Probiotics and Spirulina platensis Improved Growth Performance of Nile Tilapia (Oreochromis niloticus) by Upgrading Intestinal Morphology and Activating GH/IGF Axis
Abstract
Probiotics and spirulina are used to improve health, growth, and immunity of fish. The purpose of this study was to evaluate the effects of water additive probiotics and dietary Spirulina platensis on growth (weight gain (WG) and specific growth rate (SGR)), hematobiochemical parameters (hemoglobin (Hb) and blood glucose (Glu)), intestinal histology, and genes (growth hormone (gh) in pituitary and insulin-like growth factors: igf-1 and igf-2 in liver) expression profiles of Nile tilapia (Oreochromis niloticus). Nile tilapia was distributed to three treatment groups: Control (no probiotics or spirulina), Probiotic (probiotics 1.0 mL/L in water), and Spirulina (Spirulina platensis, 50 g/kg feed), each with three replicates for 42 days. Fish supplemented with probiotic and spirulina exhibited significantly higher (p < 0.05) WG (21.66 ± 2.92 g and 25.63 ± 3.92 g, respectively) and SGR (2.30 ± 0.16 and 2.18 ± 0.14%/day, respectively) than the control group. Hb and blood Glu levels showed negligible variation (p > 0.05) among the groups. The abundance of mucus producing goblet cells (GCs) was highest in fish reared with probiotic and spirulina treatments compared to the control group. Relative mRNA levels of gh in the pituitary were expressed profoundly (p < 0.05) in fish reared with probiotics and spirulina treatments compared to the control group. Relative mRNA levels of igf-1 in the liver expressed significantly higher (p < 0.05) in the fish fed with spirulina, whereas the relative mRNA levels of igf-2 expressed significantly higher (p < 0.05) in the fish subjected to probiotic treatment than control group. Principal component analysis (PCA) also supported the influence of probiotic and spirulina regarding growth performance over control treatment. These findings showed that probiotic and spirulina positively affect growth by improving intestine morphology and activating the GH/IGF axis in Nile tilapia.
1. Introduction
Globally aquaculture is progressively a significant source of animal protein [1]. However, intensive fish farming for maximizing the amount of yield per unit area creates many problems related to the environment [2], disease outbreaks, and the ingredients used for feed manufacture [3]. Aquaculture requires high-quality feeds rich in crude protein that must include essential nutrients and beneficial additives to ensure the health and growth of organisms [4, 5]. The insufficient supply and high cost of quality fish feed ingredients are the most critical problems that must be resolved by any possible means for better aquaculture outputs [4, 6]. Previous studies have demonstrated that the application of feed additives like probiotics and microalgae in aquaculture has shown to be an effective alternative to medicines and antibiotics, providing benefits without causing harm to the environment [7]. Efforts are ongoing to find out quality feed additives and alternative natural agents, which are vital to enrich the quality of feed and enhance healthy fish production in the aquaculture system [8]. Therefore, searching for suitable and sustainable feed additives and ingredients is an important approach to achieve continuous growth in aquaculture production.
Use of probiotics is an approach for improving intestinal environment, nutritional profiles, and an alternate viable therapeutic modality to overcome the adverse effects of toxicity, antibiotics, and drugs [9, 10]. Probiotics are microorganisms that provide health benefits to the host and are used in aquaculture as dietary supplements, enhancing growth factors, increasing immune function, and controlling disease [4, 11]. Probiotic supplements provide a multitude of other benefits, such as enhancing feed efficiency, supporting digestion through enzymatic activity, improving intestinal health by inhibiting pathogen growth through competitive exclusion for nutrients and adhesion sites, exhibiting antimutagenic and anticarcinogenic effects, secreting antimicrobial substances, neutralizing toxins, preventing and treating gastrointestinal infections, reducing intestinal inflammation, restoring normal gut mucosal function, regulating hypersensitivity reactions, and strengthening the immune response [12, 13]. The most common fish probiotics, including various genera, photosynthetic bacteria, yeast, and Bacillus, have been studied in aquaculture [1, 14–17]. Zhou et al. [18] found that using probiotics, specifically Bacillus coagulans B16 and Rhodopseudomonas palustris G06, as water additives can improve the immune and health status of Nile tilapia, leading to better growth performance. Therefore, probiotic supplementation could be a highly effective and sustainable solution for enhancing the gut health of fish by improving overall fish health and growth and contributing to a healthier and more environmentally friendly aquaculture system.
Spirulina is a filamentous algae that contains several components including phycocyanin, gamma-linolenic acid, beta-carotene, phenolic compounds, protein (up to 65%), along with a polyunsaturated fatty acid (PUFA), essential minerals (mainly, iron), B vitamins (mainly, riboflavin), essential amino acids, and a wealth of bioactive compounds [19], which all contribute as a strong antioxidant, antibacterial, immunostimulant, and disease-resistance agents [19, 20]. Additionally, spirulina can also reduce the expenses and risks associated with medication, environmental stressors, and diseases in aquaculture [20–22]. When used as a feed supplement, spirulina has demonstrated significant benefits in the growth, reproduction, carcass composition, immune responses, and disease resistance in various fish species, including common carp (Cyprinus carpio) [23], rainbow trout (Oncorhynchus mykiss) [24], and Nile tilapia (Oreochromis niloticus) [25]. Therefore, feed with naturally relevant compositions like spirulina seems to be an ideal candidate for boosting the growth and immunity in fish.
The intestinal barrier system relies on various components, including the mucus layer, epithelial layer, intercellular tight junctions, and the underlying lamina propria [26, 27]. Among these, the integrity of the mucus barrier produced by goblet cells (GCs) that secrete mucins serves as the main structural component of the mucus layer [28, 29]. These mucins are highly hydrophilic and can bind water to form a gel-like structure, which prevents direct contact between enterocytes and the intestinal contents, particularly pathogenic microorganisms [29]. If the mucus layer is absent or defective in the absence of GCs, a large number of bacteria can reach the epithelial cells, leading to an excessive immune response in the host and can play a role in pathogen antagonism [30, 31]. In a study by Harper et al. [32], feeding probiotics (Pediococcus acidilactici) to rainbow trout for 2 weeks showed a tendency to increase the number of GCs in the intestine, indicating that probiotics may promote the proliferation of GCs, thereby, enhancing mucus secretion and providing gut protection. Therefore, understanding the mechanism of GC proliferation and function in the intestine could lead to the development of increased immunity in fish in aquaculture.
A network of growth factors in the somatotropic axis such as GH, IGF-1, and IGF-2 intricately regulates fish’s growth characteristics and various physiological processes [33–37]. Previous study demonstrated that supplementing probiotic mixture significantly enhanced growth parameters, as well as the expression of growth hormone (GH), insulin-like growth factor-1 (IGF-1), and IGF receptor-1 (IGF-1R) genes in Nile tilapia [38]. This effect may be attributed to the probiotics’ ability to colonize and adhere to the intestinal wall [39], which boosts the secretion of digestive enzymes, thereby, improving metabolism and accelerating growth. Moreover, earlier studies had also demonstrated a positive correlation between the expression of gh, igf-1, and igf-2 with their diets when supplemented with spirulina in various fish species [40–43]. Spirulina supplementation also increases white blood cell count and regulates the expression of cytokine genes, which are essential signaling molecules for the immune system [44, 45].
The Nile tilapia, O. niloticus is a widely recognized aquatic product that has gained significant popularity in the global market. In 2020, global tilapia production reached nearly 7 million tons, ranking third among farmed finfish with a share of 8.33% [46]. However, tilapia growth is hampered by the limited availability of natural resources [47], necessitating the development of systems to boost production and productivity while using less water, space, and energy [46] and maintaining satisfactory economic returns. In our previous study, we found that Nile tilapia reared with probiotics at various temperatures, showed enhanced weight gain (WG), specific growth rate (SGR) and the overall physiological conditions and also a significant reduction in the frequency of cellular and nuclear abnormalities in erythrocytes compared to those without probiotics [48]. Considering all the prospects and addressing the challenges for the growth of Nile tilapia, selecting the appropriate feed ingredients or additives is a crying need. Therefore, this study is designed to have a comparative overview between Spirulina platensis and probiotics on Nile tilapia as feed additives. In this study, Nile tilapia supplemented with probiotics and spirulina as natural feed additives and their growth and immune responses are measured by evaluating the expression of growth-related genes and the number of GCs in the intestine in commercial farming systems.
2. Materials and Methods
2.1. Experimental Fish
A total of 180 uniform-sized and aged mono-sex Nile tilapia fries with an average weight (±standard deviation (SD)) of 3.49 ± 0.13 g and length (±SD) of 10.42 ± 0.45 cm were obtained from a local hatchery. The fish were acclimated in the Fish Ecophysiology laboratory at Bangladesh Agricultural University for 7 days.
2.2. Experimental Design
The experiment was conducted over a 6-week period, during which the fish were reared under three different conditions: Control, probiotics supplements in water, and fed with dietary spirulina (Spirulina platensis). Twenty acclimated fingerlings were placed in each 100 L aquarium, with three tanks randomly selected as replicates for each treatment group. Multispecies probiotics, developed in our laboratory, contained Bacillus subtilis (5 × 109 colony-forming unit; cfu/mL), Lactobacillus plantarum (5.8 × 109 cfu/mL), and L. buchneri (6.5 × 109 cfu/mL), were added to water at 1.0 mL/L [16, 17], named probiotics treatment. The required amounts of probiotics were added to the aquarium water every other day throughout the experiment. In another treatment, the fingerlings were fed with dietary spirulina (50 g/Kg feed by mixing with commercial feed) daily at a rate of 5% of their initial body weight, with the feeding amount adjusted every 2 weeks. The control group and the probiotic group fish were feed with commercial pellet feed (Mega feed Ltd, Bangladesh; protein 28%, carbohydrate 20%, ash 10%, fat 8%, fiber 3%, calcium 2%, and phosphorous 1%). Various water quality parameters, including temperature (°C), dissolved oxygen (DO; mg/L), pH, and ammonia (mg/L), were monitored throughout the experimental period and found nonsignificant (p > 0.05) differences among the treatments (Table 1). All experimental procedures strictly followed the guidelines approved by the animal welfare and ethics committee of Bangladesh Agricultural University (Approval Number: BAU-FoF/2021/002).
| Parameters | Treatments | ||
|---|---|---|---|
| Control | Pr. | Sp. | |
| Temperature (°C) | 31.40 ± 0.90 | 31.23 ± 0.94 | 31.46 ± 0.68 |
| pH | 8.60 ± 0.19 | 8.54 ± 0.43 | 8.52 ± 0.18 |
| NH3 (mg/L) | 0.28 ± 0.44 | 0.38 ± 0.14 | 0.34 ± 0.25 |
| DO (mg/L) | 6.02 ± 0.98 | 5.97 ± 1.03 | 5.90 ± 1.10 |
- Abbreviations: Pr, probiotics; Sp, spirulina.
2.3. Sample Collection
After the 42-day rearing period, the fish were anesthetized using clove oil (5 mg/L), and individual body weights (g) were recorded. Pituitary and liver samples (n = 6) were then collected, placed in RNAlater solution (Ambion, Austin, TX), and kept at 4°C for a day. The following day, the samples were transferred to the Molecular Biology and Biotechnology Laboratory at the Faculty of Fisheries, Chattogram Veterinary and Animal Sciences University, Chattogram, and stored at −80°C for further analysis. The entire intestinal tract, from the stomach to the anus, was carefully dissected from each fish under sterile conditions. A 0.5 cm section of the anterior intestine was then collected, stored in Buin’s fixative, and prepared for analysis of the number of GCs per area of the epithelium layer using transmission electron microscopy (TEM).
2.4. Growth Performance of Nile Tilapia
2.5. Haematobiochemical Parameters
The blood samples (n = 6 fish) were collected from the caudal blood vessels using a micropipette for measuring the hemoglobin (Hb; g/dL) and glucose (Glu; mg/dL) levels. Blood samples were instantaneously measured for blood Glu levels (mg/dL) and Hb levels (g/dL) by using Glu and Hb strips in a digital EasyMate GHb, blood Glu/Hb dual-function monitoring system (Model: ET-232, Bioptik Technology Inc., Taiwan 3505-7).
2.6. RNA Extraction and cDNA Synthesis
Total RNAs were extracted from the entire pituitary and liver using TRIzol reagent (Invitrogen, ThermoFisher, USA) according to the manufacturer’s instructions. The RNA pellet was dissolved in 20 μL of DEPC water and stored at −80°C for further analysis. The quality and integrity of the RNA were assessed using a Nanophotometer NP80 (Germany), with 260/280 ratio values around 2.0 being deemed acceptable for cDNA preparation. For the synthesis of the first strand of cDNA, 500–1000 ng of total RNA was used with an AddScript cDNA Synthesis Kit (Korea), following the manufacturer’s protocol. The reverse transcription reaction had a temperature profile of 25°C for 10 min, 50°C for 60 min, and 80°C for 5 min.
2.7. Real-Time PCR Assays of GH, IGF-1, and IGF-2 mRNAs
Quantitative real-time PCR was performed using a 7500 Fast Real-Time PCR System (Applied Biosystem, USA). Primers for GH, IGF-1, and IGF-2 gene expression analysis were chosen based on previous studies (Table 2). The PCR reaction mixture (10 μL) consisted of 1 μL of standard cDNA or sample, 0.4 μL of forward primer, 0.4 μL of reverse primer, 5 μL of Add SYBR Master (ADDBIO INC), and 3.2 μL of nuclease-free H2O. The amplification process included an initial step at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, 60°C for 30 s, and 72°C for 30 s. A melting curve analysis was conducted to verify the specific amplification of each cDNA. Gene expression levels were normalized using the 2−ΔΔCt method, with β-actin serving as the housekeeping gene. Each qPCR experiment was conducted in duplicate.
| Genes | Primer sequence | Accession number | Insert size | Tm (°C) | References |
|---|---|---|---|---|---|
| gh | |||||
| F | 5′CTGCTGATCAGGGCCAATC 3′ | M97766.1 | 192 | 58 | [49] |
| R | 5′ TCGACATTTAGCTACCGTCAGG 3′ | ||||
| igf-1 | |||||
| F | 5′ GTTTGTCTGTGGAGAGCGAGG 3′ | Y10830 | 97 | 58 | [50] |
| R | 5′ GAAGCAGCACTCGTCCACG 3′ | ||||
| igf-2 | |||||
| F | 5′GCTTTTATTTCAGTAGGCCAACCA 3′ | AH006117 | 90 | 58 | [51] |
| R | 5′CACAGCTACAGAAAAGACACTCCTCTA 3′ | ||||
| β-actin | |||||
| F | 5′ CGAGCTGTCTTCCCATCCA 3′ | AY116536 | 486 | 57 | [52] |
| R | 5′ TCACCAACGTAGCTGCTTTCTG 3′ | ||||
2.8. Histological Analysis of Intestine
For histological examination, the preserved intestine samples were dehydrated through a series of graded alcohol (70%, 80%, 90%, 95%, and 100%). After dehydration, the samples were embedded in paraffin wax and sectioned using a microtome (KD 2258, China). The sections were stained with hematoxylin and eosin. Once the stained sections had dried, an appropriate amount of DPX (Qualikems Fine Chemical PVT Ltd., India) was applied for mounting. The mounted slides were protected with a thin cover slip, allowed to harden, and then examined under a microscope (Carl ZEISS Microscope, Gmblt, Optica B-190 series, USA).
2.9. Statistical Analysis
The measured variables are presented as mean ± SD. To determine statistically significant differences among the treatments, data were initially analyzed using independent sample t-test for mean comparisons, performed in Microcal Origin (version 6.0). Unless otherwise specified, significant differences were indicated by p ≤ 0.05. Principal component analysis (PCA) was utilized to evaluate the overall impact of probiotics and spirulina on the growth parameters, expression of the observed genes and GC. A biplot was generated using the standardized scores of the first two principal components, which accounted for the most variation.
3. Results
3.1. Growth Performance of Nile Tilapia
In this study, probiotics and spirulina showed significant impacts on growth and feed utilization in Nile tilapia that are presented in Table 3. Fish reared with probiotics and spirulina have significantly higher (p < 0.01) WG and SGR compared to control during the 42 days of the experimental period. Moreover, a statistically (p < 0.05) reduced FCR were observed in fish reared with probiotics and spirulina when compared to fish maintained with control diets. An increased growth response was observed in probiotic treated groups compared to the spirulina, but no statistical significant differences (p > 0.05) were observed between probiotic and spirulina supplemented diet in growth performance. The survival rate of fish was not affected by different treatments.
| Parameters | Treatments | ||
|---|---|---|---|
| Control | Probiotics | Spirulina | |
| Initial body weight (g) | 3.50 ± 0.25 | 3.90 ± 0.43 | 3.97 ± 0.50 |
| Final body weight (g) | 19.23 ± 2.92 | 28.19 ± 3.69 ∗∗ | 25.63 ± 3.92 ∗ |
| Weight gain (WG; g) | 15.73 ± 2.92 | 24.29 ± 2.69 ∗ | 21.66 ± 2.92 ∗ |
| Specific growth rate (SGR; %/day) | 1.96 ± 0.14 | 2.30 ± 0.16 ∗ | 2.18 ± 0.14 ∗ |
| Feed conversion ratio (FCR) | 1.02 ± 0.34 | 0.68 ± 0.17 ∗ | 0.73 ± 0.21 ∗ |
| Survival rate (%) | 100 | 100 | 100 |
- Note: Values with different asterisk ( ∗∗p < 0.01 and ∗p < 0.05) indicates significant difference among different feeding trials. All values expressed as mean ± standard deviation (SD; n = 60).
3.2. Hematobiochemical Parameters
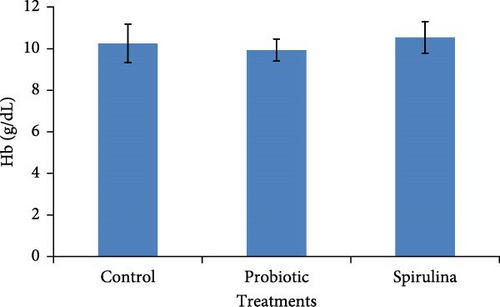
In the present study, we did not find any significant impact (p > 0.05) of probiotics and spirulina on the hematobiochemical parameters of Nile tilapia (Figure 1). A slightly elevated but statistically nonsignificant (p > 0.05) level of Hb was observed in response to spirulina fed diet compared to the control and probiotic-supplemention in Nile tilapia. In the case of Glu, probiotic-supplemented groups exhibit elevated but statistically nonsignificant (p > 0.05) levels in comparison with the control and spirulina diet. There was no statistical difference (p > 0.05) in the Hb and Glu levels among different treatments of Nile tilapia.

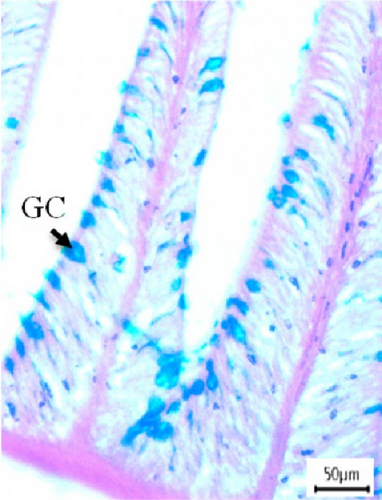
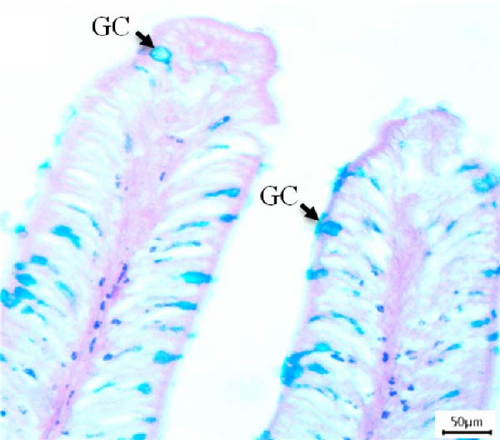
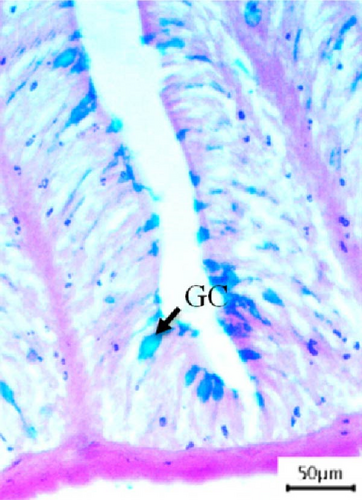
3.3. Histological Screening and Intestinal Morphometry
In this study, intestinal samples of Nile tilapiawere collected and the villi structure and presence of GCs were observed histologically from different groups (Figure 2). A significantly higher number of GCs was observed in the fish reared with probiotics (p < 0.001) and spirulina (p < 0.05) compared to control group. GC count was significantly higher (p < 0.01) in probiotics supplemented Nile tilapia compared to the spirulina supplemented groups.

3.4. Changes in the Expression of gh in the Pituitary
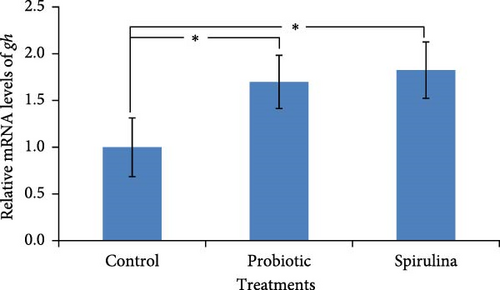
In the present study, relative expression level of genes for growth hormone (gh) at different feeds was measured from the pituitary of Nile tilapia after 42 days. Significantly (p < 0.05) higher expression of gh (1.7-fold higher in spirulina-fed fish and 1.8-fold in probiotic supplemented fish, respectively) was observed in the fish reared with probiotics and spirulina (Figure 3). No significant differences (p > 0.05) was observed between spirulina and probiotic treated groups.
3.5. Changes in the Expression of igf-1 and igf-2 in the Liver
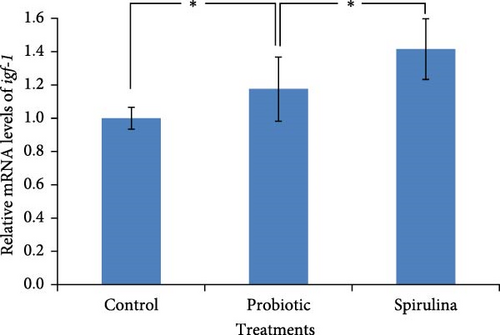
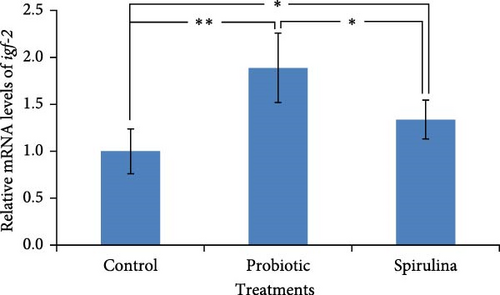
The relative expression of igf-1 mRNA was significantly (p < 0.05) higher (1.4-fold) in the fish of spirulina-fed diet compared to control feeding (Figure 4a). Additionally, a 1.2-fold increase (p < 0.05) in the expression of igf-1 was also observed in the probiotic supplemented groups. There were no significant differences between in the fish reared with probiotics and spirulina. On the other hand, the relative expression of igf-2 mRNA was 1.9-fold and 1.4-foldhigher (p < 0.05) in the fish of probiotic and spirulina treatment respectively compared to control (Figure 4b). Significant differences (p < 0.05) was also observed between probiotic and spirulina supplemented fish in the case of igf-2 mRNA with higher expression in probiotic groups.
3.6. PCA
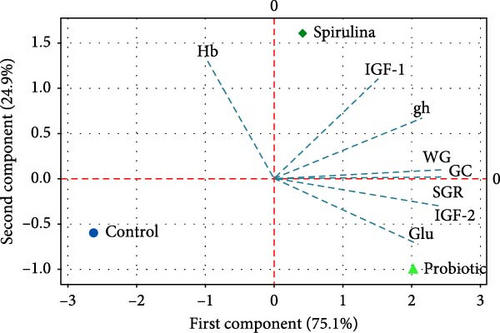
Figure 5 illustrates the PCA of gene expression, hematological parameters, growth parameters, and histology of Nile tilapia. The graph shows that the linear transformation of different parameters accounted for 100% of the variance in the first two components (PC1: 75.1% and PC2: 24.9%). The biplot indicates that the use of probiotics enhanced the expression of the igf-2 gene, SGR, and Glu levels in Nile tilapia. In contrast, the use of spirulina boosted the expression of igf-1 and gh genes, WG, and GC numbers in the regulation of growth and immunity in this studied species.
4. Discussion
The present study investigated the effects of probiotic and spirulina diets on the growth performance, hematological parameters, and gene expression in Nile tilapia (O. niloticus) and reveals significant differences in various parameters, highlighting the distinct impacts of probiotic and spirulina supplementation in dietary interventions. It is well known that the feed ingredients have a significant impact on fish growth, immunity, and overall well-being. The findings of the present study are in agreement with the previous studies [53, 54] about the effects of feed induced metabolic, physiological, and molecular enhancement in fish.
The present study found that Nile tilapia reared with probiotic supplemented diets exhibit significantly higher growth performance, improved WG and SGR compared to the control diets. This indicates that probiotics and spirulina may enhance nutrient absorption and utilization, promoting better growth in this fish. In addition, a reduced FCR observed in the spirulina and probiotic supplemented fish further supports that spirulina and probiotic also improves feed utilization efficiency. Moreover, the survival rate remained unaffected by the type of diet, indicating that both dietary interventions are safe and nonlethal to the fish over the experimental period. The result of our present study was in agreement with Aly, Mohamed, and John [53] who explored the benefits of using Bacillus pumilus as a probiotic in Nile tilapiaand found that a low dose of B. pumilus (106 g−1 in the diet) led to a significant increase in WG over a period of 2 months. Similarly, Mohamed, Fattah, and Eid [54] found that Nile tilapia fingerlings fed with probiotic-supplemented diets exhibited greater growth compared to those on a control diet. Taoka et al. [55] examined the impact of commercial probiotics (including B. subtilis, Lactobacillus acidophilus, Clostridium butyricum, and Saccharomyces cerevisiae) on the growth of Japanese flounder (Paralichthys olivaceus) and reported that adding these probiotics to the diet or rearing water enhanced the growth of the flounder. Abdelhamid, Khalil, and Seden [56] reported significantly higher growth performance, FCR, and survival rates in fish maintained on a probiotic-supplemented diet compared to those on a control diet, which also aligns with our study findings.
The hematological analysis revealed that Nile tilapiafed with spirulina diets had a higher Hb level compared to the control. This suggests that spirulina may enhance the oxygen-carrying capacity of the blood, potentially improving overall fish health and endurance. In addition, elevated levels of Hb and Glu levels was observed in probiotic supplemented Nile tilapia compared to the control. Sharma and Kumar [57] carried out a study evaluating various hematological parameters and found that Labeo rohita treated with L. acidophilus showed the highest percentage of Hb content. However, elevated Glu levels in the probiotic-supplemented fish may indicate enhanced metabolic activity and energy availability, which could contribute to the better growth performance in Nile tilapia. Increased Glu levels may also contribute to initiating the glycogenesis and glycogenolysis pathways, leading to an increase in metabolism and growth in Nile tilapia [58].
GCs throughout the intestine are responsible for producing and secreting the protective mucus, primarily composed of mucins (gel-forming glycoproteins) that cover the epithelial surface [59]. This mucus layer serves as a medium for protection from physical damage and harmful agents, lubrication, and transport between the luminal contents and the epithelial lining, forming an essential structural component of the intestine [60]. Thus, GCs play a significant role in the innate immune defense mechanism, intestinal homeostasis, activation of digestive enzymes, and overall gut health protection [61]. In this study, fish that were fed with probiotics and spirulina showed a higher abundance of GCs which is in agreement with Mohammadian et al. [62] who reported that fish-derived probiotics such as L. plantarum and Lactobacillus rhamnosus showed significant potential for enhancing digestive enzyme activity, immune responses, and the number of intestinal GCs in common carp (C. carpio). Additionally, it has been reported an increase in GC count in the intestine due to probiotic supplements in fish [63–65]. Moreover, Spirulina supplemented diet had well-structured enterocytes, a robust epithelial barrier, extensive GCs, and absence of luminal cell debris, along with no evidence of inflammation in stinging catfish (Heteropneustes fossilis, [66]) and Nile tilapia [45].
The endocrine response for growth involves GH and IGF, combined with environmental and genetic factors, could serve as an effective biomarker for growth-related studies [67]. The GH-IGF axis serves as the primary pathway for growth and tissue proliferation in most vertebrate species. Research has indicated that the growth-promoting effects of various feed additives impact growth factors and growth hormones either directly or indirectly [68]. According to the current “somatomedin hypothesis,” the tissue secretion of IGFs promotes the growth-inducing effects of pituitary growth hormone through paracrine, autocrine, or endocrine mechanisms [69]. In this study, we found that the expression levels of growth-related genes (gh, igf-1, and igf-2) varied significantly between the dietary supplements. Spirulina-fed fish showed a significantly higher expression of gh in the pituitary, indicating a potential stimulatory effect of spirulina on growth hormone production compared to the control diet. Furthermore, the expression of igf-1 mRNA in the liver was highest in the spirulina group, suggesting that spirulina enhances the igf-1 signaling pathway, which is crucial for growth and development. Findings of the present study are consistent with previous research where fish fed a diet containing spirulina demonstrated significant increases in the expression of gh, igf-1, and igf-2 in brain, liver, and muscle tissues compared to the control group [42, 70–73]. The present study found that the probiotic diet resulted in significantly higher expression of igf-2 in the liver compared to both control and spirulina diets. Our findings align with another experiment that demonstrated the ability of probiotic to modulate the expression of local growth factors (IGF-1 and IGF-2) and receptors (GHR-1 and GHR-2), which are essential for binding growth factors and hormones (growth hormone and steroid hormones), potentially leading to improved growth [69].
The PCA results, depicted in Figure 5, provide a comprehensive overview of the variations in gene expression, hematological parameters, and growth performance of Nile tilapia supplemented with probiotics and spirulina. The first two principal components (PC1 and PC2) accounted for 100% of the variance, with PC1 explaining 75.1% and PC2 explaining 24.9%. The biplot illustrates distinct clustering based on diet types, with probiotics enhancing igf-2 expression, SGR, and Glu levels, while spirulina boosted igf-1 and gh expression, WG, and GC count. These findings align with the individual analyses, corroborating the differential effects of probiotics and spirulina on Nile tilapia.
5. Conclusion
The study demonstrates that both probiotics and spirulina significantly influence the growth performance, hematological parameters, and gene expression in Nile tilapia compared to the control, albeit through different mechanisms. Probiotics primarily enhance growth performance and feed efficiency, likely through improved nutrient utilization and metabolic activity. Spirulina, on the other hand, appears to improve hematological health and stimulate growth-related gene expression. These insights suggest that both dietary interventions can be beneficial for Nile tilapia aquaculture, depending on the specific production goals. Further research is warranted to explore the long-term effects and potential synergistic benefits of combining these dietary strategies in this commercially important fish species.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by the Researchers Supporting Project number (RSP2025R144), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors would like to express their sincere appreciation to the Researchers Supporting Project Number (RSP2025R144) at King Saud University in Riyadh, Saudi Arabia.
Open Research
Data Availability Statement
The data will be available upon request to the corresponding author (Md Mahiuddin Zahangir, Email: [email protected] and Md Shahjahan, Email: [email protected]).




