Effect of Dietary Arachidonic Acid on the Broodstock Productive Performance and Egg Quality of Largemouth Bass (Micropterus nigricans)
Abstract
The influence of dietary arachidonic acid (ARA) on the reproductive performance of broodstock fish is a critical area of aquaculture research. This study investigates the impact of ARA levels in broodstock diets on broodstock hormone, fecundity, egg quality, and larval survival of largemouth bass (Micropterus nigricans). Experimental diets containing 0.9%, 2.0%, and 3.6% ARA as a percentage of total fatty acids (TFAs) were formulated and administered to broodstock over 84 days. Results indicated that a dietary ARA level of 3.6% significantly increased vitellogenin content in broodstock serum while reducing serum estradiol levels. Despite this hormonal shift, reproductive performance metrics, including fecundity, egg hatch rates, and the number of viable eggs until mouth opening, were not significantly affected by the ARA content in the broodstock diet. However, the 3.6% ARA group showed notable improvements in egg size and higher concentrations of ARA and docosahexaenoic acid in the eggs. Based on these results, 0.9% ARA in TFA content in broodstock diet could be sufficient for the reproduction of largemouth bass. A level of 3.6% ARA of TFAs can be added to the diet of largemouth bass broodstock to enhance offspring potential growth performance and healthy status.
1. Introduction
In the development of aquaculture, the supply of seeds is one of the most important constraints. The sexual maturation and the quality of seed largely determine the success of aquaculture production, and the sustainability and competitiveness of aquaculture farms or companies, due to the high costs in the management of broodfish and the maintenance of culturing facility [1]. Egg quality, the ability of the egg to be fertilized and subsequently to develop into a normal embryo [2], is determined by many factors including broodstock origin, conditioning and manipulations [2]. Broodstock nutrition is a vital factor that affects the quality of gamete since the nutrients during gametogenesis rely on the delivery from broodfish. The intake of nutrients from broodstock affects the nutritional composition of gamete, egg production, fertilization, larval survival rate, and even the growth performance of offspring [3–6].
Arachidonic acid (ARA, 20:4n-6) belongs to n-6 fatty acid long-chain polyunsaturated fatty acid (LC-PUFA) family, which has attracted more attention in recent year. ARA has been proven to play a role in maintaining the fluidity of cell membranes, regulating cellular ion channels, activating some enzymes, and regulating cell apoptosis [7]. Regarding aquatic animals, ARA is one of the essential fatty acids for aquaculture animals. ARA affects reproductive performance, stress resistance, lipid metabolism, etc. [8, 9]. The effect of ARA on reproduction of fish draws more attention. Wild captured fish with better spawning performance showed higher ARA content and EPA/ARA ration in gonads than domesticated fish in different species like cod (Gadus morhua L.) [10], European eel (Anguilla anguilla L.) [11], and white sea bream (Diplodus sargus) [12]. Blue gourami (Trichopodus trichopterus; Pallas, 1770) fed with a diet content of 2% ARA showed significantly higher fecundity and hatching rate [13].
The mechanism of the effect of ARA on the reproductive capacity relates to eicosanoid production and steroid hormone regulation. ARA is the precursor for eicosanoids, such as prostaglandins (PGs), leukotrienes (LTs), epoxy eicosatetraenoic acids (EETs), and endocannabinoids (ECs) [14], which are related with reproductive performance of fish. In yellow perch (Perca flavescens), follicles synthesize PGs, specifically prostaglandin E2 (PGE2) and prostaglandin E2α (PGE2α), which are crucial for oocyte maturation and ovulation [15]. PGE2 and LT B4 [16] are involved in the regulation of gonadal steroidogenesis via cAMP or PKC-signaling pathways [8]. The effect of ARA on gonadal steroid production seems to be a sex-dependednt process [8]. For instance, in the research on yellow catfish, female fish fed with a medium ARA levels (4.67% of total fatty acids (TFAs)) diet showed significantly lower 11-ketotestosterone (11-KT) levels than fish fed with 0.64% and 10.07 ARA content diet [17]. In Senegalese sole (Solea senegalensis), female estradiol level was not affected by dietary ARA level, while in males, 11-KT and testosterone (T) increased significantly with the increasing dietary ARA level [18].
Similar to other LC-PUFAs, ARA cannot be synthesized de novo due to the lack of ∆12 and ∆15 desaturase in vertebrate [16], but it can be synthesized from linoleic acid (LA, 18:2n-6) through a series of elongase and desaturase [19, 20]. LA is firstly catalyzed by ∆6-elongase to di-himo γ-LA (DGLA; 20:3n-8), and DGLA is converted to ARA through ∆5 desaturase [16].
Largemouth bass (Micropterus salmoides), a high-value freshwater aquaculture species, was introduced into China in 1970’s. Global production of this species was 621,326,803,809.45 tons in 2020–2022, according to the data from FAO (FAO 2025). Global Aquaculture Production. In: Fisheries and Aquaculture. [Cited Sunday, February 23, 2025]. https://www.fao.org/fishery/en/collection/aquaculture?lang=en). Requirement of largemouth bass larvae is more than 50 billion in China each year. For enhancing reproductive performance, ensuring sustainable aquaculture and reaping economic benefits, it is important to research the nutritional requirement of largemouth bass broodfish. However, due to the long period and the high cost of the experiment, few publications were found on the broodstock nutrition requirement of largemouth bass. Thus, this study investigates the effects of dietary ARA levels on the reproductive performance of broodstock largemouth bass.
2. Material and Methods
Three isoproteic and isolipidic diets were formulated, each containing ~51% protein, 10% lipid, and 14% ash (Table 1). ARA oil, containing 40% ARA, was used to adjust the experimental ARA levels of the ARA0.9, ARA2.0, and ARA3.6 diets to 0.87%, 2.05%, and 3.64%, respectively (Table 2). While the optimal levels of dietary protein and lipid remain unclear, the levels in these experimental diets were based on the optimum requirement [21] for the growth of juvenile largemouth bass. Largemouth bass broodstock was procured from Guangzhou Rongda Co. Ltd. (Guangdong, China). The experiment was conducted at the aquaculture base of Guangzhou Rongda Co. Ltd.
| Ingredients (%) | Group | ||
|---|---|---|---|
| ARA0.9 | ARA2.0 | ARA3.6 | |
| Fishmeal | 54.0 | 54.0 | 54.0 |
| Krill meal | 10.0 | 10.0 | 10.0 |
| Soybean meal | 6.0 | 6.0 | 6.0 |
| Squid hydrolysate | 5.0 | 5.0 | 5.0 |
| Corn protein | 3.0 | 3.0 | 3.0 |
| Tapioca starch | 10.5 | 10.5 | 10.5 |
| Calcium dihydrogen phosphate | 3.0 | 3.0 | 3.0 |
| Choline chloride (60%) | 0.5 | 0.5 | 0.5 |
| Vitamin mixa | 2.5 | 2.5 | 2.5 |
| Mineral mixb | 1.5 | 1.5 | 1.5 |
| Lecithin | 1.0 | 1.0 | 1.0 |
| Fish oil | 2.0 | 2.0 | 2.0 |
| Sunflower oil | 1.0 | 0.5 | 0 |
| ARA oil (40%)c | 0.0 | 0.5 | 1.0 |
| Composition (%) | — | — | — |
| Humidity | 5.3 | 5.0 | 7.9 |
| Crude protein | 51.1 | 51.8 | 49.6 |
| Crude lipid | 8.9 | 10.9 | 10.6 |
| Ash | 13.9 | 13.6 | 13.8 |
- a,bVitamin and mineral mix, designed for the growth of largemouth bass and snakehead fish were offered by Guangdong HAID Group Co. Ltd.
- cARA-rich oil was purchased from CABIO Biotech (Wuhan) Co. Ltd.
| % of total fatty acid | Group | ||
|---|---|---|---|
| ARA0.9 | ARA2.0 | ARA3.6 | |
| ∑saturated fatty acida | 30.01 | 30.61 | 30.50 |
| ∑Monounsaturated fatty acidb | 23.49 | 23.26 | 24.15 |
| 18:2n-6 | 0.12 | 0.12 | 0.13 |
| 18:3n-3 | 2.80 | 2.74 | 2.74 |
| 20:4n-6 | 0.87 | 2.05 | 3.64 |
| 20:5n-3 | 7.12 | 7.24 | 7.10 |
| 22:6n-3 | 8.19 | 7.67 | 7.30 |
- aSaturated fatty acid: 12:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 22:0, 24:0.
- bMonounsaturated fatty acid: 14:1, 16:1, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9.
The experimental procedures were performed in strict accordance with the Management Rule of Laboratory Animals (Chinese Order No. 676 of the State Council, revised March 1, 2017). The study was conducted in accordance with the local legislation and institutional requirements. In December, the sexes of largemouth bass broodstock were checked by gentle abdominal massage. The brood fish were distributed into nine net cages, each measuring 4 × 3.3 m. Each cage contained 45 fish (15 males, average weight: 711 ± 151 g, 30 females, average weight: 680 ± 187 g), and each experimental diet was allocated to three cages (Table 3). The fish were fed to apparent satiation twice daily (8:30, 16:30), and water temperature was measured daily (the average temperature during the experiment was 14.2 ± 3.2 °C). Gonad samples were taken monthly from one male and one female in each group tank to measure changes in gonadosomatic index (GSI). On March 12, the experimental broodfish was fastened for 24 h and anesthetized with MS222. Each fish was individually weighted, and blood samples from the caudal vein, as well as gonad samples from three males and four females per group, were collected for analysis. The remaining fish of each group were sex-checked, combined, and transferred to a 10 × 10 × 0.6 m concrete pond. Palm tree skins (1 × 0.5 m) were placed at the edge of the pond as nests for spawning. Water was exchanged at a rate of 100% per hour, and temperature was monitored daily.
| Group | |||
|---|---|---|---|
| ARA0.9 | ARA2.0 | ARA3.6 | |
| Male (g) | 710.7 ± 154.2 | 720.0 ± 155.7 | 751.7 ± 198.0 |
| Female (g) | 681.7 ± 173.3 | 670.2 ± 164.6 | 663.9 ± 191.5 |
| Before spawning experiment | |||
| Male (g) | 722.2 ± 143.7 | 805.9 ± 147.8 | 838.2 ± 153.6 |
| Female (g) | 751.4 ± 145.9 | 716.0 ± 225.8 | 780.3 ± 148.4 |
| Feed consumption (g/fish) | 185.3 ± 28.9 | 150.4 ± 16.9 | 162.9 ± 18.9 |
- Note: The data are presented as mean ± standard deviation (S.D.).
From March 12 to April 27, nests with eggs were collected every morning and counted according to SC-T 2039-2007. Eggs in three 2 × 2 cm squares on each nest were counted to calculate the total number of eggs spawned. Egg samples from each group were randomly taken and transferred to 96-well ELISA microplates using a micropipette (0.7 mL of fresh water and one egg per well). Each well contained only one egg, and each egg was examined under a digital microscope to confirm fertilization. All eggs were cultured at 24 °C and checked every 24 h. The viable rate after 24 h, hatching rate, and larval survival rate before mouth opening (4 days after hatching) were recorded.
2.1. Egg Diameter
Twenty eggs per spawn were collected and photos were taken under the digital microscope with a stage micrometer. ImageJ (National Institutes of Health, US) was used in the measurement of egg diameter.
2.2. Chemical Composition, Amino Acid Composition, and Fatty Acid Composition Analysis
Crude protein, lipid, and ash was measured according to AOAC [22]. Amino acid was analyzed based on GB 5009.124-2016.2 g of homogenized and weighted sample was mixed with 15 mL of 6 mol/L HCL and 0.2 mL of phenol in a hydrolyze tube. Afterwards, the tubes were incubated in an oxygen-free environment in constant boiling 6 M HCl at 110°C for 22 h. After hydrolyzation, the solution was transferred to a 50 mL volumetric flask and made up to volume. 1 mL solution was evaporated to dryness under 50 °C. 2.0 mL pH 2.2 sodium citrate buffer solution was added to the tube and then filtered × 0.22 µm membrane. Amino acid analyzer was used for the analysis. Fatty acid profile was analyzed according to Hu et al. [23]
2.3. Sex Hormone Analysis
Level of T, estradiol, and vitellogenin in serum was measured with respective ELISA kit (PYRAM, Shanghai, China) according to the instructions from the manufacturer.
2.4. Data
Unless otherwise specified, data were shown as mean ± S.D. SPSS 16.0 for Windows (IBM, New York, USA) and were used in data analysis. Boxplot graph was used for detecting outliners, Shapiro–Wilk analysis for the normality, and Levene’s test for the homogeneity of variance for the data. Once these assumptions were met, Duncan’s test was then used for the analysis of the effect of dietary ARA on the spawning performance of largemouth bass, at a level of significance of 5%.
3. Results
3.1. Changes of GSI During Feeding Experiment
Changes in GSI of experimental largemouth bass brood fish from December to March were monitored (Table 4). From December to late January, the GSI of experimental female brood fish was around 6%. The ovary developed significantly in February (p < 0.05), when the GSI increased up to 13% and kept in March. The testis grew significantly in January (p < 0.05) and stayed stable during the experimental period.
| Sampling date | Male | Female |
|---|---|---|
| 12.18 | 0.53% ± 0.04%a | 6.58% ± 0.71%a |
| 1.21 | 0.91% ± 0.11%b | 6.41% ± 3.27%a |
| 2.24 | 0.97% ± 0.17%b | 13.14% ± 2.82%b |
| 3.12 | 0.98% ± 0.27%b | 12.61% ± 3.11%b |
- Note: The data are presented as mean ± standard deviation (S.D.) (n = 3). Different superscript indicates significant difference by Duncan’s test (p < 0.05).
3.2. Sexual Related Hormone Content in the Serum of Experimental Brood Fish
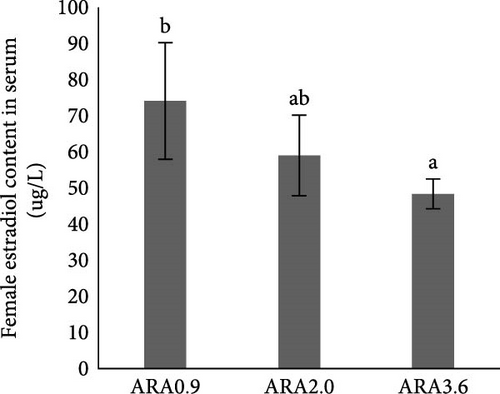
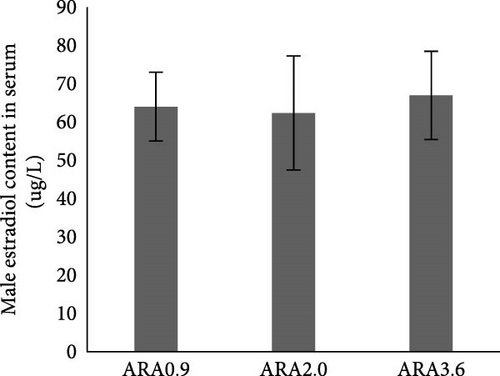
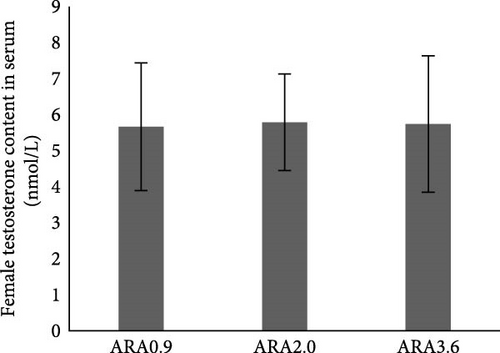
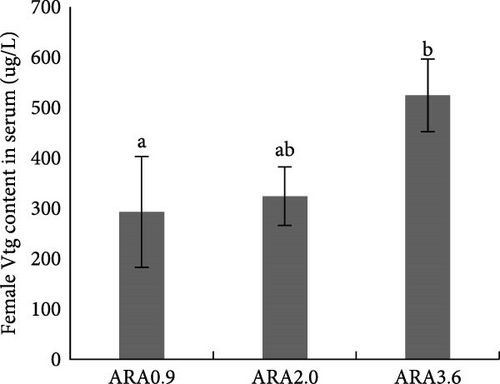
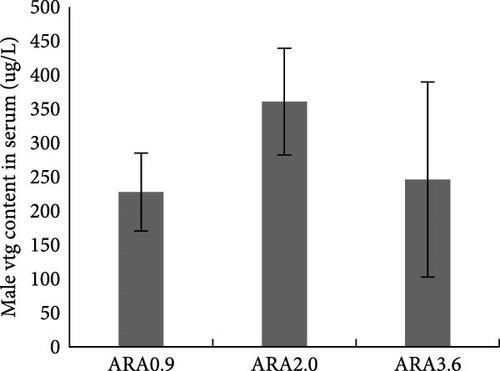
Estradiol content in serum was significantly higher in female brood fish of the ARA0.9 group than in the ARA3.6 group (p < 0.05, Figure 1A), but the vitellogenin content was significantly lower than ARA3.6 group (p < 0.05, Figure 1E). No significant difference was found in serum T content of female brood fish. Regarding the sexual hormone content in male broodfish, there was no significant difference between fish fed with different ARA content diets (Figure 1).

3.3. Spawning Performance and Diameter of Egg
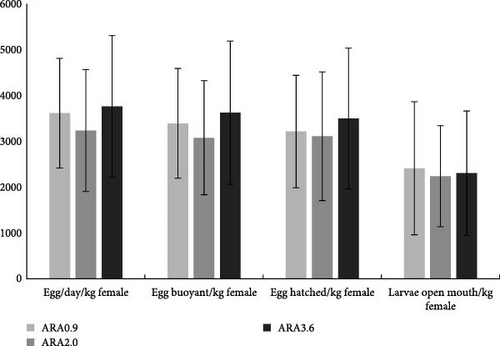
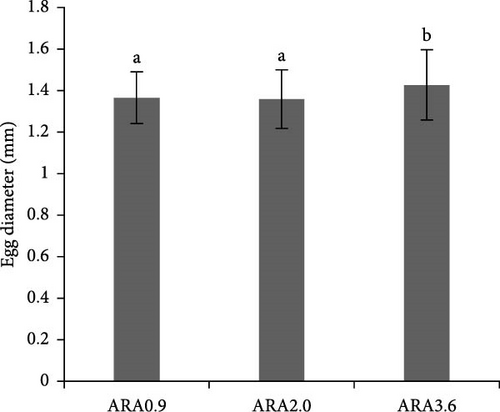
No significant difference was observed in the growth performance for both male and female broodstock (Table 3). Similarly, in the comparison of spawning performance parameters, no significant difference was observed between different experimental groups (Figure 2). However, the diameter of eggs from the ARA3.6 group was significantly higher than that from ARA0.9 and ARA2.0 groups (p < 0.05) (Figure 3).
3.4. Fatty Acid Composition
Regarding the fatty acids composition in broodstock (Tables 5 and 6), ARA content in both liver and gonad was higher in the group fed with a high ARA content diet. ARA3.6 group was significantly higher than that in the ARA0.9 group in both males and females (p < 0.05). Linolenic acid, 20:3n-6, and ARA content in the eggs from the ARA3.6 group was significantly higher than that from the ARA0.9 group and the ARA2.0 group (p < 0.05) (Table 5). Similarly, the egg docosahexaenoic acid (DHA) content of the ARA3.6 group was significantly higher than the ARA0.9 group (p < 0.05), regardless of the lower content of DHA in the ARA2.0 than in the ARA0.9 group. The content of oleic acid and eicosanoid acid was higher in the eggs from the ARA2.0 group than the ARA0.9 group (p < 0.05; Table 7).
| % of total fatty acid | Group | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| ARA0.9 | ARA2.0 | ARA3.6 | ARA0.9 | ARA2.0 | ARA3.6 | |
| ∑SFA | 25.53 ± 2.51 | 25.39 ± 1.74 | 25.91 ± 3.15 | 26.94 ± 1.02 | 26.5 ± 0.53 | 27.49 ± 1.47 |
| ∑MUFA | 34.12 ± 4.73 | 25.58 ± 5.53 | 26.02 ± 12.22 | 31.9 ± 0.62 | 24.56 ± 5.37 | 28.16 ± 1.46 |
| 18:2n-6 | 8.54 ± 1.05 | 8.97 ± 0.54 | 6.22 ± 2.86 | 12.15 ± 0.21 | 12.15 ± 0.49 | 10.21 ± 0.98 |
| 18:3n-3 | 0.71 ± 0.22 | 0.8 ± 0.03 | 0.71 ± 0.06 | 0.81 ± 0.08 | 0.77 ± 0.24 | 0.77 ± 0.14 |
| 20:4n-6 | 2.01 ± 0.51a | 3.47 ± 0.47ab | 5.17 ± 1.2b | 1.74 ± 0.13a | 2.89 ± 0.6b | 3.64 ± 0.09b |
| 20:5n-3 | 2.01 ± 0.69 | 3.56 ± 0.28 | 2.71 ± 0.52 | 1.35 ± 0.38 | 1.86 ± 0.25 | 1.63 ± 0.18 |
| 22:6n-3 | 17.45 ± 4.03 | 21.9 ± 3.39 | 21.3 ± 6.51 | 14.65 ± 1.2 | 20.1 ± 5.94 | 17.15 ± 1.06 |
- Note: Saturated fatty acid (SFA): 12:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 22:0, 24:0. Monounsaturated fatty acid (MUFA): 14:1, 16:1, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9. The data are presented as mean ± standard deviation (S.D.). Different lowercase indicates significant difference by Duncan’s test (p < 0.05).
| % of total fatty acid | Group | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| ARA0.9 | ARA2.0 | ARA3.6 | ARA0.9 | ARA2.0 | ARA3.6 | |
| ∑SFA | 29.23 ± 1.18 | 31.18 ± 1.01 | 31.81 ± 1.11 | 21.74 ± 0.34a | 22.76 ± 0.43b | 23.12 ± 0b |
| ∑MUFA | 32.81 ± 0.34 | 26.01 ± 7.19 | 20.51 ± 1.92 | 31.62 ± 0.71 | 29.62 ± 0.06 | 31.87 ± 1.9 |
| 18:2n-6 | 16.45 ± 0.78b | 13.05 ± 3.32ab | 8.27 ± 1.26a | 20.1 ± 1.56 | 20 ± 1.56 | 17.55 ± 1.63 |
| 18:3n-3 | 1.26 ± 0.12 | 0.93 ± 0.24 | 0.79 ± 0.12 | 1.94 ± 0.03 | 2.01 ± 0.21 | 1.8 ± 0.02 |
| 20:4n-6 | 1.49 ± 0.11a | 2.82 ± 1.05a | 5.84 ± 0.57b | 0.84 ± 0.03a | 1.06 ± 0.02a | 1.68 ± 0.23b |
| 20:5n-3 | 1.57 ± 0.1 | 2.77 ± 0.88 | 3.16 ± 0.57 | 1.42 ± 0.28 | 1.66 ± 0.21 | 1.62 ± 0.23 |
| 22:6n-3 | 9.75 ± 0.5 | 14.96 ± 7.27 | 18.1 ± 2.83 | 12.65 ± 0.35 | 12.95 ± 1.34 | 12.4 ± 1.98 |
- Note: Saturated fatty acid (SFA): 12:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 22:0, 24:0. MUFA (Monounsaturated fatty acid): 14:1, 16:1, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9. The data are presented as mean ± standard deviation (S.D.). Different lowercase indicates significant difference by Duncan’s test (p < 0.05).
| % of total fatty acid | Group | ||
|---|---|---|---|
| ARA0.9 | ARA2.0 | ARA3.6 | |
| ∑SFA | 34.3 ± 1.2 | 35.31 ± 0.37 | 33.92 ± 0.59 |
| ∑MUFA | 31.28 ± 0.38 | 29.22 ± 0.69 | 30.21 ± 1.55 |
| 18:2n-6 | 12.28 ± 0.45a | 11.65 ± 0.47a | 10.08 ± 0.64b |
| 18:3n-3 | 1.08 ± 0.06 | 1.09 ± 0.10 | 0.94 ± 0.10 |
| 20:4n-6 | 0.93 ± 0.14b | 1.31 ± 0.07b | 2.2 ± 0.33a |
| 20:5n-3 | 1.5 ± 0.1b | 1.95 ± 0.13a | 1.68 ± 0.28ab |
| 22:6n-3 | 16.69 ± 0.81b | 17.75 ± 0.45ab | 18.71 ± 1.31a |
- Note: Saturated fatty acid (SFA): 12:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 22:0, 24:0. MUFA (Monounsaturated fatty acid): 14:1, 16:1, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9. The data are presented as mean ± standard deviation (S.D.). Different lowercase indicates significant difference by Duncan’s test (p < 0.05).
4. Discussion
Research has shown that higher dietary ARA levels can significantly reduce serum estradiol levels in fish. For instance, in turbot broodstock, a high ARA diet (15.03% of TFAs) led to a notable decrease in estradiol production compared to the control group. Although the difference was not significant, the experimental group fed diet content of 5.63% ARA of TFA showed lower estradiol content than that fed with a diet content of 0.72% ARA of TFAs [24]. In this study, a similar trend was also shown that largemouth bass broodstock fed diet with higher ARA content had lower serum estradiol content. The reduction in estradiol is likely due to the role of ARA in the synthesis of PGs, which are involved in the regulation of hormone production. ARA can influence the activity of enzymes like aromatase, which converts androgens to estrogens [24]. However, the vitellogenin content in the serum of female broodstock fed with a high ARA content diet was significantly higher than in the serum of the broodstock fed with a low ARA content diet. The effect of dietary ARA on the serum vitellogenin content can be influenced by different factors, for instance, the stage of gonad development [25], and the optimum content of dietary ARA [26].
Given that extra ARA supplement in broodstock diet did not significantly affect the spawning performance and survival of the offspring larvae in this study, 0.9% ARA of TFAs in the diet of largemouth bass broodstock could be sufficient for reproduction. Similar results were reported in female Atlantic cod that the fecundity was not affected by dietary ARA levels from 0.5% to 4% [25]. The fertility of broodstock medaka fed with different ARA levels from 0.4% to 2.3% did not show any significant difference [27]. Absolute fecundity, relative fecundity, and hatching rate were not affected by dietary ARA level in female yellow catfish (Pelteobagrus fulvidraco) [17]. Whereas for other species, ARA content significantly affects reproductive performance. For instance, a positive correlation was found between ARA content in broodstock diet and hatching percentage in turbot [28]. 1% of the dry diet is optimum in the diet of sea urchin for growth performance, gonad development, and immunology [29]. These differences in dietary ARA content for broodstock could be related to the different ability of desaturation from C18 fatty acids to LC-PUFAs [30]. Previous studies on the LC-PUFAs requirements of juvenile largemouth bass have indicated that productive performance remains unaffected by diets containing varying ARA levels. For instance, Subhadra et al. [31] observed no significant difference in performance between fish-fed diets with 0%–2% ARA of TFAs. Similarly, Tidwell et al. [32] reported consistent productive performance across a broader range of 0.2%–28.9% ARA. Furthermore, Rossi Jr. and Habte-Tsion [33] highlighted that the essential fatty acid requirements of largemouth bass could be adequately met through the dietary supplementation of 18-carbon PUFA precursors. These findings, in together with the results of this study, suggests that the supplementation of ARA may not be strictly necessary for maintaining optimal productive performance in juvenile largemouth bass.
The size of an egg is closely related to the quality of the offspring. Larger eggs generally contain more nutrients, which can lead to better initial growth and survival rates for the hatchlings [34]. In this study, larger eggs were found from the broodstock fed with the highest ARA content diet. This influence of dietary ARA on fish egg size has been substantiated by several studies. Kowalska et al. [27] found that dietary ARA enhanced cyclooxygenase (COX) activity in medaka broodstock, which positively impacted egg size and larval survival. For Monodactylus sebae broodstock, adding 1% ARA concentrate oil to the diet significantly increased the diameter of the eggs, together with the increased hatch rates [35]. This phenomenon has been also found in other animals, for instance, in dairy cows, phosphocholine and LA participate in ARA metabolism in granulosa cells through the gut–follicle axis and regulates follicular development [36].
Regarding the fatty acid composition, the ARA content in the liver, gonad, and eggs increased with the ARA content in the broodstock diet. The eggs from high ARA content diet fed broodstock showed higher ARA content than those from the low ARA content diet group, which illustrated the nutrients that broodstock consumed did modify the nutritional composition of the broodfish and subsequently transfer to eggs. Similar results had also been found in blue gourami (T. trichopterus; Pallas, 1770) [13], and in yellow catfish (Pelteobagrus fulvidraco) [17]. Besides, in accordance with the research on blue gourami [13] and Guinean fingerfish [35], higher DHA content was found in the egg fed with a high ARA content diet in comparison with the egg from a low ARA content diet. This could be related to the preferentially transferred across the placenta of both DHA and ARA compared to other long-chain fatty acids [37]. As key components of cell membrane structure, ARA and DHA play a crucial role in the development of animals, particularly in neuron generation, growth, and immune system [38]. For instance, ARA supplements in diet increased the growth performance of different fish species, such as half-smooth tongue sole (Cynoglossus semilaevis) [39] and gilthead seabream (Sparus aurata) [40]. Thus, offspring from diet fed with ARA supplement diet might have a higher growth potential than those from broodstock fed diet without ARA supplement.
In conclusion, the dietary supplement of ARA did not affect the spawning performance and offspring viability of largemouth bass broodstock in this study. 0.9% ARA in TFA content in broodstock diet could be sufficient for the reproduction of largemouth bass. Considering the higher content of ARA, DHA level, and the diameter of the egg, 3.6% ARA of TFA can be added to the diet of largemouth bass broodstock for the potential growth performance of their offspring. In future research, the effect of dietary ARA for broodstock on the immune system and on the development and growth performance need to be conducted needs to be investigated to give a broader view on the effect of ARA on reproduction.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study “Effect of dietary arachidonic acid on the broodstock productive performance and egg quality of largemouth bass (Micropterus nigricans)” (3091729) was funded by 2020 Industrial Talent Policy Project Innovation Leading Team of Panyu District under grant number 2021-R01-4.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.




