables S2–S4 of the Supporting Information. GO enrichment analysis (Figure 4c) mapped 11 genes to GO terms, most of which were enriched in biological processes. Enriched biological processes included actin cytoskeleton organization and actin filament–based processes. The major cellular component was the cytoplasmic region. Enriched molecular functions included salt transmembrane transporter activity, inorganic molecular entity transmembrane transporter activity, and monatomic ion transmembrane transporter activity. KEGG pathway analysis revealed significant enrichment in pathways related to other types of O-glycan biosynthesis, ECM–receptor interaction, and vitamin digestion and absorption (Table 4).
| Trait | Chr | Position (bp) | SNPs number |
|---|---|---|---|
| Saponin | 4 | 33053000 | 1 |
| Saponin | 5 | 5768236 | 1 |
| Saponin | 6 | 28019380 | 1 |
| Saponin | 9 | 26459105–26461808 | 2 |
| Saponin | 10 | 20240521–27678570 | 2 |
| Saponin | 11 | 12325315–29276963 | 3 |
| Saponin | 14 | 1697507 | 1 |
| Saponin | 16 | 20745640 | 1 |
| Saponin | 18 | 7233073–7233142 | 3 |
| Saponin | 19 | 2494450 | 1 |
| Saponin | 20 | 15834809 | 1 |
| Saponin | 23 | 18103131 | 1 |
Detailed information on the SNPs and genes significantly associated with the traits of polysaccharides, collagen, and saponins in A. japonicus is provided in Table 7.
| Trait | Mark | Chr | Position | Gene | Gene symbol | Annotation |
|---|---|---|---|---|---|---|
| Polysaccharides | Chr5_2691446 | 5 | 2691446 | evm.TU.Chr5.45 | ALDH5A1 | Dehydrogenase |
| Polysaccharides | Chr13_16768309 | 13 | 16768309 | evm.TU.Chr13.447 | FAT1 | Laminin G domain |
| Polysaccharides | Chr13_16768309 | 13 | 16768309 | evm.TU.Chr13.448 | KDR | Belongs to the protein kinase superfamily. Tyr protein kinase family. CSF-1 PDGF receptor subfamily |
| Polysaccharides | Chr10_10138633 | 10 | 10138633 | evm.TU.Chr10.306 | ORC2 | Origin recognition complex, subunit 2 |
| Polysaccharides | Chr15_15946459 | 15 | 15946459 | evm.TU.Chr15.512 | PIGK | GPI-anchor transamidase activity |
| Polysaccharides | Chr2_19339577 | 2 | 19339577 | evm.TU.Chr2.619 | — | WD repeat-containing protein |
| Polysaccharides | Chr15_5717747 | 15 | 5717747 | evm.TU.Chr15.192 | — | The RING-variant domain is a C4HC3 zinc-finger like motif found in a number of cellular and viral proteins. Some of these proteins have been shown both in vivo and in vitro to have ubiquitin E3 ligase activity |
| Polysaccharides | Chr5_24071413 | 5 | 24071413 | evm.TU.Chr5.770 | IQSEC1 | Regulation of ARF protein signal transduction |
| Collagen | Chr7_28028124 | 7 | 28028124 | evm.TU.Chr7.797 | — | — |
| Collagen | Chr20_12181190 | 20 | 12181190 | evm.TU.Chr20.343 | BMI1 | Polycomb complex protein BMI-1 |
| Collagen | Chr15_12587508 | 15 | 12587508 | evm.TU.Chr15.384 | UBR4 | Ubiquitin protein ligase E3 component n-recognin 4 |
| Collagen | Chr10_15601918 | 10 | 15601918 | evm.TU.Chr10.441 | TJP2 | Tight junction protein |
| Saponin | Chr10_20240521 | 10 | 20240521 | evm.TU.Chr10.614 | CUBN | Cobalamin catabolic process |
| Saponin | Chr5_5768236 | 5 | 5768236 | evm.TU.Chr5.167 | SMC1B | Structural maintenance of chromosomes |
| Saponin | Chr10_27678570 | 10 | 27678570 | evm.TU.Chr10.828 | POFUT2 | Peptide-O-fucosyltransferase activity |
3.5. Genetic Validation Results
For polysaccharide-related traits in A. japonicus, three sample groups were selected based on content levels: high (CH30, 0.0811%), control (HC42, 0.0491%), and low (BC22, 0.0295%), with three biological replicates per group. In gene evm.TU.Chr10.306, the expression level in BC22 was higher than in CH30; in evm.TU.Chr13.447, CH30 showed higher expression than BC22; the expression level of CH30 in evm.TU.Chr13.448 was significantly higher than that in BC22 (p < 0.05); in evm.TU.Chr15.512, CH30 also exhibited higher expression than BC22. Similarly, CH30 showed significantly higher expression than BC22 in evm.TU.Chr5.45 (p < 0.05, Figure 5a).
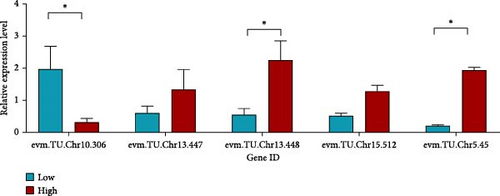
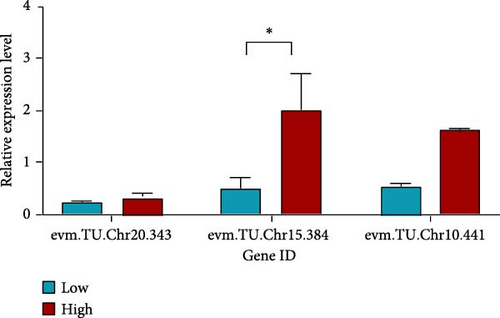
For collagen-related traits in A. japonicus, three groups were selected: high content (BC17, 13.57 µg/g), control (BC43, 10.00 µg/g), and low content (WF11, 7.67 µg/g), with three biological replicates per group. In gene evm.TU.Chr20.343, expression in BC17 was higher than in WF11; BC17 also showed significantly higher expression than WF11 in evm.TU.Chr15.384 (p < 0.05). Additionally, expression in BC17 was higher than in WF11 for evm.TU.Chr10.441 (Figure 5b).
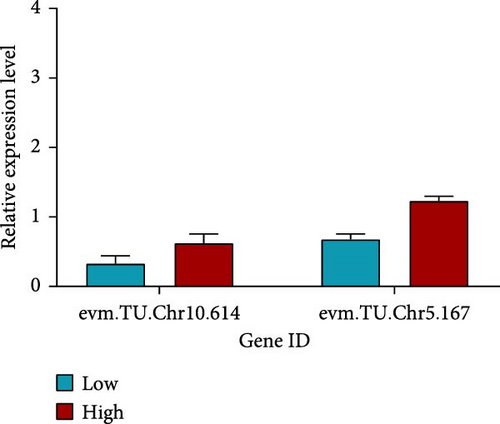
For saponin-related traits in A. japonicus, three groups were selected: high content (WF13, 207.42 mg/kg), control (BC7, 76.01 mg/kg), and low content (WF09, 10.36 mg/kg), with three biological replicates per group. In gene evm.TU.Chr10.614, expression in WF13 was higher than in WF09. Similarly, WF13 showed higher expression than WF09 in evm.TU.Chr5.167 (Figure 5c).
Primer information for the genes is provided in Table S5.
4. Discussion
GWAS was performed to investigate the genetic basis of polysaccharide, collagen, and saponin traits in A. japonicus. In gene screening, priority was given to those containing nonsynonymous SNPs, as such mutations alter amino acid sequences and can directly impact protein structure and function [48]. These mutations may lead to loss, enhancement, or alteration of protein function, ultimately affecting the associated trait.
GO and KEGG enrichment analyses were conducted on genes linked to significant SNPs. Genes enriched in multiple GO terms and KEGG pathways were selected as candidate genes for further validation by qRT-PCR.
4.1. Molecular Markers and Candidate Genes Associated With Polysaccharides Traits in Apostichopus japonicus
This study identified 37 SNPs significantly associated with polysaccharide traits in A. japonicus, resulting in the identification of five candidate genes. Among these, one SNP carried a nonsynonymous mutation.
The nonsynonymous SNP Chr5_2691446 on Chromosome 5 corresponds to the gene encoding aldehyde dehydrogenase 5 family member A1 (ALDH5A1). ALDH5A1 is involved in the oxidation of γ-aminobutyric acid (GABA) to succinate, a key reaction in the GABA metabolic pathway. GABA acts as the primary inhibitory neurotransmitter, playing a vital role in maintaining neuronal homeostasis and preventing overexcitation [49]. Succinate also serves as an intermediate in the tricarboxylic acid (TCA) cycle, underscoring the role of ALDH5A1 in cellular energy metabolism [50].
A study on fat deposition in chickens found that ALDH5A1 was significantly upregulated in the skeletal muscle of individuals with high intramuscular fat content and was closely linked to glucose and lipid metabolism pathways, including glycolysis/gluconeogenesis and starch and sucrose metabolism [51]. These findings suggest that ALDH5A1 may contribute to polysaccharide synthesis by modulating glucose metabolism.
Gene expression analysis showed that A. japonicus individuals with high polysaccharide content exhibited significantly elevated ALDH5A1 expression compared to those with low polysaccharide levels. These results suggest that ALDH5A1 may play an important role in the biosynthesis of polysaccharides in A. japonicus.
FAT1, located at the significant SNP Chr13_16768309 on Chromosome 13, encodes an atypical cadherin protein belonging to the cadherin superfamily. This protein plays essential roles in cell adhesion, regulation of cell polarity, and intracellular signal transduction [52]. FAT1 can activate multiple signaling pathways—such as Wnt/β-catenin, Hippo, and MAPK/ERK—through interactions with various partner proteins, thereby influencing cell proliferation, migration, and invasion [53].
Sulfated polysaccharides in sea cucumbers primarily include fucoidan sulfate (FCS) and heparan sulfate-like polysaccharides (HCS). Together with collagen fibers and proteoglycans, these sulfated polysaccharides (HCS/FCS) form the ECM of the A. japonicus body wall, contributing to its distinct mechanical strength and biological function [54]. FAT1-mediated cell adhesion may contribute to ECM stability in the sea cucumber body wall, suggesting that polysaccharide synthesis could be indirectly regulated by FAT1. qPCR analysis showed elevated FAT1 expression in high-polysaccharide samples, indicating that FAT1 may play a positive regulatory role in polysaccharide accumulation.
The gene corresponding to the significant SNP Chr13_16768309 on Chromosome 13 encodes vascular endothelial growth factor receptor 2 (VEGFR-2), also known as KDR, a Type III receptor tyrosine kinase. KDR functions as a primary receptor for vascular endothelial growth factor (VEGF), mediating critical processes such as endothelial cell proliferation, survival, migration, and tubular morphogenesis [55]. Upon VEGF binding, KDR activates downstream signaling pathways, including PI3K/Akt, MAPK/Erk, and PLCγ, all of which are essential for regulating cell proliferation and survival [56, 57].
Previous studies have shown that hydrolysates derived from the black milk sea cucumber (Holothuria nobilis) improve insulin resistance in diabetic rats by activating the PI3K/Akt signaling pathway [58]. While the study primarily addressed anti-diabetic effects, it also underscored the potential involvement of the PI3K/Akt pathway in mediating the biological functions of sea cucumber polysaccharides.
In the current study, KDR was found to be enriched in the HIF-1 signaling pathway, angiogenesis signaling, and the endocytosis pathway. Within the endocytosis pathway, KDR plays a role in regulating the uptake of extracellular substances, which may impact polysaccharide metabolism and transport. HIF-1 is a central regulator of glucose metabolism, promoting glycolysis, suppressing the TCA cycle, and enhancing the pentose phosphate pathway (PPP) to optimize cellular energy production under hypoxic conditions [59]. It upregulates glucose transporters (GLUT1 and GLUT3) and key glycolytic enzymes—such as hexokinase (HK2), phosphofructokinase (PFK1), and pyruvate kinase (PKM2)—to boost glucose uptake and utilization [60]. HIF-1 also downregulates pyruvate dehydrogenase (PDH) activity via upregulation of PDH kinase 1 (PDK1), thereby limiting pyruvate entry into the TCA cycle and favoring glycolysis over oxidative phosphorylation [61]. Moreover, HIF-1 stimulates the expression of glucose-6-phosphate dehydrogenase (G6PD), thereby enhancing PPP activity, increasing NADPH production for antioxidant defense, and supplying precursors for carbohydrate biosynthesis [62].
KDR is a downstream effector of the HIF-1 signaling pathway [63]. Activation of VEGFR-2, encoded by KDR, initiates signaling cascades such as PI3K/Akt and MAPK/ERK, which in turn enhance the expression and activity of enzymes involved in glucose metabolism. This promotes glycolysis, increases glucose uptake, and facilitates carbohydrate processing via receptor-mediated endocytosis [64].
It is speculated that the HIF-1 pathway may influence the transmembrane transport of polysaccharides in A. japonicus by regulating GLUT transporters or other sugar carriers. Moreover, by enhancing glycolysis and PPP activity, HIF-1 increases cellular demand for glucose, which may accelerate the degradation of sea cucumber polysaccharides to generate glycosyl precursors for metabolic processes. Gene expression analysis revealed significantly higher KDR expression in samples with elevated polysaccharide content. Taken together, these findings suggest that KDR may play a regulatory role in polysaccharide biosynthesis in A. japonicus.
ORC2, encoded by the significant SNP Chr10_10138633 on Chromosome 10, is a subunit of the origin recognition complex (ORC), which is essential for initiating DNA replication in eukaryotic cells. Loss of function or abnormalities in ORC2 can impair cell growth and differentiation [65]. For instance, in yeast, the temperature-sensitive Orc2-1 mutant leads to reduced cell growth rates [66].
Gene expression analysis showed significantly lower ORC2 expression in high-polysaccharide samples, suggesting a potential indirect role in polysaccharide synthesis via modulation of cell cycle progression. As a core component of the DNA replication initiation complex [67], reduced ORC2 expression may lead to G1 phase arrest in parietal cells (e.g., those responsible for ECM secretion), thereby lowering proliferation and reallocating metabolic resources toward polysaccharide synthesis and secretion. The downregulation of ORC2 may have pleiotropic effects, including reduced growth rates; however, its direct involvement in polysaccharide synthesis requires further validation, such as through gene knockdown or silencing experiments.
PIGK (phosphatidylinositol glycan anchor biosynthesis K-like), identified at the significant SNP Chr15_15946459 on Chromosome 15, encodes a member of the cysteine protease family C13 and is involved in the biosynthesis of GPI anchors. GPI anchors are glycolipids commonly found on the surface of blood cells, where they mediate protein attachment to the membrane and play important roles in protein localization and cellular signal transduction [68].
Lim et al. [69] reported that various PIGK gene variants were associated with growth performance and meat quality traits in pigs, indicating a potential role for PIGK in regulating porcine development.
In this study, PIGK showed elevated expression levels in A. japonicus individuals with high polysaccharide content and was functionally enriched in the GPI anchor biosynthesis pathway. It is, therefore, speculated that PIGK may enhance GPI anchoring efficiency, facilitating the membrane localization of polysaccharide-synthesizing enzymes (such as glycosyltransferases), and thereby promoting the production of polysaccharides like chondroitin sulfate. Nevertheless, the specific functional role of PIGK in A. japonicus remains to be experimentally validated.
4.2. Molecular Markers and Candidate Genes Associated With Collagen Traits in Apostichopus japonicus
In this study, 17 SNPs were found to be significantly associated with collagen-related traits in A. japonicus, leading to the identification of three candidate genes. Among these, one SNP carried a nonsynonymous mutation, but was not annotated to any known gene. Despite the lack of annotation, the SNP remains of interest, as it may reside in intergenic regions, regulatory elements, or previously uncharacterized gene loci. Such variants may still impact gene expression or protein function and thus warrant further functional investigation.
BMI1, located at the significant SNP Chr20_12181190 on Chromosome 20, encodes a polycomb group (PcG) protein that functions as a core component of the polycomb repressive complex 1 (PRC1). BMI1 is essential for chromatin remodeling and epigenetic regulation, acting primarily to modulate gene expression involved in maintaining cell self-renewal and differentiation [70]. A study in Holothuria leucospilota revealed that BMI1 expression was upregulated during the early stages of body wall regeneration, indicating a role in promoting cell proliferation and tissue repair. BMI1 functions as a transcriptional regulator of the cell cycle and cellular proliferation across multiple tissues. Its upregulation during sea cucumber body wall regeneration promotes both proliferation and differentiation, supporting tissue repair and reconstruction [71].
Given that the sea cucumber body wall is rich in collagen [28], gene expression analysis showing elevated BMI1 levels in high-collagen samples suggests that BMI1 may contribute to collagen synthesis.
In A. japonicus, collagen synthesis is regulated by transcription factors that control gene expression through interactions with collagen gene promoters [68]. The TGF-β signaling pathway plays a central role in collagen synthesis. Activation of TGF-β and its downstream SMAD cascade has been shown to enhance collagen production in A. japonicus, underscoring their importance in regulating this trait [72].
BMI1 has also been implicated in the regulation of TGF-β signaling in certain cell types. For example, in hepatocellular carcinoma, BMI1 modulates TGF-β expression and function, thereby influencing ECM remodeling and collagen synthesis [72].
UBR4 (ubiquitin protein ligase E3 component N-recognin 4), located at the significant SNPs Chr15_12587508 on Chromosome 15, encodes an E3 ubiquitin ligase involved in the N-end rule pathway [73]. This protein is essential for regulating protein degradation and maintaining cellular homeostasis. In the cytoplasm, UBR4 interacts with clathrin to form a network structure, contributing to membrane morphogenesis and cytoskeletal organization [74]. UBR4 also binds to retinoblastoma-related protein (Rb) and the calcium-binding protein calmodulin (CaM) [75]. Previous studies have demonstrated a key role for UBR4 in intestinal regeneration in sea cucumbers. As an E3 ubiquitin ligase, UBR4 facilitates protein ubiquitination, a process critical for cytoskeletal reorganization and intracellular signal transduction [76]. These functions are especially important during the rapid regeneration of internal organs in sea cucumbers under stress.
From the standpoint of protein ubiquitination and ECM metabolism (e.g., collagen), UBR4 may contribute indirectly to collagen homeostasis. Specifically, UBR4 may affect collagen synthesis, posttranslational modification, or degradation by modulating the turnover of enzymes and regulatory proteins involved in collagen metabolism. Gene expression analysis showed higher UBR4 expression in A. japonicus individuals with elevated collagen content, suggesting that UBR4 may play a role in regulating collagen synthesis in this species.
4.3. Molecular Markers and Candidate Genes Associated With Saponin Traits in Apostichopus japonicus
This study identified 18 SNPs significantly associated with saponin traits in A. japonicus, leading to the selection of two candidate genes.
CUBN, located at SNP Chr10_20240521 on Chromosome 10, encodes cubilin, a protein mainly expressed in the epithelial cells of the intestine and kidney [77]. Cubilin plays a key role in the absorption of vitamin B12 (cobalamin) [78]. It binds vitamin B12 via its 27 CUB domains and mediates endocytosis in coordination with the protein AMN. In addition, cubilin contributes to the metabolism of lipoproteins, vitamins, and iron [79].
Han et al. [80] reported breed-specific variation in CUBN expression and copy number in cattle, with significant associations observed between CUBN CNVs and growth traits such as body height and eye muscle area. Research has shown that the CUB domain in CUBN enhances the binding affinity of the AjCTL-2 protein to specific sugars, including D-galactose.
By virtue of its unique structure, the CUB domain strengthens the sugar-binding capacity of AjCTL-2, contributing to the immune defense mechanisms of sea cucumbers [81].
In this study, CUBN was enriched in pathways related to vitamin and mineral metabolism and was highly expressed in samples with elevated saponin content. These findings suggest that CUBN may influence the synthesis and accumulation of saponins in sea cucumbers by modulating nutrient uptake, glycosylation processes, and immune responses.
Structural maintenance of Chromosomes 1B (SMC1B), identified at SNP Chr5_5768236 on Chromosome 5, encodes a protein essential for meiosis. It plays roles in sister chromatid cohesion, axial element assembly, homologous chromosome synapsis and recombination, and chromosome movement. These functions underscore the essential role of SMC1B in meiosis and chromosome stability [82].
Islam et al. [82] demonstrated the importance of SMC1B in meiotic progression during their study on zebrafish, with relevance to salmon reproductive biology. Their results indicated that SMC1B is essential for the development of spermatogonia and gamete formation in salmon.
By regulating the cell cycle, maintaining chromosome integrity, and supporting cell proliferation, SMC1B may provide the cellular environment and metabolic resources necessary for saponin synthesis and accumulation in A. japonicus.
Elevated SMC1B expression, as revealed by qRT-PCR, may reflect increased metabolic activity associated with saponin biosynthesis. Thus, SMC1B may influence saponin traits in A. japonicus through its key roles in meiosis and cellular development.
5. Conclusion
In this study, genome-wide association analysis (GWAS) identified 72 significant SNPs and 10 candidate genes linked to the contents of polysaccharides, collagen, and saponins-key bioactive traits in A. japonicus. These traits are closely associated with the nutritional quality, physiological function, and commercial value of sea cucumbers, underscoring the significance of our findings for performance traits in aquaculture.
Five candidate genes were identified for polysaccharides content: ALDH5A1, FAT1, KDR, ORC2, and PIGK. These genes participate in essential metabolic and regulatory pathways—including glycolipid metabolism, ECM stabilization, and glycosylation—that contribute to polysaccharide biosynthesis. Notably, a nonsynonymous SNP (S12G) in ALDH5A1 suggests a potential functional variant influencing succinate metabolism and cellular energy balance.
For collagen content, two key genes—BMI1 and UBR4—were associated with collagen gene regulation and metabolic balance via the TGF-β pathway and ubiquitination, respectively. Additionally, a nonsynonymous SNP (Chr7_28028124) within an exon—though unannotated to a known gene-resulted in a serine-to-cysteine substitution, indicating a potential novel regulator of collagen synthesis.
For saponin traits, CUBN and SMC1B were identified as candidate genes involved in vitamin absorption, glycosylation, and energy metabolism-processes essential to saponin biosynthesis and accumulation. These genetic markers offer practical targets for selective breeding strategies to enhance product quality and boost bioactive compound yield in A. japonicus.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of Dalian Ocean University.
Consent
The authors have nothing to report.
Disclosure
All images and data in this study are original and were created by the author. No previously published images or data have been used.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jun Ding and Luo Wang designed and conceived the whole experiments. Yongjie Wang and Xianglei Zhang collected important background information and carried out literature retrieval, data collection, and data analysis. Xianglei Zhang, Shufeng Li, Ye Tian, Weiyan Li, Qi Ye, Fenglin Tian, Haoran Xiao, Junhui Wang, Wanrong Tian, and Hao Wang performed the experiments. Qi Ye and Shufeng Li provided help for data analysis. Yongjie Wang and Jun Ding wrote the paper. Yangqing Chang, Chong Zhao, Lingshu Han, Luo Wang, and Jun Ding checked the manuscript.
Funding
This research was funded by “Xingliao Talent Plan” Leading Talents Project (Grant XLYC2202001), Major Agriculture Project of Science and Technology Department of Liaoning Province (Grant 2023JH1/10200007), and the Central Guidance on Local Science and Technology Development Fund of Liaoning Province (Grant 2023JH6/100100022).
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors. The sequencing data that support the findings of this study are openly available in the NCBI Sequence Read Archive (SRA) under BioProject Accession No. PRJNA1182943.




