Immunopotential Induced in Culturable Carps Fed on Edible Bivalent Vaccine Against Aeromonas veronii and Aeromonas hydrophila
Abstract
The bacterial pathogens Aeromonas species, notably Aeromonas veronii and Aeromonas hydrophila, are major causative agents of severe infections and significant mortalities in freshwater aquaculture systems. Vaccination offers an effective prophylactic approach to enhance fish immune defenses and reduce reliance on antibiotics. This study aimed to evaluate the immunoprotective efficacy of a bivalent bacterial oral vaccine against these pathogens in carps. The vaccine was formulated by either incorporating or spraying it/onto commercial feed (30% crude protein), with 10% fish oil and mineral oil added as adjuvants. Seven experimental dietary diets were designed, comprising vaccinated feeds administered with or without adjuvants (1 mL of vaccine per gram of feed) and a nonvaccinated control. Healthy fingerlings (20 ± 5 g) were fed their respective diets over a 60-day experimental period, followed by challenge with Aeromonas spp. To evaluate the effectiveness of the vaccination, immune parameters (serum lysozyme activity, agglutination antibody titers, and IgM levels), growth performance (net weight gain [NWG], specific growth rate [SGR], and feed conversion ratio [FCR]) and survival rates were examined. In comparison to the control, the results indicated that all vaccination diets showed considerably improved immune responses and growth. However, vaccinated diet with fish oil (IVFF) showed the highest immunological stimulation and rate of survival, 83% for Ctenopharyngodon idella (C. idella) and 85% for Labeo rohita (L. rohita). These results validate the IVFF oral vaccine formulation has potential as a noninvasive, scalable method of providing protective immunization against Aeromonas infections in freshwater aquaculture.
1. Introduction
Bacterial, viral, and parasitic infections lead to disease outbreaks in aquaculture and other farmed animals. Cultured fish are more susceptible to opportunistic infections, such as Aeromonas infection, due to various factors, including high stocking density and inadequate farming practices [1, 2]. Aeromonas species are gram-negative, rod-shaped, and major pathogenic bacteria that cause motile Aeromonas septicemia (MAS) in aquaculture globally [3]. Aeromonas infection is characterized by clinical signs, such as hemorrhages on the body surface and fins, abdominal distension, exophthalmia, ulcerative lesions, and internal organ damage, especially in the liver and kidneys. The disease can result in high mortality rates, often reaching up to 70%–100% in severe outbreaks if not properly managed [4, 5]. Beyond mortality, MAS causes significant economic losses due to reduced growth rates, increased treatment costs, and decreased product quality.
The development of antibiotic-resistant strains has made the use of antibiotics to treat bacterial infections more costly and potentially inconsistent [6]. Remarkably, the development of vaccinations delivered orally through feed has advanced vaccination technology and offered an effective alternative for managing bacterial infections in aquaculture [7]. Inactivated cell vaccines are made to protect fish against a variety of bacterial diseases and they are widely used because of their financial benefits [8].
An innovative advancement in the aquaculture sector is the feed-based vaccination [9, 10]. Vaccination method is simpler and more effective, and directly added into the fish feed. This strategy improves survival rates and reduces fish stress and mortality by handling and injecting each fish [11]. Additionally, as a feed-based vaccine can provide protection against multiple pathogens with a single dose, adding two or more antigens into it is more advantageous to a monovalent vaccine [12].
Formalin-killed whole organisms of S. agalactiae and A. hydrophila were used in the development and in vivo evaluation of a feed-based bivalent vaccination in 2023 using red hybrid tilapia and 10% palm oil as an adjuvant [13]. Oral vaccination is the most feasible of the current delivery methods for large-scale operations since it is simple to administer, causes less handling stress, and used different life stages [14]. Feed-based bacterial oral vaccinations are suitable for pond and cage systems, and allow for regular dosing [15, 16]. Adjuvants are crucial for improving vaccine-induced immunity because they prolong immune responses and promote antigen presentation. Mineral oil adjuvants, such as MontanideTM, are often used because of their significant immunostimulatory effects and minimal toxicity [17].
In Pakistan and many developing countries, the vaccine development industry, skilled manpower, and high labor costs are the major constraints that cause the failure of injectable vaccines. The objective of the study was to address the need for antibiotic-free disease management and raise aquaculture production efficiency by developing an affordable, feed-based bivalent vaccine with appropriate adjuvants against Aeromonas species, and assessing its immunogenicity and protective efficacy under controlled conditions.
2. Materials and Methods
2.1. Ethical Approval
This present study has been approved by the Institutional Ethics Committee of the University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan (dr/486, dated: 15 Nov 2023).
2.2. Experimental Site
The experiment was conducted in Fish Hatchery Complex, Department of Fisheries and Aquaculture and Medical Laboratory Technology (MLT) of UVAS, Ravi Campus, Pattoki.
2.3. Bacterial Recovery
The vaccine was formulated using previously isolated and procured A. hydrophila (AH-24123) and A. veronii (AV-201022) strains, which are naturally infecting strains from carps. These strains were deposited at NCBI GenBank under accession numbers MT249822.1 and NR–118947.1, respectively [18] and belong to the UVAS, Lahore. A. veronii and A. hydrophila were reidentified based on specific morphological, physiological, and biochemical characteristics [19]. Sequences of bacteria amplified using 27F and 1492R 16S rRNA-specific primers showed 99% similarity to the standard A. hydrophila sequence in NCBI [20]. A phylogenetic tree constructed from the 16S rRNA sequences showed that the isolates were most closely related to A. veronii, with sequence similarities ranging from 98.43% to 100% [21].
2.4. Vaccine Preparation
The preserved bacterial stocks at −80°C were revived by spreading 100 µL on the tryptic soy agar plate (TSA) (Sigma-Aldrich, Switzerland). A single colony subculture of both bacteria was picked and inoculated in 5 mL of tryptic soy broth (TSB) (Sigma-Aldrich, Switzerland) and cultured [22]. This culture was used for the enrichment of bacteria and inoculated into two 500 mL flasks each having broth and incubated at 30°C for 24 h with an orbital shaker at 150 rpm. Bacterial cells from 24 h cultures in TSB were harvested by centrifugation at 1500 rpm. The cells were washed 2–3 times with 0.9% (w/v) sterile saline and the optical density (OD600) was adjusted to 0.67, to achieve a final bacterial concentration of A. hydrophila (4.1 × 109 CFU/mL) and A. veronii (3.3 × 109 CFU/mL). Serial dilution and bacterial colony counts were used to calculate the bacterial concentration after centrifugation, following the standard method [23]. Formalin (1% v/v) was added to inactivate the bacteria and kept at 30°C for 4–6 h. Subsequently, the formalin-inactivated bacteria were centrifuged again at 1500 rpm for 20 min and washed 2–3 times with sterile saline solution to eliminate residual formalin. The sterility of the inactivated bacteria was assessed using an in vitro method to ensure complete inactivation [18]. Fish oil (Nutrifactor Laboratories [Pvt] Limited, Pakistan) and Mineral oil (Acros Organics, A Thermo Fisher Scientific Brand) were added as an adjuvant at a concentration of 10%.
2.5. Bivalent Bacterial Vaccine
The bivalent vaccine was prepared by mixing the inactivated bacterial cells of the monovalent vaccines of A. hydrophila and A. veronii at a rate of 1:1 ratio by adding fish oil and mineral oil (10%) as adjuvant [23].
2.6. Formulation of Experimental Bacterial Vaccinated Feed
Commercial powder feed (30% crude protein) was purchased from Hi-Tech Aqua Feed Mill (Pvt) Ltd. of Lahore, Pakistan. Feed was divided in to two parts and vaccine was sprayed and incorporated separately at the ratio of 1 L of vaccine to 1 kg of commercial powder feed and the final concentration in each type of vaccine was maintained approximately of 109 cells g−1 of fish feed [23].
2.7. Experimental Design and Feed
- i.
Incorporated vaccinated feed (IVF),
- ii.
Incorporated vaccinated feed with mineral oil (IVFM), and
- iii.
Incorporated vaccinated feed with fish oil (IVFF).
- i.
Sprayed vaccinated feed (SVF),
- ii.
Sprayed vaccinated feed with mineral oil (SVFM), and
- iii.
Sprayed vaccinated feed with fish oil (SVFF).
A commercial diet was used as the control. Details of the experimental design and diet compositions are presented in Table 1.
| Bacterial vaccinated feed types | Control | T1 | T2 | T3 |
|---|---|---|---|---|
| Sprayed bacterial vaccinated feed | Feed (C) | Feed + bacterial vaccine without adjuvant (SVF) | Feed + bacterial vaccine with mineral oil as adjuvant (SVFM) | Feed + bacterial vaccine with fish oil as adjuvant (SVFF) |
| Incorporated bacterial vaccinated feed | Feed (C) | Feed + bacterial vaccine without adjuvant (IVF) | Feed + bacterial vaccine with mineral oil as adjuvant (IVFM) | Feed + bacterial vaccine with fish oil as adjuvant (IVFF) |
2.8. Experimental Fish Species
Experimental healthy fish Ctenopharyngodon idella (C. idella)and Labeo rohita(L. rohita) with an average weight of 20 ± 5 g were obtained from Fish Hatchery Complex, UVAS, Ravi Campus, Pattoki. The experimental trial was conducted for 60 days. Fish were first kept for 10 days to acclimatize with the hatchery conditions and fed with commercially available pelleted feed. Fish were randomly stocked in 14 tanks each having 20 fish with replication and fed at 3% body weight twice a day at 09:00 a.m. and 03:00 p.m.
2.9. Quality Tests of Feed
Before initiation feeding experiments, experimental diets were subjected to proximate analysis (AOAC, 2012), palatability, safety, and water stability tests by using established methods [24].
2.10. Growth Parameters
2.11. Immunological Analysis
Blood (10 fish per treatment) was collected on day 14, 28, 42, and 56 during trial and was centrifuged at 6000 rpm for 20 min to collect the serum and kept at 4°C. Immunity parameters were analyzed, such as mean antibody titer test following the protocol of Obaldo et al. [25], IgM analysis followed by Anderson et al. [26] and serum lysozyme activity test followed by Ruckenstein and Zeng [27].
2.12. Challenge Test
2.13. Statistical Analysis
All experimental data were expressed as mean values ± standard deviation (mean ± SD). Prior to statistical analysis, the normality of the data was evaluated using the Shapiro–Wilk test, and the homogeneity of variances was assessed using Levene’s test. A two-way analysis of variance (ANOVA) based on a randomized complete block design (RCBD) was conducted to determine statistically significant differences among the treatment groups. When significant effects were observed, Duncan’s Multiple Range Test (DMRT) was applied for post hoc pairwise comparisons. All statistical analyses were performed using SPSS version 20.0, with a significance level set at p < 0.05 [30].
3. Results
3.1. Vaccinated Feed Quality Tests
Feed palatability and feed stability tests exhibited significantly higher levels in vaccinated diets. Among the vaccinated and control diets, the diet IVFF demonstrated the highest palatability (Table 2). A feed safety test was conducted before the experiment, swabbing the samples of vaccinated diet and various fish organs (gills, intestines, and mouths) on agar plates and incubating at 30°C for 24 h did not yield bacterial growth plates. This comprehensive approach ensures the confirmation of bacterial identity, the formulation of a stable and palatable vaccine, and rigorous testing for feed safety, demonstrating the efficacy and safety of the prepared vaccine for subsequent application of fish health management. The results indicate that the vaccine is not only effective in eliciting an immune response but also safe for use in aquaculture.
| Parameters | Treatment diet | ||||||
|---|---|---|---|---|---|---|---|
| C | SVF | IVF | SVFM | IVFM | SVFF | IVFF | |
| Feed stability (%) | 78.2 ± 0.98bc | 76.1 ± 1.62c | 72.4 ± 1.62d | 77.9 ± 0.28bc | 79.8 ± 1.20ab | 81.8 ± 1.06a | 82.4 ± 1.27a |
| Feed palatability | 0.53 ± 0.01d | 0.52 ± 0.01d | 0.56 ± 0.06cd | 0.66 ± 0.09bc | 0.67 ± 0.06bc | 0.75 ± 0.04ab | 0.79 ± 0.01a |
- Note: Data are presented as mean ± standard deviation (SD), with n = 2 fish per group. Statistical differences were determined using (two-way analysis of variance [ANOVA] followed by Levene’s test); significance was set at p < 0.05. Groups with different superscript letters differ significantly.
- Abbreviations: C, control; IVF, incorporated vaccinated feed; IVFF, incorporated vaccinated feed with fish oil; IVFM, incorporated vaccinated feed with mineral oil; SVF, sprayed vaccinated feed; SVFF, sprayed vaccinated feed with fish oil; SVFM, sprayed vaccinated feed with mineral oil.
3.2. Proximate Analysis of Feed
The proximate analysis of the feed indicated elevated values in the vaccinated diets compared to the control diet. Specifically, the protein content was notably higher in diet IVFF in comparison to the other vaccinated diets and the control diet (Table 3). This analysis highlights the nutritional composition of the feed, emphasizing the enhanced protein in diet IVFF and fat contents in the vaccinated diets, particularly in diet IVFM, which may contribute to the overall efficacy and nutritional quality of the formulated vaccines.
| Compositions | Treatment diets | ||||||
|---|---|---|---|---|---|---|---|
| C | SVF | IVF | SVFM | IVFM | SVFF | IVFF | |
| Crude protein (%) | 24.8 ± 0.45d | 26.8 ± 0.42c | 24.7 ± 0.87d | 27.8 ± 0.49bc | 26.8 ± 0.91c | 28.8 ± 0.77b | 32.8 ± 0.54a |
| Crude lipid (%) | 3.9 ± 0.82b | 5.3 ± 0.28ab | 4.6 ± 1.74ab | 6.4 ± 0.27a | 5.0 ± 0.47ab | 5.9 ± 0.74ab | 5.2 ± 0.79ab |
| Moisture (%) | 5.9 ± 0.56c | 7.9 ± 0.91a | 6.8 ± 0.43ab | 6.9 ± 0.98ab | 5.9 ± 0.63bc | 6.7 ± 0.71ab | 6.8 ± 0.77ab |
| Ash (%) | 6.1 ± 0.63a | 5.4 ± 0.14ab | 5.1 ± 0.42ab | 4.8 ± 0.21ab | 4.1 ± 0.71bc | 4.3 ± 1.48bc | 2.9 ± 0.35c |
- Note: Data are presented as mean ± standard deviation (SD), with n = 2 fish per group. Statistical differences were determined using [two-way analysis of variance (ANOVA) followed by Levene’s test]; significance was set at p < 0.05. Groups with different superscript letters differ significantly.
- Abbreviations: C, control; IVF, incorporated vaccinated feed; IVFF, incorporated vaccinated feed with fish oil; IVFM, incorporated vaccinated feed with mineral oil; SVF, sprayed vaccinated feed; SVFF, sprayed vaccinated feed with fish oil; SVFM, sprayed vaccinated feed with mineral oil.
3.3. Growth Parameters of C. idella and L. rohita
Growth parameters like NWG, SGR, and FCR were recorded to check the performance of fish growth during experimental trial. Table 4 demonstrated that diet IVFF gains higher net weight as compared to the other vaccinated diets. Vaccinated diet diets showed significantly higher weight gain (WG) as compared to the control diet.
| Parameters | Fish species | Treatment diets | ||||||
|---|---|---|---|---|---|---|---|---|
| C | SVF | IVF | SVFM | IVFM | SVFF | IVFF | ||
| NWG (g) | C. idella | 6.80 ± 0.14i | 6.40 ± 0.14j | 6.90 ± 0.14hi | 7.21 ± 0.07h | 7.80 ± 0.14g | 9.62 ± 0.12d | 11.32 ± 0.14b |
| L. rohita | 7.10 ± 0.14h | 8.10 ± 0.15f | 9.10 ± 0.08e | 9.12 ± 0.08e | 10.41 ± 0.14c | 14.10 ± 0.14b | 16.11 ± 0.12a | |
| SGR (%) | C. idella | 0.75 ± 0.01def | 0.71 ± 0.01efg | 0.76 ± 0.02def | 0.79 ± 0.03cde | 0.83 ± 0.01cd | 0.94 ± 0.84ab | 1.00 ± 0.12a |
| L. rohita | 0.63 ± 0.02g | 0.67 ± 0.01fg | 0.74 ± 0.01def | 0.77 ± 0.01cde | 0.83 ± 0.02cd | 0.86 ± 0.01bc | 0.95 ± 0.01ab | |
| FCR | C. idella | 1.21 ± 0.01ab | 1.22 ± 0.01a | 1.20 ± 0.01ab | 1.20 ± 0.01ab | 1.20 ± 0.01ab | 1.14 ± 0.12ef | 1.12 ± 0.01fg |
| L. rohita | 1.21 ± 0.01ab | 1.18 ± 0.02bc | 1.16 ± 0.01de | 1.16 ± 0.02de | 1.16 ± 0.02de | 1.12 ± 0.01fg | 1.11 ± 0.01g | |
- Note: Data are presented as mean ± standard deviation (SD), with n = 2 fish per group. Statistical differences were determined using [two-way analysis of variance (ANOVA) followed by Levene’s test]; significance was set at p < 0.05. Groups with different superscript letters differ significantly.
- Abbreviations: C, control; C. idella, Ctenopharyngodon idella; FCR, feed conversion ratio; IVF, incorporated vaccinated feed; IVFF, incorporated vaccinated feed with fish oil; IVFM, incorporated vaccinated feed with mineral oil; L. rohita, Labeo rohita; NWG, net weight gain; SGR, specific growth rate; SVF, sprayed vaccinated feed; SVFF, sprayed vaccinated feed with fish oil; SVFM, sprayed vaccinated feed with mineral oil.
3.4. Serum Lysozyme Activity
The serum lysozyme activity was performed at day 14, 28, 42, and 56 after vaccinated feed. All vaccinated diets showed significantly difference with control diet (Figure 1). IVF showed highest value within the experimental diets. Diet IVFF showed significantly enhanced lysozyme activity at days 42 and 56.
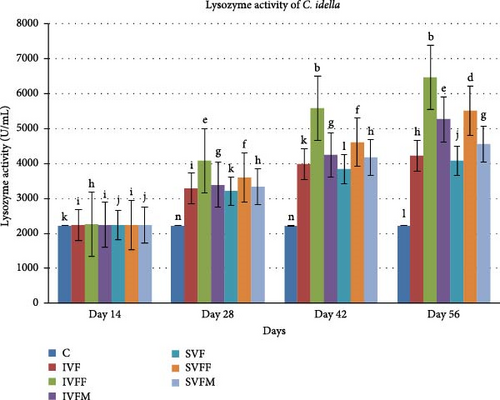
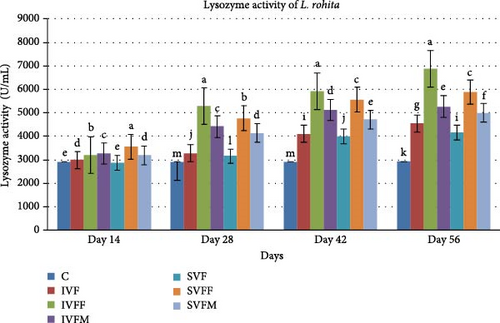
3.5. Agglutination Antibody Titer Test
An agglutination antibody titer test was conducted at various intervals (day 1, 14th, 28th, 42nd, and 56th) following the feed vaccination trial. Over the course of 56 days, mean antibody titer values displayed a gradual increase (Figure 2). Notably, vaccinated diets exhibited significantly higher values (p < 0.05) compared to the control diet. Furthermore, there was a significant difference observed between incorporated vaccinated diets and sprayed vaccinated diets. Among the incorporated diets, the IVFF diet displayed the highest mean antibody titer value (0.70) in comparison to other vaccinated diets.
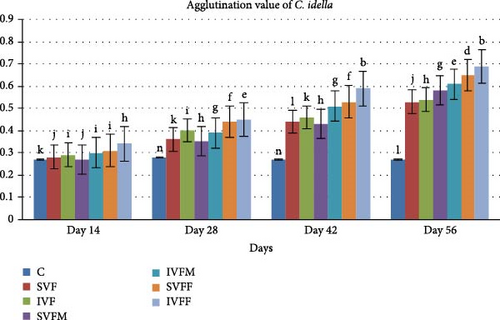
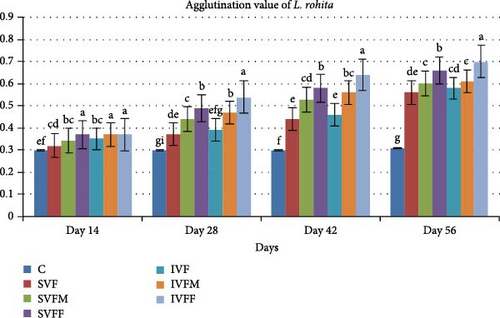
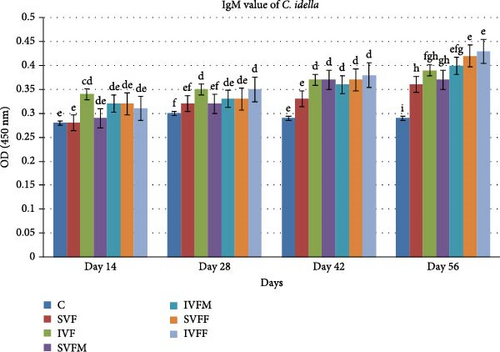
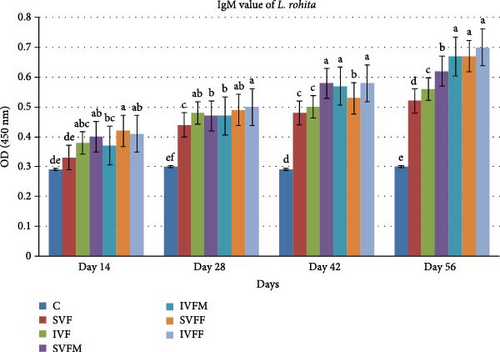
3.6. Total IgM Contents
After the vaccination trial, total IgM contents were assessed. Fish from the experimental diet fed with incorporated vaccinated diets exhibited significantly higher values (p < 0.05) compared to those fed with sprayed vaccinated diets. Additionally, vaccinated diets displayed a significant difference when compared to the control diet. By day 56, diet IVFF demonstrated the highest IgM contents among the vaccinated diets in comparison to others diets (Figure 3).
3.7. Challenge Test and RPS Rate
During 14-days observation period after challenge test, fish were monitored for symptoms of dropsy and mortality. The results indicated that the IVFM vaccinated diet exhibited lowest mortality rates and provided highest protection against A. hydrophila and A. veronii. The lowest survival rate were observed in SVF, followed by IVF, SVFM, SVFF, IVFM, and IVFF for L. rohita, whereas in C. idella the survival rate was as follows SVF, IVF, SVFM, SVFF, IVFM, and IVFF. The control diet had an 86% mortality rate. Results demonstrated that relative survival rates in IVFF offered better protection compared to other vaccinated diets (Table 5).
| Treatments | Fish species | Total number of fish | Number of death | Protection (%) | Mortality (%) |
RPS (%) |
|---|---|---|---|---|---|---|
| C | C. idella | 15 | 13 | 14 | 86 | — |
| L. rohita | 15 | 12 | 20 | 80 | — | |
| SVF | C. idella | 15 | 10 | 34 | 66 | 23 |
| L. rohita | 15 | 10 | 34 | 66 | 18 | |
| IVF | C. idella | 15 | 8 | 47 | 53 | 38 |
| L. rohita | 15 | 9 | 40 | 60 | 25 | |
| SVFM | C. idella | 15 | 9 | 54 | 46 | 70 |
| L. rohita | 15 | 8 | 47 | 53 | 34 | |
| IVFM | C. idella | 15 | 5 | 67 | 33 | 62 |
| L. rohita | 15 | 8 | 47 | 53 | 34 | |
| SVFF | C. idella | 15 | 5 | 67 | 33 | 58 |
| L. rohita | 15 | 5 | 67 | 33 | 62 | |
| IVFF | C. idella | 15 | 2 | 87 | 13 | 83 |
| L. rohita | 15 | 2 | 87 | 13 | 85 | |
- Abbreviations: C, control; C. idella, Ctenopharyngodon idella; IVF, incorporated vaccinated feed; IVFF, incorporated vaccinated feed with fish oil; IVFM, incorporated vaccinated feed with mineral oil; L. rohita, Labeo rohita; RPS, relative percentage survival; SVF, sprayed vaccinated feed; SVFF, sprayed vaccinated feed with fish oil; SVFM, sprayed vaccinated feed with mineral oil.
4. Discussion
Vaccines are the most effective way to prevent disease in aquaculture [31]. Many experimental vaccines have been studied in the literature for aquaculture in neighboring countries, particularly China [32], Bangladesh [33], Iran [34], and India [35, 36]. However, Pakistan has no literature on the development or application of fish vaccines. The primary cause of fish dropsy disease in Pakistan, A. hydrophila, has developed antibiotic resistance to many antibiotics, which can be crucial to note [37]. In order to develop and evaluate an inactivated vaccine based on montanide oil against A. hydrophila in Pakistani cultured fish species, including grass carp (C. idella), rohu (L. rohita), and mori (C. mrigala) by Sughra et al. [28]). The current study was aimed to develop the bivalent inactivated oral vaccine against Aeromonas spp. in culturable carps of Pakistan.
Stability and high acceptability are two of the good characteristics of fish feed. The intake of a sufficient diet helps to avoid growth inhibition [38]. A crucial quality factor in the production of aquaculture diets is water stability. Commercial and vaccinated pellets did not significantly differ in their water stability, showing that the vaccinated pellets are as stable in water as commercial ones [39]. Palm oil-supplemented feed improves fish feed intake, so ultimately improves total nutritional intakes [40]. In contrast, poor feed palatability can reduce fish growth and feed intake [24]. In contrast to the mineral oil, the fish oil adjuvant in the vaccinated meal in this trial demonstrated greater palatability.
The process of repelleting feed, however, can alter some nutrient compositions, which potentially have an impact on the fish’s ability to absorb nutrients. It was still in an acceptable range along with the higher moisture content. According to Terpstra [41], feed pellets that contain too much moisture can grow mold with a shorter shelf life. Commercial feed was tested in this study with a bivalent vaccine to make sure the fish, who were already used to the feed, could accept it easily. This study employed commercially available feed in conjunction with a bivalent vaccine. Protein, lipid, carbohydrate, and ash compositions were essentially unchanged, but the reformulated feed had significantly higher moisture content.
Evaluation of growth parameters is crucial in vaccination studies in order to assess the treatment’s adverse effects. The effects of vaccination on fish growth performance have been investigated as a focus of many research studies [42, 43]. In their evaluation of rainbow trout growth performance following administration of an oil-adjuvanted vaccine, Skinner et al. [44] observed the fact that the final body weight did not significantly differ from controls, there was a significant increase in WG and SGR, suggesting that increased resting metabolic rate (RMR) after vaccination did not affect overall growth. Oil-based inactivated vaccines did not negatively impact the growth performance of C. idella, L. rohita, and C. mrigala, according to Sughra et al. [28]), and vaccinated fish exhibited normal WG and provided good protection without reducing growth. In this study, the growth performance of the vaccinated fish was better than that of the control. Compared to fish fed alternative vaccine diets, fish fed the IVFF diet show superior growth.
Fish innate immunity includes lysozyme, which works in conjunction with the complement system to protect against bacterial infection. Fish lysozyme activity significantly increased by the vaccinated diet from 7 days after vaccination until the 6-week vaccination period [45]. In order to detect and eradicate any infections within the host, the complement system is essential, according to Secombes and Wang [46]. Fish fed diet IVFF revealed noticeably higher levels compared with other vaccinated diets and control.
Fish like Sparus macrocephalus were shown their immunological responses to different vaccination concentrations assessed in the past [47]. In vaccination experiments, they produced Edwardsiella tarda ghost (ETG) and executed different concentrations. According to Sughra et al. [28], three different concentrations of the vaccination showed noticeably greater serum IgM antibody titers than the control diet. However, the differences between the vaccinated diets were not statistically significant (p > 0.05). The study demonstrated that IgM antibody levels increased in the vaccinated fish serum as compared to the control. Diet IVFF showed significantly highest IgM antibody levels than the other vaccinated diet and control. The control group showed no statistically significant change over time.
Vaccinated fish exposed to A. veronii A5 and A. hydrophila A9 showed respective survival rates of 63.49% and 93.33%. The statistical test revealed no significant difference between these, but the value was lower in the fish that A. veronii challenged [48]. In diets challenged with S. iniae and A. hydrophila, the red hybrid tilapia vaccinated with feed-based bivalent vaccination demonstrated improvements in both innate and adaptive immune responses [49] and a promising relative percent survival (RPS) of up to 80%–90% and 17.78% and 22.22% [13, 50]. The bivalent vaccinated diet IVFF had a significantly higher RPS (p < 0.05) at 85% compared to the other diets. The purpose was to develop and evaluate a locally developed feed-based bivalent vaccination that would protect aquaculture fish against diseases caused on by these three infectious diseases. L. rohita and C. idella were selected as fish hosts to evaluate their immunological response.
5. Conclusion
The immunological response of L. rohita and C. idella to a bacterial bivalent oral vaccine against Aeromonas spp. was investigated in this study using spray and feed incorporation. Both fish species showed protection against single and coinfections as well as systemic and mucosal IgM responses. The highest levels of immunogenicity and protective effectiveness were produced by the incorporated bacterial vaccinated diet with fish oil (IVFF). In contrast to C. idella, L. rohita exhibited a higher level of immune responses and increased resistance to infection. For improving disease control in freshwater aquaculture, the results validate the efficacy of feed-based vaccination, especially in L. rohita.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: Nimra Mubeen, Farzana Abbas, and Muhammad Hafeez-ur-Rehman. Methodology: Nimra Mubeen, Farzana Abbas, and Muhammad Hafeez-ur-Rehman. Investigation and data curation: Nimra Mubeen and Farzana Abbas. Writing – original draft preparation: Nimra Mubeen, Farzana Abbas, and Erkan Can. Writing – review and editing: Nimra Mubeen, Farzana Abbas, Muhammad Hafeez-ur-Rehman, Iffat Amin, Samama Jalil, and Erkan Can. Supervision: Farzana Abbas and Muhammad Hafeez-ur-Rehman.
Funding
Open access funding provided by the Scientifc and Technological Research Council of Türkiye (TÜBİTAK). No funding was received from any organization.
Open Research
Data Availability Statement
No datasets were generated or analyzed during the current study.




