Effects of Pelleted Probiotic on Growth, Water Quality, and Disease Resistance in Pacific White Shrimp (Litopenaeus vannamei) in Static Biofloc Systems
Abstract
Probiotics are increasingly used in aquaculture to improve growth and water quality and boost disease resistance in farmed species. This study investigated the application of various concentrations of a commercial probiotic (mix of Bacillus subtilis and B. licheniformis) added to culture water to evaluate its effects on growth performance, water quality, and resistance to Vibrio parahaemolyticus infection. Over a 9-week trial, Pacific white shrimp (1.20 ± 0.01 g; stocked at 160 shrimp m−3) were reared in static biofloc culture systems consisting of 24 independent 156 L circular black polyethylene tanks. Shrimp were subjected to various probiotic concentrations weekly (×4, ×8, and ×16 of the recommended dose, which was one pellet per 200 g of feed) as three treatments. The control group (×0), without any probiotic addition, was used as the fourth treatment. Each treatment had six replicate tanks and shrimp were provided a commercial diet (Zeigler Shrimp Grower SI-35, CP 35%) four times per day via hand feeding. Following 9 weeks of culture, probiotic addition did not significantly impact growth or water quality (besides significant dissolved oxygen (DO) and temperature variations), but improved disease resistance. During the disease challenge, survival in all probiotic treatments (×4, ×8, and ×16) was significantly higher (regardless of dose) than that of the control group (x0; p < 0.05). These results suggest that the commercial probiotic may enhance shrimp resilience against Vibrio spp. infections. These findings suggest that although growth or water quality improvements were not confirmed in this trial, this probiotic appears to positively affect disease resistance in shrimp against V. parahaemolyticus infection in biofloc systems.
1. Introduction
Pacific white shrimp, Litopenaeus vannamei, holds a pivotal position in global aquaculture due to its rapid growth, high tolerance to varying environmental conditions, and adaptability to intensive farming approaches. These include pond-based aquaculture, biofloc technology (BFT), mixotrophic system, and recirculating aquaculture systems (RASs). Among these, BFT has been increasingly adopted for its ability to enhance water quality, reduce environmental impacts, and improve the growth performance of shrimp by fostering heterotrophic bacteria to assimilate nitrogenous waste into microbial protein [1–4]. Despite these advantages, maintaining optimal water quality and ensuring disease resistance are ongoing challenges in intensive shrimp farming.
Probiotics are increasingly used in aquaculture to enhance growth, improve water quality, and boost disease resistance in farmed species. Recent studies have underscored the potential of probiotics to enhance the health and productivity of aquaculture organisms by improving water quality (such as ammonia, nitrite, etc.) and increasing disease resistance [5–8]. The application of probiotics in shrimp culture systems, particularly within biofloc systems, has gained notable attention for its role in modulating the microbial community within the culture system. Probiotics have the potential to stabilize water quality parameters (ammonia, nitrite, and organic loads) and promote the growth and health of cultured species [9–11].
One critical issue in shrimp aquaculture is the prevalence of bacterial pathogens, particularly Vibrio spp. including Vibrio parahaemolyticus. These gram-negative bacteria are notorious for causing significant diseases in shrimp, leading to substantial economic losses worldwide. The bacterium V. parahaemolyticus is the causative agent of acute hepatopancreatic necrosis disease (AHPND), which has devastated shrimp farms due to its rapid onset and high mortalities [12, 13]. This bacterium can produce potent toxins that damage the shrimp’s hepatopancreas, resulting in swift and often widespread mortality within affected populations [14]. Given the critical threat posed by V. parahaemolyticus, exploring effective mitigation strategies is essential to enhancing production potential. In this context, the role of probiotics becomes increasingly relevant as they offer a sustainable means of inhibiting pathogenic bacteria, potentially reducing the incidence and severity of diseases like AHPND in shrimp aquaculture [13, 15].
This study investigated the effects of various concentrations of a commercial pelleted probiotic (added to culture water) consisting of a mixture of Bacillus subtilis and B. licheniformis on water quality, growth performance, and disease resistance of Pacific white shrimp reared in static biofloc culture systems. This study sought to provide insights into optimizing shrimp health, culture practices, and productivity. The findings could contribute significantly to developing improved management strategies that integrate probiotics into BFT, potentially leading to better resilience against diseases in shrimp aquaculture [16, 17].
2. Methods
2.1. Postlarvae Nursing
Pacific white shrimp postlarvae (PL16; 0.008 g) were sourced from Homegrown Shrimp USA (Indiantown, FL, USA) and acclimatized in an indoor biofloc nursery system with a volume of 3750 L. The postlarvae were initially fed commercial feeds (Zeigler Bros. Inc. Gardners, PA, USA; protein ≥50% and fat ≥15%) and later transitioned to 1.5-mm crumbled commercial shrimp feed (Zeigler Bros. Inc., Gardners, PA, USA; protein ≥40% and fat ≥9%) until they reached an appropriate size for stocking (1.20 g).
2.2. Experimental Setup
A 9-week growth trial was conducted at the E. W. Shell Fisheries Center, Auburn University, Auburn, AL, USA. The experiment utilized 24 black polyethylene tanks, each with a working volume of 156 L (Pentair Aquatic Ecosystems; 0.3 m flat bottom surface; total system volume 4140 L; flow rate 7.5 L min−1). The tanks were filled with dechlorinated water and manufactured sea salt (Crystal Sea Marinemix, Baltimore, MD, USA) was dissolved to achieve a salinity level above 10 ppt. Dissolved oxygen (DO) levels were kept near saturation (>5.5 mg L−1) using two air stones (7.62 cm, 0.35 cubic feet per minute) per tank, which were connected to a common airline and a regenerative blower (Gast Regenerative Blowers, T063ZZDER1692, 1.5 HP each, Auburn, AL). Throughout the trial, no water exchange was performed, but any water lost due to evaporation was periodically replaced with fresh water. Each of the 24 tanks was stocked at a density of 160 shrimp m−3 (25 shrimp per tank). After stocking, the water was recirculated using a fiberglass sump tank and circulation pump for 7 days to ensure uniform mixing of the newly formed biofloc community. Then, circulation was discontinued and the tanks were maintained as static systems with the addition of probiotics.
An estimated feed conversion ratio (FCR) of 1.2 [18–21] was utilized for feed input calculations. Expected growth was assessed through weekly subsampling, where 10 shrimp per tank were sampled and weighed from one replicate tank per treatment. During sampling, all treatments were handled identically and data was pooled to determine average weekly growth. Predicted growth for feed management was then estimated based on both historical data and current results [20]. Daily feed was administered by hand feeding four times a day, as shrimp are slow and continuous feeders that benefit from multiple small rations throughout the day [22–24].
2.3. Determination of Water Quality Parameters
Temperature (°C), DO (mg L−1), and salinity (ppt) were recorded in each tank twice daily in the morning (08:00–08:15) and afternoon (16:00–16:15) throughout the experimental period using a YSI Pro2030 (YSI, Yellow Springs, OH, USA). Similarly, pH levels were measured from each tank twice weekly using a digital pH meter (Vivosun, Ontario, CA, USA). For weekly measurements of total ammonia nitrogen (TAN; mg L−1) and nitrite (mg L−1), water samples were collected from each tank and filtered through 47 mm filter paper (Cytiva Whatman 42, CAT No. 1442-047). Reagents were added to the filtered water and the readings were obtained using a YSI photometer 9500 kit (YSI, Yellow Springs, OH, USA), following the manufacturer’s instructions. For total solids (g L−1) measurement, 20 mL water samples were taken from each tank weekly and vacuum filtered through 47 mm filter paper (Cytiva Whatman 42, CAT No. 1442-047). The filter paper was then dried in an oven at 60°C for 24 h. After drying, the filter paper was weighed to determine the levels of total solids present in each tank [20].
2.4. Growth Performance
2.5. Disease or Bacterial Challenge
2.5.1. Pathogen Culture for Challenge Trials
Vibrio parahaemolyticus strain A3 cryostock was kindly provided by Craig Shoemaker at the USDA-ARS Aquatic Animal Health Research Unit (Auburn, AL). The bacteria was revived via plating on HiVeg Agar (HiMedia Laboratories LLC, Kennett Square, PA, USA) and incubated at 28°C for 24 h. Following incubation, a single colony was picked and transferred into 100 mL of marine tryptic soy broth (mTSB; TSB + 2% NaCl) in a 500 L culture flask. The culture was incubated at 30°C with shaking at 100 revolutions per minute (RPM) for 6 h. For the challenge, the optical density was adjusted to 2.30 (at 540 nm), corresponding to approximately 1 × 108 CFU mL−1. Spread plating on HiVeg was used to confirm the final inoculum dose and the plates were incubated at 30°C for 24 h before counts were performed.
2.5.2. Preliminary Disease Challenge Trial
Before performing the main challenge, 50 shrimp were taken from the experimental tanks, including control and probiotic treatments and stocked in five aquaria (80 L capacity; filled to 20 L volume), with 10 shrimp each. They were then infected with two levels of bacterial culture: 25 and 35 mL (OD540 = 2.30; dose in water = 1.72 × 106 CFU mL−1). Each aquarium had one mock tank, and the pilot was conducted for up to 5 days. This preliminary trial was conducted to determine the appropriate infection volume of V. parahaemolyticus needed to challenge the shrimp.
2.5.3. Vibrio parahaemolyticus Challenge
At the end of the growth trial, the shrimp were moved to the BSL-2 challenge facility and subjected to an experimental infection with Vibrio parahaemolyticus. Each treatment had triplicate tanks that were used alongside a single mock control (noninfected) for the pathogen. This resulted in a total of 16 tanks. The system sump was filled with 10 g/L saltwater and the water quality was mirrored to have the same temperature, DO, salinity, and pH values as that of the growth trial. After that, 80 L capacity tanks were filled with water and the water level was maintained at working volume of 20 L via a recirculating sump, and the system was run for 24 h. Prior to the challenge, 10 shrimp were stocked in each tank and allowed to acclimate for 24 h. The tank water levels were lowered to 10 L for the immersion challenge. After 30 mL of V. parahaemolyticus (OD540 = 2.30; dose in water = 1.29 × 106 CFU mL−1) was introduced. After 30 min, the water was filled back to 20 L with the system switched to single pass water exchange. The mock tanks received an identical protocol to the challenged tanks but with an equivalent amount of sterile mTSB. The challenge trial was conducted for up to 7 days. The first check was done after 18 h of immersion and a water exchange (50%) was performed. Then, mortalities were checked every 6 h and water was exchanged every 12 h. Meanwhile, shrimp were fed with the same feed used during the growth trial twice a day in the morning and afternoon.
2.6. Statistical Analysis
Results were expressed as the mean for growth and mean ± standard error (SE) for water quality data. Statistical analyses were performed using SAS (V9.4, SAS Institute, Cary, NC, USA). Growth performance data were analyzed using one-way ANOVA, with all assumptions met, to determine statistically significant differences (p < 0.05) among treatments. Tukey’s multiple comparison test was used to evaluate significant differences among treatment means. All water quality data were analyzed using time series analysis, with all assumptions met. Statistical analysis for the bacterial challenge trial was performed using GraphPad Prism version 9.51 (GraphPad Software, CA, USA). For the endpoint cumulative percent mortality (CPM) analysis, the percentage values were first arcsine transformed, and then one-way ANOVA was applied. Kaplan–Meier survival curves were used to compare the survival data across the treatments.
3. Results
3.1. Water Quality
Throughout the 9-week trial, water quality parameters in the indoor static biofloc system varied among the control and various probiotic concentrations (Table 1). DO levels showed significant differences among treatments (p < 0.05). The control (×0) and ×8 treatments maintained the highest DO levels at 6.61 and 6.64 mg L−1, respectively, while the ×16 treatment exhibited the lowest DO level at 6.54 mg L−1. Temperature readings also differed significantly between treatments (p < 0.05). The control and ×4 treatments had the highest temperatures at 27.8°C, whereas the ×8 and ×16 treatments recorded slightly lower temperatures at 27.5°C. No significant differences were observed in salinity and pH levels across treatments. TAN, nitrite, and total solids concentrations varied but were not significantly different among treatments (p > 0.05).
| 1Probiotic concentration | DO (mg L−1) | Temperature (°C) | Salinity (ppt) | pH | TAN (mg L−1) | Nitrite (mg L−1) | Total solids (g L−1) |
|---|---|---|---|---|---|---|---|
| Control |
|
|
|
|
|
|
|
| ×4 |
|
|
|
|
|
|
|
| ×8 |
|
|
|
|
|
|
|
| ×16 |
|
|
|
|
|
|
|
| PSE | 0.12 | 0.43 | 0.23 | 0.14 | 0.10 | 0.03 | 0.15 |
| 2Type-III p-Value | <0.0001 | <0.0001 | 0.3886 | 0.6779 | 0.2101 | 0.2153 | 0.6954 |
- Note: Values are shown as the mean ± standard error and represent the means of six replicates. The range (minimum–maximum) is shown for each parameter within the parenthesis. Means not sharing any letter are significantly different by the Tukey’s HSD-test (parametric ANOVA) at the 5% level of significance.
- Abbreviations: DO, dissolved oxygen; PSE, pooled standard error; TAN, total ammonia nitrogen.
- 1A commercial pelleted probiotic with a mix of Bacillus subtilis and B. licheniformis (Pro4000X, Aquaintech Inc., Lynnwood, WA, USA) added at ×4, ×8, and ×16 of the recommended dosage.
- 2Repeated measures mixed-effects ANOVA.
3.2. Growth Performance
No significant differences (p > 0.05) were observed among treatments for any of the growth parameters measured (Table 2). A numerically higher final biomass was recorded in the ×16 treatment (409.01 g), while the lowest was observed in the ×8 treatment (396.49 g). The final mean weight of shrimp followed a similar pattern, with the ×4 treatment yielding the higher mean weight (20.11 g) and the control group (×0) having the lower (18.87 g). Despite this range, none of the differences were statistically significant (p > 0.05).
| aProbiotic concentration | Final biomass (g) | Final mean weight (g) | Survival (%) | Weight gain (%) | Weight gain/week (g) | FCR |
|---|---|---|---|---|---|---|
| Control (×0) | 398.39 | 18.87 | 84.67 | 1477.13 | 1.96 | 1.43 |
| ×4 | 400.59 | 20.11 | 80.00 | 1553.28 | 2.10 | 1.48 |
| ×8 | 396.49 | 19.38 | 82.00 | 1504.66 | 2.02 | 1.49 |
| ×16 | 409.01 | 19.04 | 86.00 | 1499.04 | 1.98 | 1.45 |
| PSE | 12.02 | 0.49 | 3.26 | 63.45 | 0.06 | 0.05 |
| bp-Value | 0.8137 | 0.2514 | 0.4858 | 0.8294 | 0.2780 | 0.7186 |
- Note: Values represent the means of six replicates.
- Abbreviations: FCR, feed conversion ratio; PSE, pooled standard error.
- aA commercial pelleted probiotic with a mix of Bacillus subtilis and B. licheniformis (Pro4000X, Aquaintech Inc., Lynnwood, WA, USA) added at ×4, ×8, and ×16 of the recommended dosage.
- bOne-way ANOVA, followed by Tukey’s multiple comparison tests to evaluate significant differences between treatment means.
Survival was consistent across all treatments, ranging from 80.0% in the ×4 treatment to 86.0% in the ×16 treatment. These differences in survival rates were not statistically significant (p > 0.05). Weight gain percentages also showed variation, with the control group achieving a weight gain of 1477.13% and the ×4 treatment attaining a higher weight gain percentage of 1553.28%. However, these differences were not statistically significant (p > 0.05). Weekly weight gain was higher in the ×4 treatment (2.10 g per week) and lower in the control group (1.96 g per week). As with other parameters, these differences were not statistically significant (p > 0.05). FCR values ranged from 1.43 in the control group to 1.49 in the ×8 treatment, with no significant differences observed between the treatments (p > 0.05). All growth parameters were similar among the treatments.
3.3. Pathogen Challenge
The control group (×0), which did not receive probiotic treatment, exhibited the lowest survival, with a sharp decrease in survival starting around 18 h postchallenge. Survival dropped progressively over time, with less than 20% of the shrimp surviving by the end of the 7-day trial (Figure 1a). The CPM of the aforementioned group of shrimp reached approximately 60% by 84 h postchallenge. In contrast, all the probiotic-treated groups showed significantly higher survival than that of control (p < 0.05). Survival in these groups remained above 80% throughout the challenge (Figure 1a), showing much lower mortality, with CPM curves indicating only a slight increase in mortality over time. The highest recorded mortality in the probiotic groups was under 20%, and this level was consistent across all probiotic dosages (Figure 1b). Notably, there was no significant difference in survival (p > 0.05) among the various probiotic doses (×4, ×8, and ×16), as all these doses provided similar levels of protection against the pathogen. The mock groups, which did not receive the pathogen, but underwent the same handling procedures, showed only one mortality in the control mock (Figure 1b), confirming that the observed effects were due to the V. parahaemolyticus challenge and not the other stress factors.
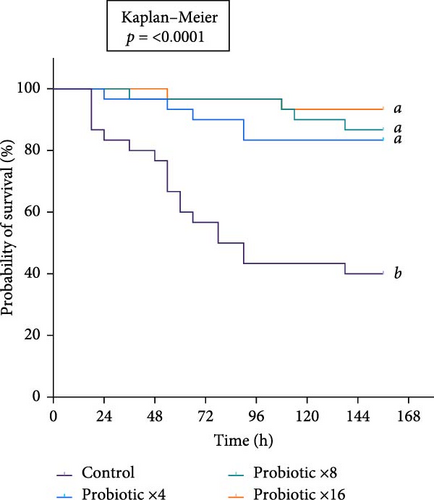
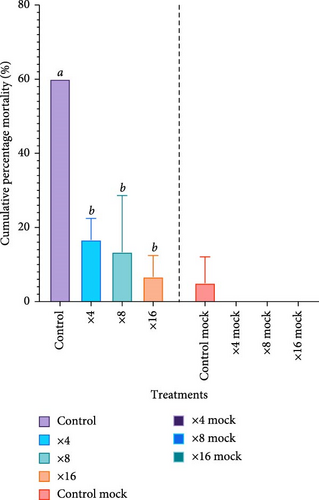
CPM was measured in four treatment groups for 7 days after exposure to Vibrio parahaemolyticus. The average CPM values were control (60%), ×4 (16.7%), ×8 (13.3%), and ×16 (6.7%). One-way ANOVA was performed for the arcsine-transformed data and showed significant differences (p = 0.0002). The highest mortality occurred in the control group (60%), while the probiotics ×16 group had the lowest mortality (6.7%). Meanwhile, survival data showed a significant difference in tested treatments. The Log-rank (Mantel–Cox) test showed a significant difference (p < 0.0001) compared to the control group. The pairwise comparison was performed to find the difference between treatment groups. Significant differences were observed when comparing the control group with each treatment group (×4 (p = 0.002), ×8 (p = 0.0002), and ×16 (p < 0.001), indicating that the probiotic treated groups notably improved survival outcomes relative to the control. However, the pairwise comparisons among the treatment groups themselves (×4 vs. ×8 (p = 0.4277), ×4 vs. ×16 (p = 0.2293), and ×8 vs. ×16 (p = 0.6591) revealed no significant differences, suggesting that while probiotic treatment as a whole was effective, the specific treatment intensities did not result in markedly different survival benefits.
4. Discussion
During the 9-week trial, there were significant differences among water quality parameters in DO and temperature levels. The ×16 treatment showed lower DO levels compared with the control, ×4, and ×8 treatments, probably resulting from higher microbial activity due to higher probiotic concentrations associated with more oxygen consumption. However, we do not expect these differences in DO to have any biological effect on the shrimp growth. DO above 5 mg L−1 has to be maintained for optimum shrimp growth; their metabolism and overall health may be adversely affected below this. While the temperature differences were statistically significant, all values were still within the optimum range, which minimizes possible thermal stress on the shrimp [25, 26]. In both cases, the shifts in water quality would not be considered biologically significant albeit they were statistically significant.
pH levels remained stable across treatments, which indicated that probiotic supplementation did not affect the system’s alkalinity, which is in accordance with the findings of Arias-Moscoso et al. [27], Amjad et al. [28], and Huang and Li [29]. TAN, nitrite, and total solids concentrations were slightly variable, but not significantly different in this study. This demonstrated the capacity of the biofloc system to manage nitrogenous waste compounds through efficient waste recycling [4, 30].
In terms of growth performance, no statistically significant differences (p > 0.05) were observed between the control and probiotic-treated groups across all measured parameters, including final biomass, mean shrimp weight, survival, weight gain, and FCR. These results suggest that the probiotics used in this study did not markedly enhance the growth of shrimp under the reported conditions. For instance, while probiotics have been often reported to improve feed efficiency and growth rates [10, 31–33], other studies have shown minimal or inconsistent effects on growth performance, potentially due to differences in probiotic strains, dosages, environmental conditions, health of the shrimp, presence of floc as a natural food, or the baseline nutrition provided by the diet [19, 34–38]. The meta-analysis of various studies conducted by Toledo et al. [35] revealed that while probiotics can have positive effects in some cases, there are instances where no significant growth improvements were observed [27, 28, 36, 39]. This suggests that the benefits of probiotics might be more related to health and disease resistance, rather than direct growth enhancement under certain conditions.
Vibrio strains, particularly, V. parahaemolyticus, naturally present in marine environments, are a significant bacterial pathogen in shrimp aquaculture, causing slow growth, anorexia, necrosis, and early mortality syndrome (EMS) with mortality rates reaching up to 100% within 30 days poststocking [12, 40]. Probiotics, particularly Bacillus spp., have proven effective in enhancing both cellular and humoral immune responses while promoting competitive exclusion of harmful bacteria [41]. As bio-friendly agents, probiotics are considered nontoxic and safe, offering a sustainable solution to managing diseases in shrimp farming [9].
Our study found that probiotic supplementation had a profound impact on the survival of shrimp following the V. parahaemolyticus challenge. This enhanced survival in the probiotic groups suggests that probiotics can effectively bolster the immune response in shrimp, reducing mortality in the face of pathogenic infections [42–44]. The consistent survival across the various probiotic dosages (×4, ×8, and ×16) indicates that even the lowest level of supplementation provided substantial protection against Vibrio parahaemolyticus.
The finding of this research is important for the aquaculture industry, as it indicates that lower dosages of probiotics may be just as effective as higher ones, potentially reducing the cost of probiotic supplementation without compromising health benefits. These results are consistent with other studies that have demonstrated that probiotics can enhance disease resistance in shrimp by modulating the gut microbiota, enhancing immune function and inhibiting pathogenic bacteria [45–47]. The mechanisms through which probiotics confer disease resistance may include competitive exclusion, where beneficial bacteria outcompete harmful bacteria for resources and attachment sites in the gut, as well as the production of antimicrobial compounds that directly inhibit pathogen growth [6, 48–50]. Additionally, probiotics are known to enhance the immune response in shrimp by upregulating the expression of immune-related genes, which can improve the ability to respond to and neutralize pathogens [36, 50, 51].
5. Conclusion
The findings of this study highlight probiotics’ vital role in improving shrimp survival when exposed to pathogens, especially V. parahaemolyticus. All probiotic treatments (×4, ×8, and ×16) showed a significant increase in shrimp survival throughout the challenge phase. This resilience against V. parahaemolyticus emphasizes the important role probiotics could serve as a crucial component of disease management strategies within intensive aquaculture systems. These findings will be very useful for shrimp farmers, as they emphasize the importance of integrating probiotics into farming practices not only for their growth benefits but, more importantly, for their ability to significantly reduce mortality during disease outbreaks. The enhanced survival observed in this study suggests that probiotics can provide a vital line of defense, enabling shrimp to better withstand disease infection. This can lead to more stable production cycles, reduced economic losses, and ultimately, a more profitable shrimp farming operation. The application of probiotics, therefore, should be considered a key element in the comprehensive management of shrimp health, particularly in environments prone to disease infections like those caused by Vibrio spp. Future studies could explore the immunomodulatory mechanisms or combine probiotics with prebiotics (synbiotics) with the aim of improving both growth and survival.
Ethics Statement
The experiments comply with the Auburn University Animal Care and Use Committee. All pathogen work was also approved under Auburn University’s Biological Use Authorization #926 (Bruce).
Disclosure
The reference to trade names or commercial products in this study is solely for the purpose of providing specific details and should not be interpreted as an endorsement. This study was previously presented at the Aquaculture 2025 conference (https://www.was.org/Meeting/Program/PaperDetail/166541).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by the United States Department of Agriculture-Agriculture Research Service (USDA-ARS) Specific Cooperative Agreements (Grant 58-6010-0-007) and the Institute of Food and Agriculture Hatch Program (Grant ALA016-08027).
Acknowledgments
We extend our gratitude to the students, staff, and faculty at Auburn University who contributed to the success of this project. We thank Craig Shoemaker (USDA-ARS) for providing the bacterial strain used in the challenge trials.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




