Detailed Early Life History of the Giant Muricid Snail Chicoreus ramosus in the Southern China Sea
Abstract
Tropical marine life is highly diverse, but the development and utilization of many species remain insufficient. The giant muricid snail Chicoreus ramosus, notable for its large size and high-quality meat, holds significant potential for aquaculture. Previous descriptions of its seedling production were incomplete and sometimes inaccurate. This study details the early life history and aquaculture potential of C. ramosus. Experiments conducted from December 2023 to October 2024 covered parental cultivation, spawning, and developmental tracking. Key findings include female parents spawning 191–531 egg capsules per event, each containing 189–520 fertilized eggs, with up to three spawnings and decreasing capsule numbers in subsequent events. The early life stages are: (1) Inner egg capsule development period: Fertilized eggs progress through stages including polar body release, two-cell, four-cell, eight-cell, morula, blastocyst, gastrula, and veliger larvae stages; (2) Outer egg capsule development: Postveliger larvae emerge and transition to juvenile and youth stages. Specifically, eggs develop into middle veliger larvae in about 4 weeks, followed by a 1–2 week postveliger stage, juvenile metamorphosis around 2 weeks later, and growth into the youth stage in 2–3 weeks. This study provides a successful framework for artificial incubation and seedling production, advancing the potential aquaculture applications of C. ramosus.
1. Introduction
The giant muricid snail, Chicoreus ramosus, is an intriguing member of the Gastropoda class, belonging to the Muricidae superfamily, within the Muricidae family, specifically the subfamily Muricinae [1]. As one of the largest marine gastropods, second only to Syrinx aruanus and comparable to Charonia tritonis and Cassis cornuta, it inhabits tropical and subtropical waters across the Indo–Pacific, from the Red Sea to Eastern Polynesia (Mostafa et al. 2013; [2]. Its massive, spiny shell, and high-protein flesh have driven demand in global markets for handicrafts and seafood, yet unsustainable harvesting practices threaten wild populations [3, 4].
While prior studies have established foundational knowledge about C. ramosus, critical gaps persist in understanding its reproductive biology, field observations describe egg capsule deposition in clusters and larval hatching sizes [1, 5]. Preliminary attempts at mass culture identified bottlenecks in larval survival and metamorphosis induction [6]. No studies have fully characterized embryonic development stages, quantified larval growth dynamics, or achieved successful settlement under controlled conditions.
To address these gaps and advance aquaculture potential, this study aims to document embryonic development through detailed morphological and temporal characterization of egg capsules to hatching, describe larval development by tracking growth, survival, and morphological changes in early veliger stages test induction methods for larval settlement and metamorphosis using substrate-specific cues, and evaluate artificial breeding feasibility by assessing reproductive success under laboratory conditions.
2. Materials and Methods
2.1. Parental Collection and Culture
Parental specimens of C. ramosus were collected from Zhanjiang, Guangdong Province, China, in early December 2023. Healthy, shell-intact adult snails were carefully selected and transported to the Daya Bay Marine Biological Comprehensive Experimental Station of the Chinese Academy of Sciences. At the experimental station, the adult snails were temporarily housed in cement pools, where water temperature (25.2–28.6°C) and salinity (28–30 ppt) were artificially controlled to promote their maturation and natural mating. During this period, the snails were fed with white clams and Manila clams.
2.2. Mating and Hatchery
From June 2024 onwards, several parents began mating and subsequently spawned. During the spawning stage, the egg capsules were incubate under the same temperature and salinity conditions until larvae were released from the capsules. The larvae were then transferred to dedicated hatching buckets for further cultivation. The water temperature and salinity during this stage were maintained at 26.3–28.7°C and 27–30 ppt, respectively.
2.3. Progeny Rearing
To ensure healthy larval growth, water quality in the hatching buckets was rigorously maintained. During the egg capsule stage, early veliger larvae rely on yolk reserves and do not feed. After hatching, larvae were fed Isochrysis zhanjiangensis and Chlorella pyrenoidosa twice daily at a ration of 2.0 × 104 to 4.0 × 104 cells/mL, adjusted to developmental stages. Post-metamorphosis juveniles received the same algal diet, while Ruditapes philippinarum was introduced during the youth stage. Microinjection methods optimized feeding conditions.
Water quality management: Temperature: Maintained at 27.2°C–28.8°C to support metabolic activity. Salinity: Controlled at 27–30 ppt to mimic natural estuarine conditions. pH: Stabilized at 7.8–8.2 using buffered seawater and monitored daily to prevent acidosis/alkalosis stress. Dissolved oxygen (DO): Kept ≥5.8 mg/L via gentle aeration, critical for larval respiration and avoidance of hypoxia-induced mortality. Ammonia/nitrite: Maintained below 0.05 mg/L and 0.1 mg/L, respectively, through regular water changes and biofiltration to prevent toxicity. Light: Diffuse illumination (200–400 Lx) provided 12 h:12h light–dark cycles to regulate larval phototaxis.
Water was replaced every 3 days (50% volume exchange) using pretempered and filtered seawater. Real-time sensors tracked DO and pH, with manual verification twice daily. This protocol ensured optimal ionic balance and minimized osmotic stress during shell formation and metamorphosis.
2.4. Data Collection
During the incubation period within the egg capsules, daily sampling of the same batch of capsules was conducted for observation. The larvae sampling protocol involved systematically selecting 30 individuals through stratified random sampling from intact egg capsules exhibiting normal morphological characteristics (translucent appearance with uniform texture, Figure 1B). Damaged capsules, identified by visible purple discoloration and structural compromise, were excluded. Larvae were selected using a random number generator applied to gridded capsule sections to ensure spatial representation. Water quality parameters (temperature: 25 ± 0.5°C, salinity: 27–30 ppt, and pH: 7.8–8.2) were continuously monitored using calibrated digital sensors (Hanna Instruments HI98194) with automated data logging every 15 min. Manual verification occurred three times daily using portable meters, with a <5% deviation tolerance requiring adjustment through preconditioned seawater exchange (10% volume/h) and buffered mineral supplements (Seachem Stability). This dual monitoring system maintained ionic stability (Ca2+ 420 ppm and Mg2+ 1280 ppm) throughout larval development stages. After hatching, more than 30 larvae from each experimental group were randomly sampled daily from the hatching buckets. Their developmental stages and morphological characteristics were recorded in detail using an optical microscope (40–100x magnification), accompanying by photographs.
To measure the shell height of C. ramosus larvae accurately, an ocular micrometer was employed, with the larvae submerged in seawater during measurement to enhance visibility. For size measurements at different developmental stages, micrometers or vernier calipers with a precision of 0.01 mm were used.
2.5. Data Analysis
SPSS 27.0 software was utilized to statistically analyze the collected data, including variance analysis and correlation analyses using the one-way ANOVA method. These analyses provided valuable insights into the reproductive characteristics and larval development patterns of C. ramosus.
3. Results
3.1. Spawn and Hatchery
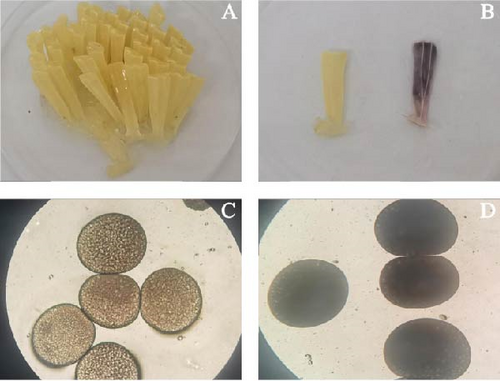
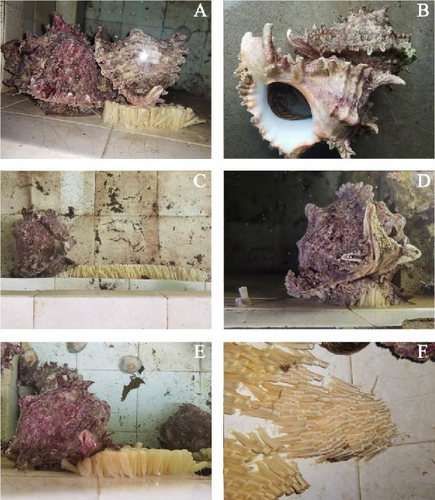
The sizes of five female parents are summarized in Table 1. They spawned egg capsules on the wall of the pool below the sea water level between June 18 and August 6, 2024 (Figure 2A,B). During the spawning process, they first laid the bases of the egg capsules, which adhered to the pond wall (Figure 2C,D). The egg capsules were distributed in clusters (Figure 2F), with each spawning cycle lasting 7–10 days. Generally, several egg capsules spoiled and turned purple due to unfertilized eggs, while normal egg capsules appeared buff-colored, indicating the presence of fertilized eggs (Figure 1A–D). Only three females produced normal egg capsules, with the spawning dates being June 18–28 June, July 4–12 July, and July 31–August 6.
| Items | Shell length (cm) | Shell height (cm) | Shell width (cm) | Fresh weight (g) |
|---|---|---|---|---|
| 1 | 21.10 | 13.10 | 14.50 | 1075.61 |
| 2 | 20.30 | 14.50 | 13.50 | 1189.82 |
| 3 | 21.50 | 16.30 | 14.10 | 1264.29 |
| 4 | 20.70 | 15.90 | 15.80 | 1466.91 |
| 5 | 23.60 | 15.90 | 18.50 | 1586.88 |
| Average | 21.44 ± 0.89 | 15.14 ± 1.07 | 15.28 ± 1.50 | 1316.70 ± 168.15 |
The number of egg capsules produced in these three batches was 531, 290, and 191, respectively, totaling 1012 egg capsules. The lengths of the egg capsules ranged from 24.11 to 25.13 mm and the number of eggs per capsule varied from 313.00 ± 33.23 to 397.90 ± 122.90 (n = 10). The egg diameter ranged from 351.30 ± 23.30 to 356.30 ± 19.40 μm, with maximum fecundity reaching 211,338 eggs (Tables 2 and 3). Each egg capsule was an elongated rod, wider at the top and narrower at the bottom, with a smooth surface. The interior was filled with a transparent gelatinous liquid that evenly distributed the fertilized eggs, maintaining the shape of the egg capsules and protecting the fertilized eggs.
| Spawning times | Egg capsules | Spawning days | Fecundity (ind.) | ||
|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Number (ind.) | |||
| 1st time | 24.76 ± 0.52 | 7.16 ± 0.50 | 531 | 10 | 211,338 |
| 2nd time | 25.13 ± 0.89 | 5.97 ± 0.65 | 290 | 8 | 105,560 |
| 3rd time | 24.11 ± 0.67 | 6.16 ± 0.42 | 191 | 7 | 59,974 |
| Total | — | — | 1012 | 25 | 376,872 |
| Spawning times | Average number of eggs | Egg diameter (μm) | ||
|---|---|---|---|---|
| Eggs/capsule (n = 10) | Min | Max | ||
| 1st time | 397.90 ± 122.90 | 269 | 520 | 351.30 ± 23.30 |
| 2nd time | 363.60 ± 89.80 | 189 | 481 | 356.30 ± 19.40 |
| 3rd time | 313.00 ± 33.23 | 280 | 362 | 343.60 ± 14.70 |
During the hatchery stage, the color of the egg capsules transitioned from buff to the deep yellow, then, to ash black and finally to black. As incubation progressed, a hole appeared at the top of the capsule, serving as a larval release outlet (Figure 3). The entire incubation period lasted about 4 weeks, with hatchery times varying among batches due to different environmental conditions.

3.2. Embryonic and Larval Development Within Egg Capsules
Yellow fertilized eggs were round, with sizes ranging from 343.60 to 356.30 μm (Figure 4A). After 1–3 days, the first polar body and the second collective were released. The zygotes then entered the cleavage phase, undergoing disc cleavage. The stages included the two cell stage (452.00–454.83 μm; Figure 4B), four cell stage (461.67–465.00 μm; Figure 4C), and multicellular stage (471.67–492.67 μm; Figure 4D). By the fourth day, the embryos developed into the blastocyst stage (Figure 4E), with blurred boundaries between the mitotic balls and rapid cell number increases, averaging 419.33–431.33 μm in size. On the ninth day, the embryo reached the gastrula stage (Figure 4F), characterized by the animal pole overturning to cover the plant pole, forming indentations in the middle. The average size was 420.67–434.33 μm. By the 11th day, embryo developmented into the trochophore larva (Figure 4G), where the embryos gradually elongated from round to oval, averaging 471.33–479.33 μm. The veliger had not yet fully formed, transitioning to intramembranous veliger larvae within deep yellow egg capsules.
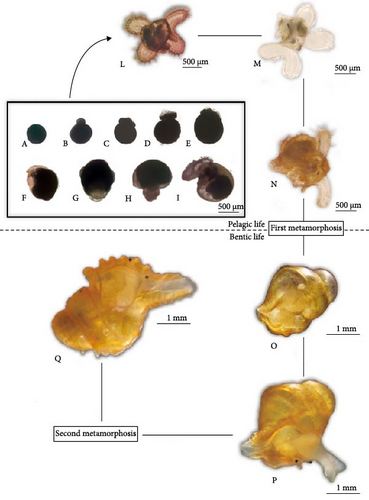
On the 17th day, the embryos entered the intramemellar veliger larvae stage. At this stage, the embryos remained elliptical, with the veliger visible in the middle and lower part. Cilia appeared on the edges of the veligers, with sizes averaging 481.67–490.67 μm (Figure 4H). By the 21st day, the embryos developed into early veliger larvae. Their embryonic shells were formed, with obvious shell textures. The veliger area expanded and internal organs began to form. The larvae measured 515.67–550.00 μm, with frequently oscillating cilia (Figure 4I). On the 25th day, the embryos reached the middle veliger larval stage. The egg capsules turned dark purple and inner granular larvae were visible. The left and right lobes of the veliger varied in size. The shell height of the larvae ranged from 663.23 to 671.29 μm, with cilia covering the surface edges, enabling free movement (Figure 4L). Meanwhile, the egg capsules ruptured, releasing the middle veliger larvae into sea water, where they entered the planktonic stage.
3.3. Development of Plan-Tonic Larvae, Juveniles, and Youth
When the small hole at the top of the egg capsules ruptures, veliger larvae are released, marking the smooth transition from the embryonic stage to planktonic larva stage (Figure 5A). The life cycle of these newborn gastropod is divided into two stages: a planktonic stage, followed by a benthic stage.
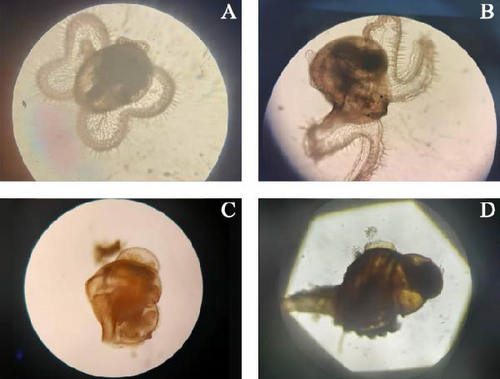
Middle veliger larvae develop a thin and transparent primary shell with a convex edge on one side, forming a prosulci structure (Figure 5B). Podoprimordia and the operculum differentiate beneath the veliger. These larvae swim freely using cilia and feed on planktonic algae. The food groove connects the base of veliger to its mouth, directing food into the esophagus. This narrow channel leads to an enlarged, cystic stomach, where food particles rotate rapidly, indicating an active digestive process. The stomach is connected to a slender intestine, which loops at the top of the shell and extends toward the opening of the shell, near which the anus is located. In the apical cavity near the intestine, clusters of colorless or brown round globules, remnants of nutrients from the egg capsules are visible. These nutrient globules provide additional energy for early growth. The heart, an oval cystic structure near the esophagus, and the shell opening pulsates independently, supporting the larva’s vital functions. A pair of black eye spots is located at the base of the veliger, with a pair of balance sacs beneath the eye spots. Antennae extend from the center of the veliger. The balance sacs are prominent and the shell height ranges from 663.23 to 671.29 μm (Figure 4L). The veliger has a depression in the middle of its body, giving it strong swimming abilities. As the two-leaf veliger stage progresses, the depression deepens, forming four-leaf structures. This stage is also known as the “butterfly larva” stage.
In the late veliger larvae stage, the larvae develop two spiral layers of the shell. The foot primordium differentiated into anterior and posterior feet, while the antennae and black eye spots develop rapidly. The center of the veliger become more concave, showing a distinct four-leaf butterfly shape. The upper and lower leaves of the veligers differ in length and the individual disc-like leaves elongate, almost matching the shell height (721.00–728.13 μm; Figure 4M; Tables 4 and 5). Later stage planktonic larvae exhibit strong swimming abilities. When stimulated by external factors, the veliger retracts into its shell, remaining motionless but still able to feed. Once the external stimulus is removed, the larvae extend their outer discs and resume swimming activities.
| Developmental stage | Exp. 1 | Exp. 2 | Exp. 3 | Times (days) |
|---|---|---|---|---|
| Intracapsules stage | ||||
| Fertilized eggs | 356.13 ± 23.28 | 356.30 ± 19.41 | 343.63 ± 14.65 | 12–24 h |
| Two cells stage | 453.67 ± 20.42 | 452.00 ± 24.83 | 454.83 ± 24.74 | 24–48 h |
| Four cells stage | 463.67 ± 20.42 | 461.67 ± 20.86 | 465.00 ± 21.46 | 36–60 h |
| Multicellular stage | 492.67 ± 27.53 | 482.00 ± 30.44 | 471.67 ± 25.88 | 48–72 h |
| Blastocyst stage | 419.33 ± 44.09 | 425.67 ± 24.45 | 431.33 ± 40.66 | 4–8 day |
| Gastrula stage | 434.33 ± 27.00 | 420.67 ± 41.52 | 429.00 ± 39.60 | 9–10 day |
| Trochanter larvae | 479.33 ± 31.40 | 476.00 ± 54.18 | 471.33 ± 30.82 | 11–16 day |
| Intracapsules veliger larvae | 490.00 ± 28.45 | 481.67 ± 30.41 | 490.67 ± 49.55 | 17–20 day |
| Early veliger larvae | 515.67 ± 33.08 | 548.33 ± 44.57 | 550.00 ± 44.33 | 21–24 day |
| Middle veliger larvae | 671.18 ± 51.85 | 663.23 ± 38.18 | 671.29 ± 50.82 | 25–28 day |
| Outside capsules stage | ||||
| Middle veliger larvae | 671.18 ± 51.85 | 663.23 ± 38.18 | 671.29 ± 50.82 | 25–28 day |
| Late veliger larvae | 721.00 ± 36.04 | 728.13 ± 35.63 | 723.00 ± 30.53 | 28–30 day |
| Newly Juvenile | 952.67 ± 20.99 | 965.00 ± 23.01 | 960.33 ± 22.36 | 30–40 day |
| Juvenile | 1109.33 ± 67.97 | 1112.67 ± 68.17 | 1127.67 ± 80.92 | 40–50 day |
| Newly Youth | 1259.16 ± 66.46 | 1257.50 ± 54.99 | 1245.00 ± 58.50 | 45–55 day |
| Youth | 1806.43 ± 136.92 | 1801.60 ± 121.33 | 1807.23 ± 114.67 | 50–60 day |
| Items | Exp. 1 (μm) | Exp. 2 (μm) | Exp. 3 (μm) | Average (μm) |
|---|---|---|---|---|
| Day0 | 356.13 ± 23.28 | 356.30 ± 19.41 | 343.63 ± 14.65 | 352.02 ± 19.11 |
| Day7 | 419.33 ± 44.09 | 425.67 ± 24.45 | 431.33 ± 40.66 | 425.44 ± 36.40 |
| Day14 | 479.33 ± 31.40 | 476.00 ± 54.18 | 471.33 ± 30.82 | 475.55 ± 38.80 |
| Day21 | 515.67 ± 33.08 | 548.33 ± 44.57 | 550.00 ± 44.33 | 538.33 ± 40.66 |
| Day28 | 671.18 ± 51.85 | 663.23 ± 38.18 | 671.29 ± 50.82 | 668.57 ± 46.95 |
| Day35 | 721.00 ± 36.04 | 728.13 ± 35.63 | 723.00 ± 30.53 | 724.04 ± 34.07 |
The newly formed juvenile possess three snail layers. The head features antennae and black eye spots, while the feet are well-developed, enabling rapid movement (Figure 5C). These juveniles feed on planktic algae. The veliger structure is gradually degraded, allowing the juveniles to both swim and crawl (Figure 4N). At this stage, the larvae adopt a prostrate lifestyle but can turn over when necessary and still retain the ability to swing. The first metamorphosis is completed during the juvenile stage. The shells become brown-red, developing distinct anterior furrows, and the surface veligers are entirely degenerated. Juveniles can crawl on surfaces using their feet and occasionally swim on the water’s surface through foot motion. The mantle forms a siphon that extends out of the shell along the anterior groove for breathing. The snout also protrudes, allowing them to feed on single-celled algae. Juveniles at this stage reach a shell height of 1109.33–1127.67 μm (Figure 4O; Tables 4 and 6).
| Items | Exp. 1 | Exp. 2 | Exp. 3 | Average | Key trait’s description |
|---|---|---|---|---|---|
| Newly hatchery larvae (μm) | 721.00 ± 36.04 | 728.13 ± 35.63 | 723.00 ± 30.53 | 724.04 ± 34.07 | The veliger has one large and one small lobe in the left and right leaves. At the base of the veliger has a pair of black eye points (a) pair of antennae and a pair of balance sacs. |
| Metamorphosis size (Juvenile) (μm) | 1259.16 ± 66.46 | 1257.50 ± 54.99 | 1245.00 ± 58.50 | 1253.89 ± 59.98 | Veligers undergoes completely degeneration, forming a secondary shell, with movement relying on foot. |
| Newly youth (μm) | 1806.43 ± 136.92 | 1801.60 ± 121.33 | 1807.23 ± 114.67 | 1805.09 ± 120.31 | The foot becomes further thickened and elongated, capable of large scale expansion and deformation, and enabling activities such as turning over, crawling and other movements. The secondary shell thickens, and spirals begin to form. The diet shifts from herbivorous to carnivorous. |
The surface veliger of juvenile is fully degraded and the secondary shell begins to grow, forming four snail layers. This development marks their transition to a benthic lifestyle, producing a new generation of young snails. The secondary shell starts forming at a shell height of approximately 1175 μm. Initially, it is white, thin, and transparent, but becomes opaque as the snails grow (Figure 4P; Tables 4 and 6). Once the veliger is fully degraded, the snout and radula of the juveniles develop further, becoming their primary feeding organs, capable of consuming shellfish meat. The foot also thickens, elongates, and gains the ability to expand and deform significantly, facilitating activities such as turning over and crawling (Figures 4Q and 5D).
4. Discussions
4.1. Larval Incubation Way
The reproductive cycle of C. ramosus highlights its unique biological characteristics. Upon fertilization, the fertilized egg promptly expels polar bodies and enters the discoidal cleavage stage, a process markedly different from that of other gastropod mollusks and aquatic animals. During embryonic development, the brief trochophore larval stage is quickly succeeded by the veliger larval stage, demonstrating an efficient developmental strategy [1, 6]. Chicoreus ramosus adopts an indirect development mode, where embryos undergo hatching, planktonic life, and crawling within the egg capsules, ultimately maturing into juvenile snails. This developmental strategy is consistent with various species within the Prosobranchia, Mesogastropoda, and Neogastropoda, emphasizing the species’ broad ecological adaptability. The developmental trajectory of C. ramosus aligns closely with that observed in congeneric muricids like C. brunneus and C. torrefactus, all of which share indirect development through planktonic larval phases [7]. This conserved reproductive strategy within the genus suggests evolutionary maintenance of larval dispersal mechanisms, though subtle variations in larval duration or settlement behavior among these species may reflect niche-specific adaptations to distinct coastal environments.
Mating activities typically occur at night and last for several hours. During courtship, female accept mating attempts from multiple males during, demonstrating intricate mating behaviors [5]. This study reveals that the period from mating to oviposition spans approximately 3 months, highlighting the uniqueness of the species’ reproductive strategy and its sensitivity to environmental factors, though the underlying mechanisms require further investigation. The reproductive cycle of C. ramosus is influenced by environmental factors such as temperature and salinity, which can affect the timing of spawning and larval development [8].
Notably, C. ramosus demonstrates remarkable fecundity, producing thousands to tens of thousands of eggs per spawning event, encapsulated within 200–600 egg capsules. Maternal incubation behavior has limited influence on larval development, both maternally carried and naturally occurring egg capsules exhibit similar hatching rates and embryonic organogenesis, suggesting that maternal egg capsule carriage primarily serves cleaning and protective functions, akin to those observed in C. tritonis [9]. The high fecundity of C. ramosus is similar to that of other large gastropods, such as C. cornuta, which also produce a large number of eggs to ensure the survival of offspring [2].
Regarding intracapsular development, C. ramosus exhibits considerable variability, consistent with the overall characteristics of marine gastropods within the Heterobranchia [1]. Incubation time is significantly influenced by temperature, with higher temperatures accelerating development and lower temperatures delaying it. This study confirms the critical role of temperature regulation in embryonic development, identifying it as a key factor contributing to the species’ reproductive success. The effect of temperature on the development of C. ramosus is similar to that observed in other marine gastropods, such as Haliotis diversicolor, where temperature plays a crucial role in larval development and survival [10]. According to Nugranad [11], the incubation period of C. ramosus varies from 22 to 35 days and temperature significantly influences this period. Higher temperatures accelerate development, while lower temperatures delay it. The study by Nugranad [11] also highlighted that the optimal temperature range for the development of C. ramosus larvae is between 27.21 and 31.97°C, with an average temperature of 29.3°C. Temperatures outside this range can hinder development and reduce survival rates. For example, temperatures below 27.21°C can delay the hatching of larvae, while temperatures above 31.97°C can lead to developmental abnormalities and increased mortality.
4.2. Metamorphosis Process
In examining the metamorphosis and settlement patterns of C. ramosus larvae, researchers have expressed divergent views, particularly regarding the precise timing and markers of settlement metamorphosis in this species [12]. Mostafa and Gawish [13] reported that the metamorphosis of pelagic larvae into juvenile snails occurs at approximately day 40 of intracapsular development, followed by their release from egg capsules. These released juveniles exhibited a shell height of 1.54 mm. In contrast, Nugranad [11] found that the transition to settlement metamorphosis occurs when pelagic larvae develop into juvenile snails with a shell height of 1.375 μm.
Our current study further refines this process, revealing that the critical transition from the late veliger stage to the juvenile snail phase occurs at a shell height of approximately 721 μm. This transition is marked by distinct morphological changes, including the pronounced regression of the velum, enhanced functionality of the foot, and the initial formation of the secondary shell. Notably, when the shell height reaches 1.259 μm, the juvenile snail is distinctly formed, signaling the end of the pelagic phase and the commencement of settlement metamorphosis. The metamorphosis process of C. ramosus is similar to that of other muricid species, such as C. palmarosae, which also exhibit a transition from the veliger stage to the juvenile snail phase at a specific shell height [7].
Morphological transformation: During this phase, the larval morphology undergoes profound transformations. The number of whorls increases to four and the foot elongates and thickens significantly, enabling agile retraction and extension. This development makes the foot the primary locomotive force for the juvenile snail. As the shell grows, the secondary shell initially appears white and translucent, but gradually thickens and becoming opaque, enhancing the juvenile’s protective capabilities. Following the degeneration of the velum, the development of the proboscis and radula improves the snail’s feeding abilities, particularly its proficiency in preying on bivalves [14]. Additionally, the two pairs of anterior tentacles, similar to those of C. tritonis, function as vital sensory organs. Combined with the eye spots, these features strengthen the juvenile’s capacity to perceive and explore its surroundings [9].
Behavioral adaptations: At the initial stages of juvenile formation, the larvae exhibit a characteristic floating and sinking behavior, possibly be related to their adaptation to new habitats and searching for attachment substrates. However, when attachment substrates were introduced to these juveniles during experiments, a crucial observation emerged; while most individuals appeared to attach, they were actually passively retained by impurities adhering to their shell surfaces, failing to achieve genuine settlement metamorphosis. In this state, the juveniles, hampered by the regression of their velum and their inability to roll over or crawl, as well as limited mobility, experienced a sharp increase in mortality. Substantial losses typically occurring within 3–4 days. The behavioral adaptations of C. ramosus larvae during the settlement process are similar to those of other marine gastropods, such as Trochus niloticus, which also exhibit floating and sinking behavior during the early stages of juvenile formation [10]. The high pre-metamorphic mortality rates in C. ramosus are primarily due to heavy cannibalism after settlement and the impact of environmental factors such as water quality, temperature, and salinity. To address this, future research and aquaculture practices should focus on optimizing larval rearing techniques to reduce cannibalism and enhance survival rates, improving feeding protocols by providing a sufficient and appropriate food supply such as Isochrysis galbana at a concentration of 5000 cells per larva per day, utilizing advanced aquaculture technologies like factory-based recirculating aquaculture systems (RASs) to better control environmental conditions and investigating genetic factors that influence survival and growth to identify and select for traits that enhance resilience and survival rates. By implementing these practices, the high pre-metamorphic mortality rates in C. ramosus can be significantly reduced, thereby enhancing the overall success of large-scale cultivation [15].
4.3. Aquaculture Potential
The C. ramosus is a large snail similar in size to C. tritonis and C. cornuta, with a maximum individual fresh weight of up to 3 kg. Its meat is delicious and highly valued, making it suitable for the high-end market. Furthermore, its shell is a popular high-grade handicraft, occupying an important share of the global market. However, due to the widespread use of diving equipment, most natural resources have been overfished, resulting in a severe global shortage. Therefore, the seed production of this species is urgently needed for resource recovery and the development of the aquaculture industry [8]. This larger snail has significant aquaculture potential in tropical and subtropical seas worldwide. The aquaculture potential of C. ramosus is also supported by its high fecundity and adaptability to various environments, which make it a promising candidate for large-scale cultivation [2].
To elaborate on the economic importance of C. ramosus, the potential markets for both meat and shells are significant. The meat of C. ramosus is highly valued in the high-end market due to its delicious taste and nutritional composition. It is considered a luxury food item in many regions, particularly in Asia, where there is a growing demand for high-quality seafood products. The shell of C. ramosus is also a popular high-grade handicraft, with a significant share in the global market. The unique shape and distinctive features of the shell make it a sought-after item for collectors and artisans. The shell is often used in the production of perfumery, jewelry, and other decorative items, further increasing its market value. For example, the shell of C. ramosus is used in the manufacturing of perfumery products in Vietnam, highlighting its economic value in the global market.
Second, the adaptability of C. ramosus to various environments is a significant advantage for aquaculture. However, environmental factors such as temperature and salinity can affect the timing of spawning and larval development. Therefore, temperature regulation and salinity control are crucial for the reproductive success of this species. The use of advanced aquaculture technologies, such as factory-based RAS, can help improve the efficiency and sustainability of C. ramosus cultivation. These systems allow for better control of environmental conditions, reducing the impact of external factors on larval development and survival.
In conclusion, C. ramosus has significant aquaculture potential due to its high fecundity, adaptability to various environments, and the economic value of its meat and shells. Addressing the challenges of high mortality before metamorphosis and optimizing larval rearing techniques are essential for the successful large-scale cultivation of this species. Future research should focus on improving feeding protocols, understanding genetic factors that influence survival and growth, and exploring the use of advanced aquaculture technologies to enhance the overall success of C. ramosus cultivation.
5. Conclusion
We described the early life history of the giant muricid snail C. ramosus in the Southern China Sea and clarified the discrepancies between the previous two reports [6] (Mostafa et al. 2013). Interestingly, embryos within egg capsules developed directly into middle veliger larvae, which were then released into seawater and entered the plank-tonic stage. Notably, the switch in feeding habits occurred during the youth stage rather than the juvenile stage. Although some young seedlings were obtained, the high mortality rate before metamorphosis remains a critical challenge. This issue needs to be addressed to establish a technical foundation for the large-scale production of seedlings.
This research contributes significantly to the understanding of the early life stages of C. ramosus, providing valuable insights for conservation and aquaculture practices. By elucidating the developmental process and identifying the critical challenge of high mortality before metamorphosis, this study lays the groundwork for future efforts to optimize larval rearing techniques and improve survival rates. Future research should focus on optimizing larval rearing techniques, understanding genetic factors that influence survival and growth, and improving feeding protocols to enhance the overall success of artificial breeding. Additionally, further investigation into the environmental factors that affect larval development and settlement could provide additional strategies for improving aquaculture practices and conservation management.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Gan Wang: data collection, writing–original draft, formal analysis, investigation. Yu Zhong: data collection, investigation, first draft revision, funding acquisition. Yanping Qin: designed the experiments, investigation, first draft revision, funding acquisition. Qishuai Wang, Chao Yue, Weitao Wan, and Jingyue Huang: data measurement and organization. Haitao Ma: investigation, data curation. Jun Li: resources, software. Ziniu Yu: writing–review and editing. Xiangyong Yu: supervision, writing–review and editing. Yuehuan Zhang: funding acquisition, designed the experiments, data collection, validation, writing–review and editing. Gan Wang and Yu Zhong contributed equally to this work.
Funding
This work was supported by National Key Research and Development Program of China (2022YFC3103403); Research on Breeding Technology of Candidate Species for Guangdong Modern Marine Ranching (2024-MRB-00-001); Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) (SML2023SP201); Hainan Provincial Key R&D Programme (ZDYF2024XDNY175 and ZDYF2025XDNY088); Guangdong Science and Technology Plan Programme (2024B1212050006); Guangzhou Science and Technology Project (202206010133 and 2023B03J00165); Guangdong Provincial Key Research and Development Program (2021B0202020003); Guangdong Basic and Applied Basic Research Foundation (2023A15150109443); National Marine Genetic Resource Center; the Earmarked Fund for CARS-49; and the Science and Technology Planning Project of Guangdong Province, China (2023B1212060047).
Open Research
Data Availability Statement
The data will be made available upon request.




