Effects of Different Dried Chlorella pyrenoidosa Preparations on Fatty Acid Profiles and Reproductive Outcomes in Daphnia carinata
Abstract
Daphnia carinata is an important biological live feed in aquaculture. Conventionally, D. carinata is cultivated and fed with fresh Chlorella pyrenoidosa, which is inconvenient for storage and transportation. After undergoing vacuum freeze-drying or spray-drying, C. pyrenoidosa exhibits advantages such as reduced weight and easier storage and transport. This study investigated the effects of feeding freeze-dried and spray-dried Chlorella on the reproductive performance and fatty acid composition of D. carinata. The results indicated that there was no significant difference in reproductive performance between the two groups of D. carinata fed with freeze-dried Chlorella and fresh Chlorella. However, a significant decline in reproductive performance was observed in the group fed with spray-dried Chlorella. The contents of crude fat and unsaturated fatty acids in freeze-dried Chlorella were significantly higher than in spray-dried Chlorella. Additionally, D. carinata fed with freeze-dried Chlorella exhibited significantly higher levels of unsaturated fatty acids (especially ALA and EPA) than those fed with spray-dried Chlorella. In conclusion, freeze-dried Chlorella can be used as an alternative to fresh Chlorella for cultivating D. carinata. Unsaturated fatty acids are a key factor influencing the reproductive performance of D. carinata.
1. Introduction
Live feed consists of various zooplankton and phytoplankton that have been artificially selected and optimally cultured to be fed to aquaculture organisms [1]. It is considered an important natural feed for many aquatic animals. Most fish and shrimp rely on zooplankton during certain stages of their life cycle [2], while Arrhenius and Hanson [3] reported that some species, such as Clupea harengus, feed exclusively on zooplankton during their entire life. Owing to the advantages of small size, slow movement, rapid growth, strong fecundity, high nutritional value, and ease of cultivation, zooplankton-based live feed has been widely employed in the seedling cultivation of economically important aquatic animals [4]. With the rapid development of the aquaculture industry, the demand for zooplankton-based live feed has increased annually. However, the low yield and seasonal fluctuations of natural zooplankton populations cannot meet production demands, making their artificial mass cultivation extremely important.
D. carinata is a commonly filter-feeding freshwater cladoceran in ponds during spring, summer, and autumn. With a body length of ~1.5–3.8 mm, D. carinata is suitably sized for feeding fish and shrimp larvae [5]. Characterized by a relatively slow swimming speed, averaging at 1.34 cm/s and reaching a maximum of 2.04 cm/s, and Bengtson [6] has proved that its movement in water can stimulate the feeding response of larvae. Additionally, D. carinata grows quickly and has a high reproductive capacity. Its juveniles typically mature within 5–10 days. Under favorable conditions, it reproduces parthenogenetically, laying eggs every 2–3 days, with each clutch consisting of 10–30 eggs. This enables D. carinata to rapidly increase its population density in a short period, making it very easy to cultivate [7]. Moreover, D. carinata is rich in proteins, essential amino acids, fatty acids, vitamins, and calcium required for the growth and development of fish and shrimp larvae [8]. A study has reported carbohydrate, protein, and lipid content in D. carinata is, respectively, 57.62%, 36.10%, and 24% [9], and the 17 amino acids present in D. carinata account for 29.82% of its dry weight, with nine of these being essential amino acids for fish, which constitute 18.91% of the dry weight [10]. These nutrients are essential for the larval and juvenile stages of most aquaculture species and are also more readily digested and absorbed [11, 12]. Furthermore, D. carinata has a wide temperature tolerance and is easy to cultivate, and using D. carinata as feed for fish and shrimp larvae can enhance breeding survival rates and disease resistance without negatively impacting water quality [13, 14]. Therefore, D. carinata is an ideal zooplankton-based live feed and has been proven to be applicable in the cultivation of Carp, Tilapia, Shrimp, and Ornamental Fish [15–18].
Food plays an extremely important role in the artificial cultivation of D. carinata. Traditionally, fresh Chlorella are used as feed in the cultivation of D. carinata. Chlorella is not only highly adaptable and fast-growing but also easy to cultivate [19]. In addition to using carbon dioxide and water for heterotrophic photosynthesis, it also can utilize organic carbon sources, such as glucose, for growth without relying on light, making it suitable for high-density cultivation in fermenters [20]. Additionally, it is rich in various proteins, minerals, and trace elements. Studies have shown that the protein content in Chlorella cells can exceed 55% [21], with 18 different amino acids present and essential amino acids account for 42% of the total amino acid content [22]. This nutrient-rich composition plays a significant role in the growth and reproduction of D. carinata [23]. However, fresh Chlorella faces challenges in transportation and storage in practical production [24]. To address this, Chlorella is commonly processed through high-temperature spray drying or rapid vacuum freeze-drying. High-temperature spray drying uses hot, high-velocity air to rapidly evaporate moisture, converting the feed into powder form. In contrast, vacuum freeze-drying removes moisture by directly sublimating ice crystals from solid to gas [25]. Both drying techniques effectively solve transportation and storage issues by transforming fresh Chlorella into algae powder, reducing transport weight while enabling long-term storage at room temperature.
Current research has demonstrated that fresh Chlorella is an ideal feed for cultivating D. carinata [26]. The suitability of freeze-dried and spray-dried Chlorella for cultivating D. carinata remains insufficiently explored. It is well established that the quality of feed plays a crucial role in the growth and reproduction of D. carinata [27]. Moreover, the quality of feed is closely linked to the type and quantity of essential fatty acids [28]. A study demonstrated that different drying methods affect the nutritional composition of Chlorella, particularly its fatty acid content [29]. Consequently, D. carinata fed with different types of dried Chlorella powder may exhibit differences in reproductive performance. Therefore, in this study, freeze-dried Chlorella powder and spray-dried Chlorella powder were selected as feed for the culture of D. carinata. The effects of these feeds on the growth, reproduction, digestive enzyme activity, and nutritional composition of D. carinata were determined, and the results will be compared with those observed in D. carinata cultured with fresh Chlorella, in order to explore the feasibility of using Chlorella powder as a substitute for fresh Chlorella in the cultivation of D. carinata. This study will provide a theoretical basis for large-scale cultivation of D. carinata in the future.
2. Materials and Methods
2.1. Experimental Materials
The test subjects, D. carinata, were collected from the Yangzhou section (32°23′N, 119°29′E) of the Beijing-Hangzhou Grand Canal and were maintained in multiple generations of monoclonal cultures in the laboratory [30]. The D. carinata was placed in an incubator at about 25°C, with a light cycle of 13:11 h and a light intensity of 1000 Lx, and were fed with fresh Chlorella pyrenoidosa at a concentration of 1 × 105 cells/L. Tap water served as the experimental medium, which underwent boiling, cooling, and oxygenation before being utilized for D. carinata culture. All experiments were conducted under the same constant temperature and light conditions.
The feeds used during the experiment included fresh Chlorella, freeze-dried Chlorella, and spray-dried Chlorella. The fresh Chlorella was obtained from the Institute of Hydrobiology, Chinese Academy of Sciences, and cultured in Shuisheng NO.4 medium at 28°C in an incubator [31]. Freeze-dried Chlorella powder was obtained by centrifuging fresh Chlorella liquid (12,000 r/min) followed by processing with a vacuum freeze-dryer (SCIENTZ-10N/A, SCIENTZ, China). Spray-dried Chlorella powder was obtained by rapidly drying fresh Chlorella liquid at an inlet air temperature of 200°C using a small-scale spray dryer (SD-1000, EYELA, Japan).
2.2. Reproduction Performance of D. carinata
Before the experiment, D. carinata (aged >9 days) were collected and placed under the same conditions. The newborns in 24 h were collected for experiments. These newborns were divided into three groups: Chlorella, Freeze-dried Chlorella (F-d Chlorella), and Spray-dried Chlorella (S-d Chlorella). Each group consists of 18 replicates, with 18 D. carinata individuals placed into three six-well plates, ensuring that each well contained only one individual. The reproductive performance of each D. carinata was observed and recorded. The average total offspring, reproductive frequency, time to first oviposition, and survival time were calculated for each group. During the experiment, each well was filled with 10 ml of 25°C experimental water and under 13:11 h light/dark cycle. A 50% water change was conducted every 24 h to prevent deterioration in water quality. Prepared as 1 × 105 cells/ml solutions using the experimental water, the three types of feed were administered to the D. carinata twice every 24 h, with each feeding involving 1 ml of the feed. The survival and reproduction of D. carinata were observed every 6 h, and newborn larvae were removed and counted.
2.3. Digestive Enzyme Activity Measurement
D. carinata was cultured in 5 L cylindrical transparent glass tanks within a constant temperature and light incubator under conditions specified in Section 2.2. The feeding was halted 12 h prior to sample collection to allow the D. carinata to empty their digestive tracts. Before collection, the samples were rinsed to avoid residual excrement, and other impurities in the water were collected together. The activity of five digestive enzymes—lipase (A054-1-1), cellulase (A138-1-1), trypsin (A080-2-1), α-amylase (C016-1-1), and β-amylase (C016-2-1)—was measured with the kit of Nanjing Jiancheng Bioengineering Institute.
2.4. Nutrition Composition Analysis
Compositions of diets and D. carinata were analyzed using Association of Official Analytical Chemists (AOAC) method [32]. Moisture was measured by freeze-drying the sample to constant weight for 48 h. Crude protein was calculated from the determination of the total nitrogen (N × 6.25) using the Kjeldahl method (2300-Autoanalyzer, FOSS, Denmark). Crude lipid was determined by chloroform–methanol method [33]. After freeze-drying the samples for 48 h, methylation was performed [34]. The samples were then extracted with 1 mL of n-hexane, and the upper yellow layer containing the fatty acid methyl esters was collected for fatty acid analysis. The fatty acid content was determined using a gas chromatography-mass spectrometry (GC-MS) system (QP2010 Plus, SHIMADZU, Japan). The relative percentage of each fatty acid component was obtained using the area normalization method. The total carbohydrate content was analyzed using the phenol–sulphuric acid method [35].
2.5. Statistical Analysis
All experimental results are presented as mean ± SD and were statistically analyzed using SPSS 26.0. The data analysis concerning proximate composition and fatty acid analysis of Chlorella was conducted using the independent samples t-test. While the data analysis related to survival, reproductive performance, enzyme activity, proximate composition, and fatty acids of D. carinata employed one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons to assess intergroup differences, with significance set at p < 0.05. Correlation analysis was conducted using SPSS, where p < 0.05 indicates a significant difference, and p < 0.01 indicates a highly significant difference.
3. Result
3.1. Survival and Reproductive Performance of D. carinata
As shown in Table 1, feeding D. carinata with Chlorella prepared using different drying methods had a significant impact on its reproductive performance. The reproductive performance of the D. carinata in the freeze-dried Chlorella group was not significantly different from that in the fresh Chlorella group (p > 0.05). However, the D. carinata in the spray-dried Chlorella group had significantly fewer reproductive frequencies, lower average reproductive number, and a reduced total offspring compared to the freeze-dried and fresh Chlorella groups. Additionally, the time to first oviposition and the reproductive interval were significantly longer in the spray-dried Chlorella group (p < 0.05).
| Parameters | Chlorella | F-d Chlorella | S-d Chlorella |
|---|---|---|---|
| Time to first oviposition | 6.50 ± 0.50a | 7.33 ± 0.47a | 7.75 ± 0.83b |
| Reproductive interval | 1.93 ± 0.07a | 2.03 ± 0.10a | 2.57 ± 0.18b |
| Reproductive frequency | 12.75 ± 0.63b | 12.67 ± 0.33b | 9.50 ± 0.50a |
| Average reproductive number | 9.13 ± 0.21b | 8.61 ± 0.18b | 7.66 ± 0.13a |
| Total offspring | 116.45 ± 2.03b | 109.00 ± 1.53b | 72.80 ± 3.40a |
- Note: Values are mean ± standard error of mean (n = 18). Means in the same row sharing the same superscript letter are not significantly different by Tukey’s multiple test (p > 0.05).
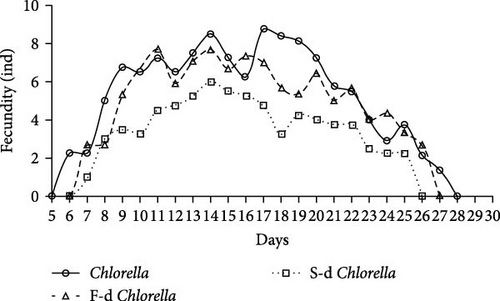
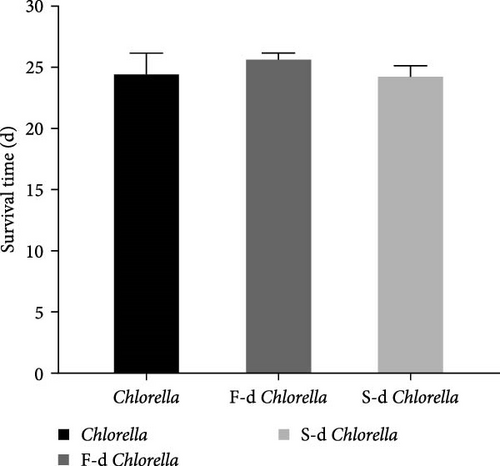
A reproductive curve was plotted based on the daily reproductive counts of D. carinata in the three groups (Figure 1). The D. carinata in the fresh Chlorella group reached maturity and began reproducing from day 5, and the reproductive peak occurred between days 9 and 20, reaching a maximum of 8.5 individuals per day on day 14, after which the reproductive output gradually declined. In the freeze-dried Chlorella group, reproduction began on day 6, with a peak between days 10 and 18, showing similar reproductive output to the fresh Chlorella group. In contrast, in the spray-dried Chlorella group, reproductive output peaked on day 14 with a maximum of only 6 individuals per day and declined thereafter, with the total offspring significantly lower than the other two groups throughout the reproductive period. Feeding D. carinata with Chlorella processed using different drying methods did not significantly affect its survival time (Figure 2).
3.2. Enzyme Activity Measurement
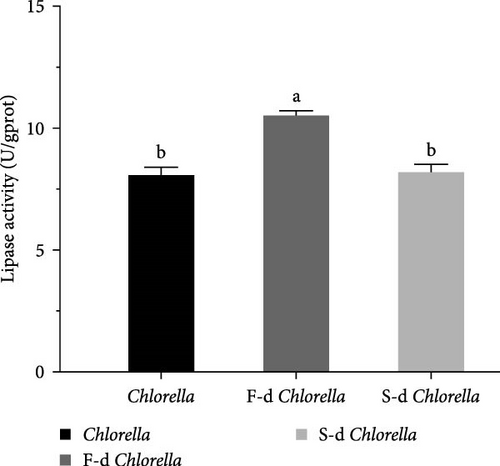
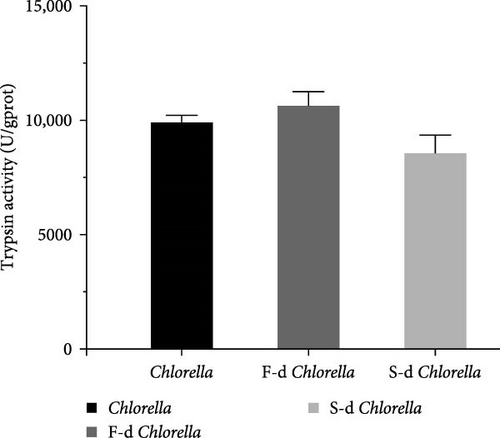
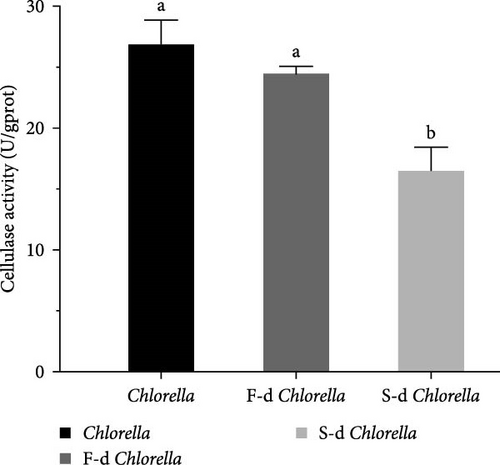
Digestive enzyme activity is a key indicator for assessing an organism’s ability to break down and digest food. The activities of five digestive enzymes—lipase, cellulase, trypsin, α-amylase, and β-amylase—were measured. As shown in Figure 3, the lipase activity of D. carinata fed with freeze-dried Chlorella was significantly higher than that of the other groups (p < 0.05) (Figure 3A). The cellulase activity in the spray-dried Chlorella group was significantly lower than in the other groups (p < 0.05) (Figure 3C). Additionally, α-amylase activity in the freeze-dried Chlorella group was significantly higher than in the spray-dried Chlorella group (p < 0.05) (Figure 3D). However, there were no significant differences among the three groups in trypsin (Figure 3B) or β-amylase activity (Figure 3E) (p > 0.05).

3.3. Proximate Composition and Fatty Acid Analysis of Chlorella
The analysis of the proximate composition and fatty acid profile of Chlorella powder obtained from two different drying methods indicates that the drying method significantly affects its nutritional content. As shown in Table 2, the crude fat and carbohydrate content in freeze-dried Chlorella powder were significantly higher than in spray-dried Chlorella powder (p < 0.05), while the protein and moisture content were significantly lower (p < 0.05). Table 3 shows that the fatty acids in Chlorella powder mainly consist of C16 and C18 chains. The freeze-dried Chlorella powder had significantly higher levels of both monounsaturated and polyunsaturated fatty acids (PUFAs) compared to the spray-dried Chlorella powder (p < 0.05).
| Proximate composition | F-d Chlorella | S-d Chlorella |
|---|---|---|
| Crude protein (%) | 53.47 ± 0.53a | 57.40 ± 0.03b |
| Crude fat (%) | 11.28 ± 0.35b | 4.40 ± 0.30a |
| Carbohydrate (%) | 15.79 ± 0.93b | 9.37 ± 0.01a |
| Moisture (%) | 4.59 ± 0.09a | 5.45 ± 0.08b |
- Note: Values are mean ± standard error of mean (n = 3). Means in the same row sharing the same superscript letter are not significantly different by independent samples t-test (p > 0.05).
| Fatty acid profile | F-d Chlorella | S-d Chlorella |
|---|---|---|
| C16:0 | 22.87 ± 0.11a | 27.35 ± 0.07b |
| C16:1 | 7.05 ± 0.16b | 3.85 ± 0.09a |
| C17:0 | 6.95 ± 0.11 | 7.41 ± 0.09 |
| C17:1 | 13.26 ± 0.10 | 12.74 ± 0.19 |
| C18:1 trans | n.d. | 2.00 ± 0.07 |
| C18:2 n-6 | 16.84 ± 0.21b | 15.85 ± 0.13a |
| C18:3 n-3 | 33.04 ± 0.03b | 30.80 ± 0.05a |
| ∑SFAs | 29.82 ± 0.10a | 34.76 ± 0.11b |
| ∑MUFAs | 20.31 ± 0.13b | 18.59 ± 0.02a |
| ∑PUFAs | 49.88 ± 0.21b | 46.65 ± 0.16a |
| n-6/n-3 | 0.51 ± 0.01 | 0.51 ± 0.01 |
- Note: Values are mean ± standard error of mean (n = 3). Means in the same row sharing the same superscript letter are not significantly different by independent samples t-test (p > 0.05).
- Abbreviations: MUFA, monounsaturated fatty acid; n.d., not detected; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
3.4. Proximate Composition and Fatty Acid Analysis of D. carinata
As shown in Table 4, the crude fat content of D. carinata was highest in the freeze-dried Chlorella group, followed by the fresh Chlorella group, and lowest in the spray-dried Chlorella group (p < 0.05). The spray-dried Chlorella group had the highest crude protein content, exceeding that of the fresh Chlorella group and significantly higher than the freeze-dried Chlorella group (p < 0.05). The carbohydrate content in the freeze-dried Chlorella group was significantly lower than in the other two groups (p < 0.05). There were no significant differences in moisture content among the three groups.
| Proximate composition | Chlorella | F-d Chlorella | S-d Chlorella |
|---|---|---|---|
| Crude protein (%) | 24.90 ± 0.36b | 22.18 ± 0.83a | 25.97 ± 0.73b |
| Crude fat (%) | 34.99 ± 0.42b | 35.38 ± 0.25c | 28.27 ± 0.17a |
| Carbohydrate (%) | 9.38 ± 0.53b | 7.05 ± 0.05a | 8.40 ± 0.19b |
| Moisture (%) | 92.41 ± 0.10 | 90.66 ± 0.10 | 92.78 ± 0.84 |
- Note: Values are mean ± standard error of mean (n = 3). Means in the same row sharing the same superscript letter are not significantly different by Tukey’s multiple test (p > 0.05).
Feeding D. carinata with Chlorella processed using different drying methods had a significant impact on the fatty acid composition in the organisms (Table 5). The results showed that the saturated fatty acid (SFA) content was higher in the spray-dried Chlorella group than in the freeze-dried Chlorella group, but there was no significant difference compared to the fresh Chlorella group (p < 0.05). The PUFA content in the freeze-dried Chlorella group was significantly higher than in both the spray-dried and fresh Chlorella groups, with a marked increase in ∑n-6 fatty acids in both dried Chlorella groups compared to the fresh Chlorella group. The freeze-dried Chlorella group had significantly higher levels of ∑n-3 long-chain PUFAs (particularly α-linolenic acid and EPA) than the other groups (p < 0.05). There were no significant differences in monounsaturated fatty acids (MUFAs) among the three groups.
| Fatty acid profile | Chlorella | F-d Chlorella | S-d Chlorella |
|---|---|---|---|
| C4:0 | 3.50 ± 0.32b | 2.31 ± 0.11a | 3.06 ± 0.19ab |
| C6:0 | 1.71 ± 0.14b | 1.11 ± 0.02a | 1.48 ± 0.02b |
| C8:0 | n.d. | n.d. | 2.65 ± 0.17 |
| C10:0 | 3.11 ± 0.34b | 1.85 ± 0.03a | 2.40 ± 0.04a |
| C11:0 | 2.55 ± 0.25b | 1.23 ± 0.04a | 2.46 ± 0.44b |
| C12:0 | 2.75 ± 0.27b | 1.96 ± 0.04a | 2.24 ± 0.05ab |
| C13:0 | 5.20 ± 0.31b | 3.22 ± 0.24a | 4.39 ± 0.26b |
| C14:0 | 3.07 ± 0.18b | 2.46 ± 0.03a | 2.80 ± 0.04ab |
| C14:1 | 2.42 ± 0.05 | 3.56 ± 0.68 | 2.31 ± 0.46 |
| C15:0 | 2.01 ± 0.16b | 1.80 ± 0.04a | 1.51 ± 0.07ab |
| C15:1 | 3.10 ± 0.31 | 3.43 ± 0.71 | 3.09 ± 0.13 |
| C16:0 | 4.25 ± 0.35 | 4.19 ± 0.05 | 4.56 ± 0.07 |
| C16:1 | 1.54 ± 0.12a | 2.08 ± 0.03b | 1.69 ± 0.03a |
| C17:0 | 3.95 ± 0.02c | 0.93 ± 0.03a | 1.42 ± 0.03b |
| C17:1 | 2.90 ± 0.06b | 1.56 ± 0.05a | 1.47 ± 0.07a |
| C18:0 | 2.77 ± 0.17b | 2.00 ± 0.04a | 2.41 ± 0.10b |
| C18:1 | 16.39 ± 0.51b | 15.03 ± 0.15a | 15.30 ± 0.13ab |
| C18:2 n-6 | 12.09 ± 0.28a | 15.56 ± 0.24b | 14.76 ± 1.59ab |
| C18:3 n-6 | 1.76 ± 0.10b | 1.18 ± 0.01a | 1.12 ± 0.04a |
| C18:3 n-3 | 14.14 ± 0.34a | 18.69 ± 0.08b | 13.30 ± 0.19a |
| C20:0 | 1.55 ± 0.78 | 1.68 ± 0.01 | 2.18 ± 0.04 |
| C20:1 n-9 | 0.50 ± 0.50 | 1.26 ± 0.09 | 1.20 ± 0.04 |
| C20:2 | n.d. | 0.59 ± 0.30 | n.d. |
| C21:0 | n.d. | 0.31 ± 0.31 | 0.42 ± 0.42 |
| C20:4 n-6 (ARA) | 2.39 ± 0.08a | 3.36 ± 0.05b | 4.13 ± 0.13c |
| C20:3 n-9 | 0.39 ± 0.39a | 0.98 ± 0.01ab | 1.30 ± 0.03b |
| C20:5 n-3 (EPA) | 1.72 ± 0.10a | 2.38 ± 0.03b | 1.70 ± 0.52a |
| C22:0 | 0.75 ± 0.75 | 1.79 ± 0.02 | 2.68 ± 1.34 |
| C22:1 | 1.85 ± 0.11b | 1.32 ± 0.17ab | 0.46 ± 0.46a |
| C22:2 | n.d. | n.d. | n.d. |
| C23:0 | n.d. | 0.98 ± 0.02 | n.d. |
| C24:0 | n.d. | n.d. | n.d. |
| C24:1 | 1.63 ± 0.12 | 1.19 ± 0.02 | 1.02 ± 0.51 |
| C22:6 n-3 (DHA) | n.d. | n.d. | n.d. |
| ∑SFAs | 37.19 ± 0.97b | 27.83 ± 0.57a | 36.66 ± 1.48b |
| ∑MUFAs | 30.33 ± 0.36 | 29.44 ± 1.28 | 26.54 ± 1.38 |
| ∑PUFAs | 32.48 ± 0.62a | 42.73 ± 0.72b | 36.31 ± 2.29a |
| ∑n-3 | 15.86 ± 0.25a | 21.07 ± 0.11b | 15.01 ± 0.34a |
| ∑n-6 | 16.24 ± 0.09a | 20.69 ± 0.61b | 20.01 ± 1.42b |
| n-6/n-3 | 1.02 ± 0.01a | 0.98 ± 0.03a | 1.33 ± 0.08b |
- Note: Values are mean ± standard error of mean (n = 3). Means in the same row sharing the same superscript letter are not significantly different by Tukey’s multiple test (p > 0.05).
- Abbreviations: ARA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MUFA, monounsaturated fatty acid; n.d., not detected; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
3.5. Correlation Analysis
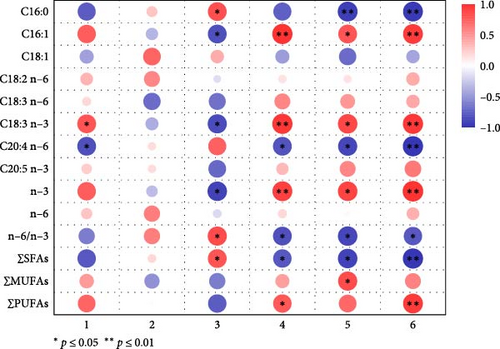
The heatmap of correlation analysis between essential fatty acid content and reproductive performance in D. carinata showed that survival time was significantly positively correlated with accumulated C18:3 n-3 (ALA) content and significantly negatively correlated with C20:4 n-6(ARA) content (Figure 4). C16:1, ALA, and n-3 fatty acids significantly shortened the average reproduction interval. The number of reproductive events was highly positively correlated with C16:1, ALA, and n-3 fatty acids, significantly positively correlated with PUFAs, and significantly negatively correlated with ARA and SFAs. The average reproductive number was significantly positively correlated with C16:1, ALA, n-3 fatty acids, and MUFAs, but highly negatively correlated with C16:0 and significantly negatively correlated with ARA and SFAs. Total offspring was highly positively correlated with C16:1, ALA, n-3 fatty acids, and PUFAs, while highly negatively correlated with ARA, C16:0, and other SFAs. The n−6/n-3 ratio was negatively correlated with reproductive frequency, average reproductive number, and total offspring, while significantly positively correlated with the reproduction interval.
4. Discussion
The development and reproduction of D. carinata rely on the intake of nutrients from external feed sources, making feed a crucial factor in regulating population dynamics [36]. Currently, Chlorella is considered one of the most promising feeds for culturing D. carinata [37]. In practice, drying fresh Chlorella into powder extends its shelf life and facilitates transport. This study compared the effects of freeze-dried and spray-dried Chlorella powder on the reproductive performance and fatty acid composition of D. carinata, aiming to explore the potential of using Chlorella powder as a substitute for fresh Chlorella in D. carinata culture. The findings provide scientific evidence and technical guidance for large-scale D. carinata cultivation.
Different drying methods affect the composition and content of nutrients in feed. The rapid freeze-drying technique causes ice crystals to sublimate directly from solid to gas, effectively preventing cell structure damage and nutrient loss. This ensures the integrity of feed appearance, color, flavor, and nutritional value [38, 39]. In contrast, spray-drying uses high-temperature, high-speed air to rapidly evaporate moisture. While this process is highly efficient in dehydration and sterilization, high temperatures can degrade heat-sensitive components such as enzymes and some unsaturated fatty acids [40]. It also promotes cell wall rupture, causing the leakage of soluble substances like proteins, carbohydrates, and certain fats [41]. In this experiment, freeze-dried Chlorella was found to have significantly higher levels of key nutrients, including carbohydrates, crude fat, and unsaturated fatty acids, compared to spray-dried Chlorella.
Lipids are considered a more efficient energy source than proteins and carbohydrates in organisms, playing a crucial role in maintaining normal biological functions and supporting life activities [42]. Given that D. carinata has a limited capacity to synthesize its own lipids, it relies almost entirely on exogenous lipids to meet its physiological needs [43]. Thus, the type and content of lipids in feed can indirectly regulate its reproductive performance. Lipids are primarily broken down by enzymatic reactions into free fatty acids and glycerides, which are then absorbed and utilized by D. carinata [44]. In this study, we found that freeze-dried Chlorella contained higher levels of crude fat and PUFAs compared to spray-dried Chlorella. Additionally, D. carinata fed with freeze-dried Chlorella exhibited significantly higher lipase and cellulase activities than the spray-dried group, indicating a stronger capacity for digesting and absorbing lipids from freeze-dried Chlorella. As a result, D. carinata fed with freeze-dried Chlorella accumulated higher levels of unsaturated fatty acids in their bodies.
We also found that D. carinata fed with freeze-dried Chlorella not only reached sexual maturity earlier and shortened their egg-laying intervals but also significantly increased their reproductive frequency and total reproductive output. This may be due to the significant influence that certain essential fatty acids have on gonad development in the parent organisms [45]. Previous studies have highlighted the role of PUFAs in enhancing reproductive output [46, 47]. For example, Sangk et al. reported that n-3 PUFAs significantly promote reproduction in large Daphnia species [48]. The n-3 PUFAs mainly include ALA, EPA, DHA, and DPA, with ALA serving as the precursor for the synthesis of all n-3 PUFAs. In D. carinata, the importance of ALA has been demonstrated, as it promotes growth and reproduction [49, 50]. EPA, in particular, is recognized as a crucial fatty acid for the growth and reproduction of water fleas [51, 52], and research has shown that an EPA-rich diet leads to higher reproduction rates in Daphnia [53]. In our study, the n-3 PUFA content (especially ALA) in Moina was significantly positively correlated with their reproductive performance. Moina fed with freeze-dried Chlorella had significantly higher levels of ALA and EPA compared to those fed with spray-dried Chlorella, and this diet notably accelerated reproduction and increased reproductive output in the freeze-dried group.
In addition to n-3 PUFAs, the content of n-6 PUFAs can also influence reproductive performance. However, excessive intake of n-6 PUFAs can promote inflammation and generate harmful substances [54]. This may explain why ARA levels in D. carinata showed a negative correlation with reproductive performance in this experiment. D. carinata fed with spray-dried Chlorella converted more ARA, potentially triggering inflammatory responses, which could lead to oxidative stress in D. carinata.
Moreover, due to the metabolic competition between n-6 and n-3 fatty acids [54], where n-6 fatty acids tend to promote inflammation while n-3 fatty acids can suppress it, some studies suggest that lowering the n-6/n-3 ratio is beneficial for overall health [55]. In this experiment, the n-6/n-3 ratio in D. carinata fed with spray-dried Chlorella (1.33) was significantly higher than that in the freeze-dried Chlorella group (0.98) and the fresh Chlorella group (1.02). As a result, D. carinata fed with freeze-dried Chlorella showed no significant difference in reproductive performance compared to those fed with fresh Chlorella, whereas the reproductive capacity of those fed with spray-dried Chlorella significantly declined.
5. Conclusion
The freeze-dried Chlorella contained higher crude fat and PUFAs contents than the spray-dried Chlorella, and D. carinata fed with freeze-dried Chlorella contains a higher content of PUFAs and demonstrates significantly superior reproductive performance compared to those fed with spray-dried Chlorella. There is no significant difference in reproductive performance between D. carinata cultured with freeze-dried Chlorella and fresh Chlorella. Therefore, freeze-dried Chlorella can be used as a substitute for fresh Chlorella in the culture of D. carinata.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research is financially supported by the Jiangsu Provincial Department of Science and Technology (SZ-HA2021022).
Open Research
Data Availability Statement
Data will be made available on request.




