Exploring the Potentials of Sajana (Moringa oleifera Lam.) as a Plant-Based Feed Ingredient to Sustainable and Good Aquaculture Practices: An Analysis of Growth Performance and Health Benefits
Abstract
As aquaculture increasingly seeks sustainable alternatives to traditional feed ingredients such as fishmeal and fish oil, Moringa oleifera, an eco-friendly and widely available plant, has emerged as a promising plant-based feed ingredient. This review synthesizes research from 1988 to 2024, sourced from PubMed, Google Scholar, and Research Gate, to evaluate the nutritional and medicinal potentials of M. oleifera in aquaculture feeds. Rich in protein, essential amino acids, vitamins (A, C, and E), minerals (calcium, iron, and potassium), and polyunsaturated fatty acids, M. oleifera offers significant benefits. Its leaves, flowers, and rhizomes utilized as crude extracts or bioactive compounds promote growth, enhance immunity, and provide antimicrobial defense against parasites, fungi, bacteria, and viruses. Studies demonstrate its effectiveness in improving growth, nutrition, and hematology in aquaculture species while lowering production costs. Furthermore, its antioxidant properties attributed to phenolic and bioactive compounds, bolster fish health, and resilience. This review underscores the potential of M. oleifera to advance sustainable aquaculture practices through its dual nutritional and medicinal benefits.
1. Introduction
The rapid expansion of aquaculture is accompanied by significant challenges, particularly in sourcing high-quality, sustainable feed ingredients. Fishmeal, a traditional protein source in aquafeeds, is renowned for its digestibility and nutritional value. However, its escalating demand over the past three decades has driven up costs, posing economic challenges to commercial aquaculture [1]. Additionally, the reliance on fishmeal as the primary protein source is unsustainable due to its limited availability [2]. These challenges underscore the need to explore alternatives, plant-based protein sources that can support fish growth, reproductive efficiency, and overall health [3].
M. oleifera (commonly known as the “drumstick tree” or “Tree of Life”) has emerged as a promising candidate for inclusion in aquaculture diets [4]. Its rapid growth, adaptability to tropical and subtropical climates, and nutrient-rich composition make it an appealing alternative. Various parts of the plant, including leaves, seeds, flowers, and roots, are rich in proteins, vitamins, minerals, and bioactive compounds, earning it the title of “superfood” in global markets [5]. Moreover, M. oleifera possesses therapeutic properties such as anti-inflammatory, antioxidant, hepatoprotective, and antimicrobial effects [6–8]. Its potential to support fish health and improve feed efficiency has gained attention among aquaculture researchers [9].
In addition to nutritional challenges, aquaculture faces rising incidences of disease outbreaks, often linked to intensive production methods [10]. Stressors such as poor water quality, overcrowding, and inadequate nutrition can suppress immune responses in fish, making them more vulnerable to infections [11]. The use of conventional veterinary medications, while effective, can leave harmful residues in fish tissues and the surrounding environment, ultimately impacting human health [12]. As an alternative, medicinal plants like M. oleifera are increasingly being explored for their ability to combat diseases with minimal environmental and health risks [13]. These plants have demonstrated bioactivities such as immune stimulation, growth promotion, stress reduction, and antimicrobial defense [14, 15].
M. oleifera shows potential as a sustainable, environmentally friendly, and economical component for aquafeeds. It is rich in amino acids, antioxidants, and macronutrients, which not only enhance growth and feed conversion but also improve health and disease resistance in fish [16]. However, its application comes with some challenges, as certain antinutritional compounds in Moringa leaves can interfere with nutrient absorption and metabolic processes if not properly managed [17]. The effectiveness of M. oleifera supplementation in aquafeeds depends largely on the optimized dosage and feeding duration. Research indicates that dietary inclusion levels ranging from 0.5% to 10% have varying effects on fish performance and health. Lower levels, such as 1% to 2%, are effective in enhancing immune responses and antioxidative enzyme activities without introducing adverse effects, while higher levels, between 5% and 10%, have shown to improve resistance against pathogens like Aeromonas hydrophila [18, 19]. However, exceeding certain thresholds may result in antinutritional effects that could impair nutrient absorption and metabolism [17].
In terms of duration, studies often recommend feeding M. oleifera-supplemented diets for 60–90 days to observe significant improvements in growth performance, feed utilization, immunity, and disease resistance [14]. Prolonged feeding beyond this period can be beneficial but requires careful monitoring to prevent nutrient imbalances. This highlights the importance of tailoring supplementation protocols to specific aquaculture species and farming conditions to maximize the benefits of M. oleifera. Addressing these issues and optimizing its inclusion levels in fish diets will be critical to its success in aquaculture.
While M. oleifera offers significant potential as a sustainable aquafeed ingredient, environmental and sustainability challenges must be addressed. Large-scale cultivation may lead to land-use changes, competition with food crops, and ecological imbalances in non-native regions [20, 21]. Improper cultivation could deplete soil nutrients and threaten biodiversity, while processing generates agricultural waste that requires effective management to prevent pollution. Additionally, the carbon footprint of large-scale production and transport should be evaluated. Integrating M. oleifera into circular bioeconomy frameworks, such as composting byproducts or bioenergy production, and adopting sustainable practices can help mitigate these challenges.
This review aims to assess the potential of M. oleifera as a plant-based feed ingredient for aquaculture, focusing on its nutritional, and health-promoting properties. It evaluates the effects of dietary M. oleifera on fish growth performance, body composition, and health indices such as hematobiochemical parameters, immune responses, and resistance to diseases. Furthermore, the review explores strategies to enhance the utilization of M. oleifera in aquaculture and identifies knowledge gaps that require further research to ensure its long-term sustainability and efficacy.
2. Materials and Method
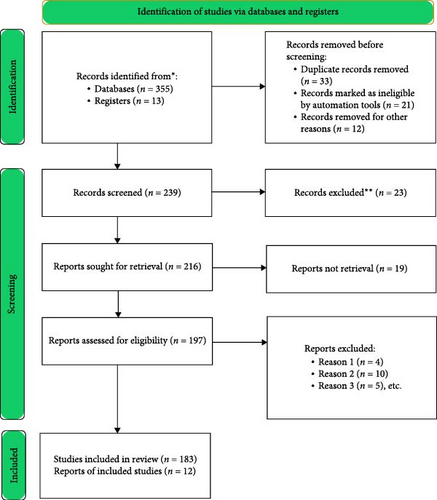
The comprehensive literature search was conducted across databases, including PubMed, Google Scholar, Scopus, ResearchGate, and PubMed Central, using targeted keywords such as M. oleifera + fish feed, M. oleifera + growth performance, M. oleifera + health benefits, M. oleifera + antibiotic/disease resistance, and M. oleifera + hematology parameters in fish (Figure 1). The search was performed between September 2023 and October 2024, considering articles published between August 1988 and 2024 and focused on studies published from August 1988 to October 2024. The inclusion criteria for selecting studies were as follows:
- 1.
Peer-reviewed publications.
- 2.
Articles published within the specified date range.
- 3.
Studies reporting the use of M. oleifera leaves as an ingredient in pellet feeds for different aquaculture species.
The primary screening process involved reviewing titles and abstracts to identify relevant articles, followed by a detailed evaluation of the full text (Figure 1). Data extraction focused on outcomes related to growth performance, health benefits, hematological indices, and disease resistance in aquaculture species fed with M. oleifera. Additionally, a word cloud was generated to visualize the most frequently discussed terms in the reviewed literature, highlighting key concepts such as “fish,” “growth,” “feed,” and “Moringa,” which reflect the core focus of this study (Figure 2). The extracted data were systematically reviewed and collaboratively revised by all authors to ensure accuracy and completeness.

2.1. Geographical Distribution
M. oleifera plant is widely distributed globally, as shown in Table 1. Known for its versatility, the plant thrives in diverse climates, making it a valuable resource for combating malnutrition, promoting health, and ensuring food security in communities worldwide. Originally native to the sub-Himalayan regions of India, Pakistan, Bangladesh, and Afghanistan, M. oleifera is now extensively cultivated in tropical and subtropical regions across Asia, Africa, the Americas, the Caribbean, and Oceania [22–24].
| Species | Country/region | Trivial name | References |
|---|---|---|---|
| M. arborea Verdcourt | Kenya, Somalia | — | www.theplantlist.orgwww.bihrmann.com |
| M. borziana Mattei | Kenya, Somalia | — | www.theplantlist.org |
| M. concanensis Nimmo | India | — | www.bihrmann.com |
| M. drouhardii Jumelle | Southern Madagascar | — | www.theplantlist.org |
| M. hildebrandtii Engler | Southwest Madagascar | Hildebrandt’s Moringa | www.bihrmann.com |
| M. longituba Engler | Kenya, Southeast Ethiopia, Somalia | — | www.theplantlist.org |
| M. oleifera Lam. | India | Horseradish, Ben-oil Drumstick, Kelor | [7] |
| M. ovalifolia Dinter ex Berger | Namibia, Southwest Angola | Phantom tree, Ghost tree, African Moringo | [7] |
| M. peregrina Forssk. Ex Fiori | Red Sea, Arabia, Northeast Africa | The Ben tree, also known as the wispy-needled Yasar tree, Wild drumstick tree, Yusor, Al Yassar, or Al Ban | www.theplantlist.org |
| M. pygmaea Verdcourt | North Somalia | — | www.theplantlist.org |
| M. rivae Chiovenda | Kenya, Ethiopia | Swanjehro | — |
| M. ruspoliana Engler | Kenya, Ethiopia, Somalia | — | [7] |
| M. stenopetala (Baker f.) Cufodontis | Kenya, Southwest Ethiopia, Somalia | Cabbage tree, Haleko, Shelagda, Shiferaw | [7] www.worldfloraonline.org |
The extent of its native distribution has been a subject of debate. While Northern India is consistently identified as its origin [25], other sources suggest that M. oleifera may also be native to Bangladesh, East Africa, and Southeast Asia [26, 27]. Regardless of its origins, the plant’s adaptability and nutritional richness have made it a cornerstone for food security in various regions. In Bangladesh, M. oleifera is extensively grown and its leaves have been evaluated for their qualitative and quantitative nutritional content. M. oleifera is valued for its high levels of vitamins, minerals, amino acids, and antioxidants. It serves as a dietary supplement, water purifier, ingredient in cosmetics, and animal feed [28]. Historically, it was prized by ancient Egyptians, Romans, and Greeks for its medicinal properties, oil, and use as food [29]. This multifaceted utility underscores its importance in both ancient and modern contexts.
2.2. Extract Preparation From Moringa Plants
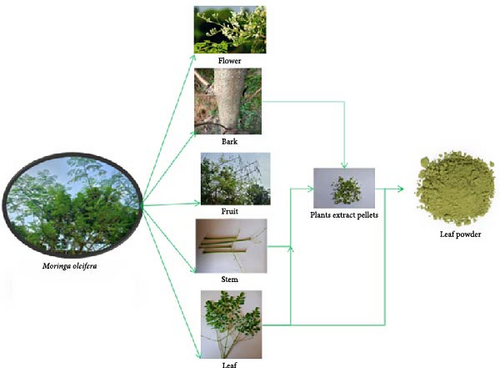
Various techniques are employed to prepare leaf extracts from fresh Moringa leaves, tailored to specific applications. Figure 3 outlines the process for powder formation from different parts of the Moringa plant:
- 1.
General extraction technique: Fresh Moringa leaves are gathered, cleaned, and washed thoroughly before being stored overnight at freezing temperatures. After 24 h, the frozen leaves are crushed using a mortar and pestle or a blender and then sieved through cheesecloth to collect the extract. The resulting extract is diluted as needed based on plant requirements [30].
- 2.
Water-based grinding method: According to Fuglie [31], Moringa leaves are ground in water at a ratio of 1 L per 10 kg of fresh leaves. The young leaves are harvested from mature plants, washed, and frozen for 2 days at 4°C [32]. The frozen leaves are then ground with a mortar and pestle or blender, adding a small amount of water (1 mL per 10 g of fresh leaves). The resulting juice is filtered through Whatman filter paper for further use [33].
- 3.
Dilution and application: Prepared extracts are diluted with distilled water following the method outlined by Fuglie [34]. For application, direct foliar sprays are used, employing calibrated equipment such as a knapsack sprayer or pressure spray bottle [32].
- 4.
Leaf sorting and drying: Leaves are sorted based on their color and maturity. Tender leaves, which are light green and 1–1.5 months old, are separated from mature leaves, which are dark green and 3–3.5 months old, following the classifications by Shuib et al. [35] and Ahmed et al. [18]. The leaves are washed thoroughly in water and dried using a freeze dryer at −56°C for 32 h until a constant weight is achieved, ensuring accurate moisture determination.
2.3. Biochemical Compound of Moringa oleifera
M. oleifera is a rich source of diverse biochemical compounds, contributing significantly to the health and well-being of both aquatic and terrestrial animals. These compounds make plant extracts an excellent alternative to traditional fish meal in commercial feeds. Additionally, M. oleifera offers numerous health benefits, such as relief from gastrointestinal issues, protection against arsenic poisoning, cholesterol reduction, and anti-inflammatory effects. Its extracts are particularly valued for their abundance of amino acids and potent antioxidant properties [36]. The chemical composition of dried M. oleifera plants is detailed in Table 2, highlighting its nutritional value. Key components include crude protein (CP), fiber, lipids, vitamins, minerals, carbohydrates, moisture content, calorie count, Vitamin C, and humidity [54]. These attributes underline the potential of M. oleifera as a sustainable and nutritionally rich feed ingredient in aquaculture.
| Crude protein (%) | Crude lipid (%) | Crude fiber (%) | Moisture (%) | Ash (%) | Carbohydrate (%) | Calories (cal) | Vitamin C (mg) | Humidity (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| 27.22 | 8.65 | 10.84 | 4.89 | 9.97 | — | — | — | — | [37] |
| 24.34 | 2.45 | 19.65 | 9.32 | 7.23 | — | — | — | — | [38] |
| 29.4 | 5.2 | 12.5 | — | — | 41.2 | 3290 | 15.8 | — | [39] |
| 38.9 | 10.79 | — | — | 5.84 | — | — | — | — | [40] |
| 35.4 | 43.6 | 4.7 | — | 5 | 9.2 | — | — | — | [41] |
| 29.56 | 36.34 | 4.7 | 3.78 | 4.08 | 21.54 | — | — | — | [42] |
| 26.7 | — | 1.4 | — | 2.6 | 32 | — | — | 6.8 | [43] |
| 28 | 45.8 | — | — | 4.1 | 10.6 | — | — | 4.7 | [44] |
| 26.47 | 5.45 | 7.55 | — | 11.35 | 45.89 | 3290 | — | — | [45] |
| 27.59 | 6.48 | 8.54 | — | 9.36 | 48.03 | 4120 | — | — | [46] |
| 28.95 | 2.83 | 19.95 | — | 10.87 | 37.4 | 3840 | — | — | [47] |
| 22.60 | 13.40 | 8.07 | — | 11.24 | 44.69 | — | — | — | [48] |
| 28.2 | — | 6.5 | — | 11.9 | — | — | — | — | [49] |
| 28.9 | 6.73 | 8.51 | — | — | — | — | — | — | [50] |
| 28.65 | 7.09 | — | — | 10.9 | 44.36 | — | — | — | [51] |
| 26.60 | — | 7.00 | — | 5.00 | — | — | 21.3 | — | [52] |
| 26.62 | 5.34 | 18.97 | 10.32 | 12.01 | — | — | — | — | [53] |
- Note: An arrow (—) indicates data not reported or not available.
2.4. Nutrient Profile of M. oleifera
M. oleifera is recognized for its rich and diverse nutrient profile, particularly its fatty acid and amino acid composition, which contribute significantly to its nutritional and health benefits. Table 3 presents the various nutritional elements of Moringa plants as reported in previous studies.
| Elements | Amounts (mg) | References |
|---|---|---|
| Carbon (C) | 45.86 ± 0.16 | [42] |
| Hydrogen (H) | 10.65 ± 0.15 | |
| Nitrogen (N) | 1.96 ± 0.22 | |
| Sulfur (S) | 3.31 ± 0.25 | |
| Organic carbon | 0.33 ± 0.07 | |
| Magnesium (Mg) | 147.5 ± 10.5 | [41] |
| Calcium (Ca) | 111.0 ± 7.00 | |
| Potassium (K) | 559.0 ± 14.05 | |
| Sodium (Na) | 21.50 ± 1.50 | |
| Zinc (Zn) | 0.125 ± 0.05 | |
| Copper (Cu) | 0.53 ± 0.09 | |
| Zinc (Zn) | 0.00329 | [55, 56] |
| Iron (Fe) | 0.0282 | |
| Calcium (Ca) | 2003 | |
| Copper (Cu) | 0.57 | |
| Magnesium (Mg) | 368 | |
| Phosphorus (P) | 204 | |
| Potassium (K) | 1324 | |
| Phosphorus (P) (g/100 g) | 0.25–0.6 | [21, 57–62] |
| Iron (Fe) (mg/100 g) | 25.6–49.0 | |
| Zinc (Zn) (mg/100 g) | 0.82–5.9 | |
| Sulphur (S) | 363–630 | [40] |
- Note: Organic carbon (%) = Organic matter (%)/1.72.
2.5. Fatty Acid Composition
- •
α-Linolenic acid (C18:3ω3): An essential omega-3 fatty acid found in significant amounts, providing substantial nutritional value [63].
- •
Palmitic acid (C16:0): A saturated fatty acid present in noteworthy proportions, contributing to the overall fatty acid profile [64].
- •
Linoleic acid (C18:2ω6): An essential omega-6 fatty acid, also found in appreciable quantities [64].
- •
Oleic acid (C18:1ω9): A monounsaturated fatty acid enhancing the nutritional quality of M. oleifera [65].
A comprehensive analysis by Moyo et al. [39] identified a spectrum of 17 fatty acids in M. oleifera. Among these, α-linolenic acid was the most abundant, followed by heneicosanoic acid, γ-linolenic acid, palmitic acid, and capric acid.
2.6. Amino Acid Composition
- •
Essential amino acids: tryptophan, threonine, isoleucine, leucine, lysine, methionine, cystine, phenylalanine, tyrosine, and valine.
- •
Nonessential amino acids: arginine, histidine, alanine, aspartic acid, glutamic acid, glycine, proline, and serine.
The presence of these nutrients highlights M. oleifera’s potential as a high-value ingredient in both aquaculture feed and human nutrition.
2.7. Effects of M. oleifera on Growth Performance in Aquaculture Species
The integration of M. oleifera into aquaculture diets has gained significant attention due to its rich nutritional profile, including high-quality protein, essential amino acids, vitamins, and bioactive compounds. Numerous studies have demonstrated its potential to enhance growth performance, improve feed utilization, and support the health of various aquaculture species [9, 67, 68].
For Nile tilapia (Oreochromis niloticus), dietary inclusion of M. oleifera leaf meal (MOLM) or aqueous extract has consistently resulted in improved specific growth rate (SGR), weight gain (WG), and feed conversion ratio (FCR) at optimal dosages. For instance, Emam et al. [67] found that a dietary dose of 200 mg/kg of M. oleifera aqueous extract significantly enhanced growth while supporting hematological parameters and organ function. Similarly, Sharker et al. [9] observed marked increases in final weight, survival rates, and growth metrics in Heteropneustes fossilis fed a diet containing 10% MOLM.
The effects of M. oleifera are not universal, as antinutritional factors, particularly at higher inclusion levels, may limit its efficacy. For example, Mehdi et al. [69] reported a decline in growth performance for Labeo rohita when diets contained over 30% MOLM. However, strategies such as fermentation [70] or protein hydrolysate preparation [71] have been shown to mitigate these limitations, enhancing both growth and nutrient absorption.
Across different species, optimal inclusion levels and forms of M. oleifera vary. While moderate levels (5%–10%) of leaf meal generally yield favorable results, aqueous extracts, and protein hydrolysates provide alternative means of supplementation. For example, Stadtlander et al. [72] observed efficient feed conversion rates in common carp (Cyprinus carpio) and tilapia with dietary supplementation of M. oleifera plant extract (MPE) at 400–600 ppm. Similarly, fermented M. oleifera leaves were successfully used to replace 40% of fishmeal in Carassius auratus diets, enhancing both growth performance and immunity [70].
A detailed summary of findings across species, dosages, and durations is presented in Table 4. This compilation underscores the versatility of M. oleifera in aquafeeds and highlights its role as a sustainable and effective alternative to conventional protein sources. However, further studies are necessary to optimize its application, address species-specific requirements, and evaluate long-term impacts on fish health and aquaculture productivity.
| Dosage | Duration (days) | Experimental fish | Results | References |
|---|---|---|---|---|
| 10% | 90 | H. fossilis |
|
[9] |
| 200 mg/kg feed | 90 | O. niloticus | Enhanced SGR, WG, FW; caution on exceeding recommended dosage | [67] |
| 100 g/kg | 60 | Pangasius bocourti | No negative impacts on growth, feed utilization, and digestibility | [73] |
| 10% M. Oleifera and 20% shrimp meal | 42 | Clarias gariepinus | Improved growth performance and nutritional quality | [74] |
| 5.80 g/kg | 60 | O. niloticus | Improved growth performance and health benefits with no adverse effects | [68] |
| Fermented leaves, 40% of the fishmeal | 50 | C. auratus gibelio var. CAS III | Improved growth performance and immune response | [70] |
| 10 g of MOLs/kg; 20 mL MOL extract/kg | 60 | Cyprinus Carpio | Enhanced growth and immune function | [75] |
| Up to 60% MPH | 70 | O. niloticus | Increased growth parameters, reduced glucose and leptin levels, and boosted growth hormone activity | [71] |
| 10% MOLM | 28 | Sparus aurata | Significant improvements in growth parameters and immune status | [76] |
| 30% MOLM | 56 | L. rohita | Higher inclusion levels negatively impacted growth performance | [69] |
| 1.5% MOLM | 90 | O. niloticus | Enhanced growth and hematological parameters | [77] |
| 0.5% MOL extract | 60 | Macrobrachium rosenbergii | Enhance the growth rate and improve both the physiological and immune functions | [78] |
| 19.3–25.0 g/kg | 56 | Oncorhynchus mykiss | Boosted growth rate | [79] |
| 5% and 10% MOLM | 42 | O. niloticus | Enhance the growth performance | [80] |
| 2.5 g/kg diet | 60 | O. niloticus | Significantly enhanced growth and feed intake | [81] |
| 5% moringa leaf powder | 56 | O. niloticus | Higher final body weight and improved FCR | [82] |
| 20 mg/g feed | 28 | Puntius altus | Significantly increased body weight | [83] |
| 10% | 49 | O. niloticus | Exhibited normal growth performance | [84] |
| 15% | 150 | Heterobranchus longifilis | Enhance growth performance | [85] |
| 15% | 150 | H. longifilis | The final body weight and feed intake were significantly improved | [86] |
| 30% | 84 | C. carpio | Growth parameters have been substantially enhanced | [87] |
| 15% | 56 | Clarias garipenus | Weight increase, FCR, and SGR all showed significant improvement | [88] |
| 20% | 56 | C. garipenus | Showed best performance in case of FCR, weight gain, and SGR | [89] |
| 8.2 g | 84 | C. garipenus | The addition of moringa enhanced the FCR, AWG, and SGR | [90] |
| 10% | 56 | C. garipenus | Mean weight gain and FCR showed better performance | [91] |
| 20% | 90 | O. mykiss | Showed the highest growth rate | [92] |
| 3% | 90 | Cirrhinus mrigala | Improved weight gain, SGR, and nutrient digestibility | [93] |
| 30% | 90 | L. rohita | Enhance weight gain, SGR and improve FCR | [94] |
| 20 mg/g diet | 28 | Puntius altus | Fish health, growth performance, and muscle protein profile all exhibited considerable improvements | [95] |
- Abbreviations: AWG, average weight gain; FCR, feed conversion ratio; FW, final weight; MOL, M. oleifera leaves; MOLM, M. oleifera leaf meal; MPH, M. oleifera protein hydrolysate; SGR, specific growth rate; WG, weight gain.
2.8. Hematological Changes in Fish Species
Hematological parameters are vital indicators of fish health and physiological status, offering insights into stress levels, immune responses, and nutritional adequacy in aquaculture. Studies have shown that dietary supplementation with MOLM positively affects hematological indices, although the impacts vary depending on dosage and species.
For C. gariepinus, a significant increase in red blood cell (RBC) count and hemoglobin (Hb) levels was observed with dietary inclusion of MOLM, highlighting its potential to enhance immunological strength [38]. Similarly, Ayoola, Kuton, and Shokefun [90] and Dienye and Olumuji [91] reported a gradual rise in Hb, RBCs, and white blood cells (WBCs) with increasing MOLM levels, up to an optimal threshold. However, excessive inclusion (beyond 20%) was associated with hematological abnormalities, suggesting a dosage-dependent effect [96].
The substitution of fishmeal with MOLM in diets for L. rohita demonstrated that up to 10% replacement enhanced CP content, fat deposition, and hematological parameters such as RBC and Hb levels. Higher substitution levels, however, led to reduced hematological performance and abnormalities, emphasizing the need for balanced inclusion levels [96].
In Oreochromis mossambicus, a diet containing 9%–12% MOLM significantly increased levels of RBCs, Hb, hematocrit (HCT), and other hematological indices, supporting its immunomodulatory potential [97]. Other studies, including those on H. fossilis and C. mrigala, also reported enhanced RBC and Hb levels with MOLM supplementation at 10% inclusion, marking it as an optimal dosage for improved fish health [9, 46]
Table 5 summarizes the findings across various studies, highlighting the effects of M. oleifera supplementation on hematological parameters in different aquaculture species. These results demonstrate its potential to support fish health through nutritional and immunological enhancements, provided the inclusion levels are carefully optimized.
| Recommended dosage | Duration (days) | Experimental fish | Results | References |
|---|---|---|---|---|
| 10% | 90 | H. fossilis | Significant increase in Hb and RBC levels | [9] |
| 9%–12% | 45 | O. mossambicus | Increased WBC, RBC, Hb, and HCT levels | [97] |
| 60% | 56 | C. gariepinus | Decrease in RBC levels; increase in MCHC, MCH, and MCV | [89] |
| 0.5% | 30 | O. niloticus | Significant rise in RBC, WBC, Hb, HCT, MCV, MCH, and MCHC values | [98] |
| 10% | 180 | C. mrigala | Highest RBC, Hb, and optimum MCHC, MCV, MCH, and PCV values | [46] |
| 10% | 70 | L. rohita | Highest RBC, Hb values, and lower WBC, MCHC values | [96] |
| 10% | 90 | C. mrigala | The highest values for RBCs, WBCs, Hb, PCV, and MCHC, while the lowest values for PLT, MCH, and MCV | [45] |
| 36% | 70 | O. niloticus | The highest values for RBCs, PLT, and Hb were found | [99] |
| 10% | 84 | C. gariepinus | Maximum RBC count, Hb levels, and remarkable increase in platelet values | [53] |
| 1.5% | 56 | C. gariepinus | Enhanced PCV, Hb, RBC; higher WBC | [52] |
| 0.5% | 30 | O. niloticus | Improved RBC, Hb, HCT, and WBC counts; significant enhancement in WBC and lymphocyte counts | [100] |
| 10% | 56 | C. gariepinus | Maintained PCV, RBC, and Hb within healthy ranges; WBC and lymphocytes increased with higher MOLM inclusion | [91] |
| 200 mg/kg | 90 | O. niloticus | Significant increase in RBC, Hb, PCV, WBC, and total serum protein | [67] |
| 2.5 g/kg | 60 | O. niloticus | Significant improvement in RBC, WBC, HGB, and HCT | [81] |
| 4.1 g/kg diet | 84 | C. gariepinus | Significant increase in PCV, HGB, RBC, and WBC | [90] |
| 12.40 mg/L | 7–35 | C. carpio | Significant increase in WBC, MCV, MCH, and AST, ALT, ALP activities. Significant decrease in plasma protein levels. Biphasic trend in Hb, HCT, RBC, and MCHC | [101] |
| 0.5% | 30 | Nile tilapia | Significant increase in RBC, Hb, HCT, MCV, MCH, MCHC, and WBC | [98] |
| 1.5%–5% | 60 | O. niloticus | Significant increase in WBC count, no significant change in RBC count and Hb levels | [102] |
- Abbreviations: Hb, hemoglobin; HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MOLM, M. oleifera leaf meal; PCV, packed cell volume; RBC, red blood cell; WBC, white blood cell.
2.9. Effects of MOLM on Immune Gene Expression
Dietary supplementation with MOLM has been shown to influence immune gene expression in various aquaculture species, enhancing immune system function and stress resistance. Fish fed a 10% MOLM-supplemented diet showed considerably higher mRNA levels of the HSP70 and SOD2 gene, indicating increased immune system and antioxidant defence against microbial infection [9]. Fingerlings of crucian carp were fed with 0.5% M. oleifera leaf extract diet improved physiological and immunological function, as well as resistance to pathogenic infections [103]. Before the starvation test, Nile tilapia fed with 1.5% M. oleifera integrated feed exhibited significantly higher (p < 0.05) expression of insulin-like growth factor 1 (IGF-1), transforming growth factor-b (TGF-b), and SOD genes than the control group. Following starvation stress, the group fed a meal supplemented with 1.5% M. oleifera showed substantial upregulation (p < 0.05) in the expression of IGF-1, TGF-b, and SOD genes [77]. Moreover, the relative expression of hepatopancreas HSP70 in M. rosenbergii was considerably influenced by the moringa-supplemented group compared to the control group, both before and after exposure to ammonia stress [78].
2.10. Bioactive Compounds From M. oleifera and Their Bioactivity
M. oleifera, often referred to as the “miracle tree,” is renowned for its diverse and abundant bioactive compounds. Various parts of the plant are rich in glucosinolates, flavonoids, phenolic acids [104], carotenoids [105], tocopherols, polyunsaturated fatty acids [106], bioavailable minerals [105], and folate. These compounds exhibit significant pharmacological potential, offering a wide array of health benefits, including antioxidant, anti-inflammatory, and immunomodulatory effects.
The leaves of M. oleifera are particularly notable for their high concentrations of flavonoids, saponins, tannins, catechol tannins, anthraquinones, and alkaloids, which collectively enhance the plant’s nutritional, therapeutic, and water-purifying properties [107]. Furthermore, the specific composition and bioactivity of these phytochemicals can vary depending on geographical and environmental factors, influencing their efficacy in addressing nutritional deficiencies, promoting health, and combating diseases. Table 6 summarizes previous studies on bioactive compounds and their biological activities.
| Bioactive compound | Biological activities | References |
|---|---|---|
| Vitamins A, vitamin C | Plays vital roles in reproduction, embryonic development, immunological function, and cell differentiation | [108] |
| Glucosinolates | Numerous health-promoting advantages | [109] |
| Phenolic compounds | Antioxidant, antidiabetic, antitumor, anti-inflammatory, anticancer, hepatoprotective, cardioprotective, and antiasthmatic effects. Improves metabolic performance and has cytotoxic properties | [110–112] |
| Ethanol extract | Inhibits neurotoxins, acts as an antivenom, neutralizes toxins, and prevents bleeding caused by venomous snakebites or other venomous creatures | [113, 114] |
| γ-Tocopherol | Prevent lipid peroxidation and promote peroxide metabolism | [115] |
| Kaempferol | Prospective therapeutic applications | [116] |
| Hexadecanoic acid, oleic acid | Prevent damage from oxidative stress | [117] |
| Morphine, moriginine | Reduces inflammation and prevents ulcers | [115] |
2.11. Multifunctional Protective Agent of M. oleifera
2.11.1. Antinutritional Factors
The inclusion of M. oleifera leaf extract as a feed ingredient in aquaculture has shown promising results. However, diets incorporating plant sources like M. oleifera often face challenges related to antinutritional factors, which can affect nutrient absorption and overall fish growth. These factors include alkaloids, glycosides, oxalic acid, phytates, protease inhibitors, lectins, saponins, cyanoglycosides, and imbalances in essential amino acids, fatty acids, and micronutrients [116, 117].
Higher substitution levels of MOLM for fish meal have been associated with reduced growth performance, primarily due to antinutritional components such as phenols, tannins, phytates, and saponins [84]. Laheng et al. [118] reported that antinutrients in Moringa leaf meal contributed to decreased body weight in tilapia. Similarly, Hlophe and Moyo [119] highlighted that saponins, polyphenols, phytic acid, and tannins are the primary antinutritional compounds in M. oleifera leaves.
While M. oleifera leaves are largely free of antinutritional factors except for saponins and phenols [120], minimizing their presence is crucial for optimizing fish growth and nutrient absorption [121, 122]. The low levels of antinutrients in M. oleifera are generally considered safe for consumption by humans and animals [123]. However, even minimal levels of these compounds can interfere with the activity of digestive enzymes, reducing nutrient absorption and inhibiting growth rates in fish [124, 125].
2.11.2. Antioxidant Properties of M. oleifera
M. oleifera is a plant known for its high antioxidant content. M. oleifera exhibits antioxidant activity, as confirmed by in vitro studies. Rutin compounds are found in M. oleifera which exhibited antioxidant and anticancer properties in bioactivity tests [126]. Qualitative phytochemical screening showed that M. oleifera contains flavonoids, saponins, and steroids and has antioxidant capabilities [127]. M. oleifera flowers were extracted and evaluated for antioxidant activities. According to the findings, the ethyl acetate fractions of M. oleifera flowers have anti-inflammatory and antidiabetic characteristics and are a good source of antioxidants [128]. The antioxidant properties of M. oleifera may be attributed to the presence of phenolic compounds, as confirmed by the phytochemical analysis of the hydroethanolic extract.
2.11.3. Antifungal
M. oleifera exhibits notable antifungal properties, making it effective against a variety of fungal species. During the fermentation of MOLM, fungi such as Aspergillus flavus, Aspergillus niger, Saccharomyces cerevisiae, Alternaria alternata, Penicillium citrinium, Neurospora crassa, and Rhizopus stolonifera were identified [129]. These fungi contributed to a reduction in nitrogen-free extract (NFE) levels during fermentation, which was accompanied by an increase in CP content as the fungi utilized carbohydrates for growth [130].
Ariyani, Asmawit, and Utomo [131] also linked the increase in CP content to fungal growth during fermentation. Jimoh et al. [129] further demonstrated that MOLM at a concentration of 12.08 g/kg exhibited the strongest antiyeast effects in C. gariepinus, while a concentration of 10.6 g/kg served as an effective antifungal agent. These findings emphasize the dual impact of fungal activity on the nutritional enhancement of MOLM during fermentation and its application as a bioactive antifungal agent in aquaculture species like C. gariepinus.
2.11.4. Antiparasitic Properties of M. oleifera
M. oleifera has shown promise as a natural antiparasitic treatment in aquaculture, offering a sustainable alternative to synthetic chemicals for managing parasitic infections. Its potential in treating parasitic diseases in animals has been highlighted by Pareek et al. [132].
The aqueous leaf extract of M. oleifera (ALEMO) is a natural, safe, and cost-effective option for controlling parasitic infections, such as Ichthyophthirius multifiliis (Ich), in aquaculture systems. Chika et al. [133] demonstrated ALEMO’s efficacy in reducing infestations in C. gariepinus, emphasizing its potential as a natural remedy against Ich. Similarly, Valladão, Gallani, and Pilarski [134] reported that extracts from M. oleifera and Morinda citrifolia are highly effective against Argulus spp., a parasitic crustacean that infects freshwater fish.
Saponins, a bioactive compound found in M. oleifera, have shown strong antiparasitic properties. Zhou et al. [135] demonstrated their anthelmintic effects against Gyrodactylus kobayashii, a monogenean ectoparasite in goldfish (C. auratus). However, the study also highlighted the potential for acute toxicity in fish, indicating that the chemical structure of saponins is critical in determining their antiparasitic efficacy and safety.
2.11.5. Antibacterial
M. oleifera extracts have demonstrated antibacterial properties that contribute to healthier fish growth by combating bacterial infections. Firdaus et al. [136] examined the antibacterial effects of edible coatings made from pectin of mandarin orange peel, supplemented with M. oleifera leaf extract, and assessed its impact on the freshness of Nile tilapia (O. niloticus). This research highlights the potential role of M. oleifera in food preservation and antibacterial interventions.
Using natural extracts like M. oleifera in aquaculture offers a promising alternative to synthetic antibiotics, improving fish health and reducing reliance on chemical treatments. Jimoh et al. [129] determined an optimum effective concentration of 11.2 g/kg for Moringa seed marinade, exhibiting antibacterial activity against identified bacteria such as Enterobacter spp., Staphylococcus spp., Proteus spp., Streptococcus spp., and Bacillus spp. Ethanolic extracts of M. oleifera have demonstrated effectiveness against both Gram-positive and Gram-negative bacteria [137]. The antibacterial mechanism of secondary metabolites in M. oleifera leaves, particularly flavonoids, involves inhibiting nucleic acid synthesis, damaging the cytoplasmic membrane, inhibiting bacterial metabolism, and interfering with the synthesis of bacterial cell membranes, leading to bacterial cell aggregation [138].
Furthermore, Oladeji, Odelade, and Oloke [139] recognized the antibacterial properties of M. oleifera, attributing its flavonoids with antibacterial effects through mechanisms such as binding to extracellular proteins, deactivating bacterial cell metabolism, and interacting with bacterial cell walls [136]. The antibacterial potential of M. oleifera is highlighted by the identification of specific targets for emodin, a compound in M. oleifera, against A. hydrophila [140]. This underscores M. oleifera’s multifaceted antibacterial properties, positioning it as a valuable candidate for further research and potential applications in combating bacterial infections. M. oleifera leaf extract is very effective for treating Argulus spp. infection in goldfish. It was reported that a concentration of 62.5 mg/L resulted in a 30% reduction in infestation [141]. This suggests the potential of M. oleifera leaf extract, particularly at this concentration, as a promising treatment for Argulus spp. infections in goldfish.
Considering these findings, Laheng et al. [118] recommend further research on M. oleifera leaves. They propose subjecting the leaves to various processing steps, including solvent extraction, enzyme treatment, soaking, heat treatment, drying, and fermentation. The goal is to reduce the presence of antinutrients in the leaves, indicating an interest in optimizing extraction and preparation methods to enhance the nutritional quality of M. oleifera leaves for potential applications, possibly for consumption or as a therapeutic agent.
2.11.6. Antiviral Properties of M. oleifera
M. oleifera extracts exhibit potent antiviral properties, making them a promising natural alternative for enhancing fish health and controlling viral infections in aquaculture systems. In aquaculture, berberine hydrochloride has been found effective in treating infections caused by cyprinid herpesvirus 2 (CyHV-2) across various fish species, including silver carp [142]. Additionally, geniposidic acid has shown antiviral activity against white spot syndrome virus (WSSV) in red swamp crayfish Procambarus clarkii, as indicated by Huang et al. [143].
2.12. Disease Treatment
Fish diseases pose a significant threat to successful aquaculture. In response to food security concerns, the world is now moving from synthetic medicine to natural medication. Researchers find hope in M. oleifera leaves. They are highly effective against the disease caused by harmful bacteria A. hydrophila. Hammed et al. [144] conducted research on C. gariepinus and reported that feeding the fish with 50% Moringa leaf extract produced favorable outcomes. There were no detrimental effects from the antinutritional factors, and the fish had a rise in RBC numbers, indicating better blood oxygen levels. Furthermore, their WBC counts also increased which suggests a strong immune system. The rise in both red and WBCs indicates that the leaf extract of M. oleifera helps fish fight against infections and stay healthy. Similarly, incorporating M. oleifera leaf powder as a dose of 15% could significantly improve the skin mucus immunity of guppy [145]. Moreover, M. oleifera leaf extract contains natural bioactive compounds with bacteriostatic and antibacterial properties which inhibit the growth of Edwardsiella tarda [146]. Further, M. oleifera leaf powder supplements can strengthen O. niloticus fry immune responses and help in the prevention of the disease caused by A. hydrophila.
2.13. Antistressor
In intensive aquaculture, aquatic animals face various environmental stressors, such as poor water quality, high temperatures, overcrowding, and pathogen infections. These stressors can weaken the immune system of animals, leading to negative impacts on growth performance, metabolic balance, and antioxidant levels, and may result in disease outbreaks and high mortality rates.
Azhar et al. [147] reported significant improvements in fish-fed diets supplemented with 2% and 4% moringa extract along with 1/10th of the arsenic LC50 concentration. These groups exhibited recovery and increased activity of the catalase enzyme in the liver, gills, and muscles of the fish, while oxidative stress levels were reduced. Emodin, an active constituent of anthraquinone extract, demonstrated protective effects in herbivorous fish (Megalobrama amblycephala) under various stress conditions, including crowding stress and oxidative stress induced by dietary oxidized fish oil. Gbadamosi, Fasakin, and Adebayo [148] showed that adding M. oleifera leaf extract to fish diets considerably reduced the excessive cortisol, glucose, LDH, MDH, AST, and ALT levels caused by stressors.
The conventional approach to managing diseases in the presence of external stressors typically involves the use of antibiotics or other drugs. However, the overuse of such medications can contribute to the development of drug resistance [149]. M. oleifera extract, particularly with emodin as a key component, shows promise in mitigating the negative effects of environmental stressors on fish health, offering a potential alternative to conventional medication use in aquaculture.
As shown in Table 7, numerous studies have demonstrated the positive effects of M. oleifera as an antistressor in various aquaculture species, improving growth performance, immunity, and stress tolerance [75, 77, 78, 148, 150, 151]. The incorporation of M. oleifera extracts or leaf meals into aquaculture diets has been shown to mitigate the adverse impacts of environmental stressors, demonstrating its potential as a natural, effective solution to enhance fish health.
| Authors and reference | Experimental species | Key findings |
|---|---|---|
| Shourbela et al. [150] | Nile tilapia (O. niloticus) | M. oleifera reduces hypoxia-related stress in tilapia, as demonstrated by a consistent decrease in cortisol levels in fish exposed to hypoxia |
| Elabd et al. [77] | Nile tilapia (O. niloticus) | Significant improvement in antioxidant levels (e.g., superoxide dismutase, catalase) and reduced stress indicators (glucose, cortisol, AST, ALT) |
| Kaleo et al. [78] | Freshwater prawn (M. rosenbergii) | A 0.5% inclusion rate of M. oleifera leaf extract enhances growth performance and helps protect prawns from ammonia stress |
| Khalıl and Kornı [75] | Common carp (Cyprinus Carpio) | M. oleifera leaf extract improved growth and immunity, mitigating stress effects in common carp |
| Gbadamosi, Fasakin, and Adebayo [148] | Nile tilapia (O. niloticus) | Addition of M. oleifera leaf extract reduced stress-induced increases in cortisol, glucose, and several biochemical markers (MDH, AST, ALT) |
| Faheem et al. [151] | Grass carp (Ctenopharyngodon Idella) | A 5% inclusion of Moringa leaf meal improved growth rate, physiological functions, and protected against heat stress |
2.14. Future Perspective and Concluding Marks
M. oleifera is a viable, sustainable alternative for improving aquaculture feeds owing to its abundant nutritional composition and prospective health advantages. Its elevated essential amino acids, vitamins, and antioxidants benefit fish nutrition, enhancing growth and reproductive performance. However, caution is needed due to antinutritional factors, which can sometimes hinder growth. Strategies such as blanching can help to reduce these compounds, enhancing the suitability of M. oleifera as a feed ingredient. The lack of standardized research on M. oleifera makes it challenging to compare results across various studies. Moreover, inconsistencies in nutrient composition due to varying cultivation and processing methods make data interpretation more difficult. A thorough assessment of its potential benefits is also hindered by the limited understanding of M. oleifera action mechanisms. Further research is necessary to understand its long-term effects on fish growth and health. Overall, M. oleifera presents an exciting opportunity to meet the increasing demands of the aquaculture industry while promoting sustainability, good aquaculture practices, and economic viability for farmers. In vitro assessment of feed additives, including identifying and quantifying substance viability and activity postinclusion in feed and after storage, the activation mechanisms of the immune pathway, and cloning to enhance M. oleifera efficiency, warrants further investigation. Consequently, studies focusing on growth performance, immunology, and artificial pathogen challenges should be regarded as vital in forthcoming research endeavors to ascertain and measure the impact of additives on aquaculture species. Additionally, analysis of proximate composition, determination of transcription levels of several immune-related genes, calculation of serum biochemical characteristics, and measurement of digestive enzyme activity and release of nutrients would significantly enhance the research.
Disclosure
As this is a review article, no consumables or materials (such as cells, animals, and reagents) were used in the preparation of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Md. Abdullah Al Mamun Hridoy, Fardin Shahriar, and Md. Abu Kawsar conceived and conceptualized the study and prepared the original draft. Md. Abdullah Al Mamun Hridoy, Md. Abu Kawsar, Fatema Jannat Munny, Fardin Shahriar, Md. Moshiur Rahman, Md. Fakhrul Islam, and Abeer Kazmi contributed to literature research, drafting, reviewing, and editing the manuscript.
Funding
This study did not receive any financial support or funding from external sources, institutions, or organizations.
Open Research
Data Availability Statement
As this is a review article, no primary data were generated or analyzed. All conclusions are based on previously published studies, which are cited accordingly within the manuscript.




