Impulsivity in Male Episodic Cluster Headache
Abstract
Background: Cluster headache (CH) is the most prevalent trigeminal-autonomic cephalalgia. Research evidence supports the hypothesized involvement of the posterior hypothalamus, the trigeminal-vascular system, and other central pain-processing regions in the pathogenesis of pain. Because of the role of the hypothalamus, CH patients should be at greater risk of developing an altered emotional response. Impulsivity is associated with depression, bipolar disorders, suicide attempts, and addictive disorders, which can be frequent in CH.
Objective: Our objective is to evaluate the prevalence of impulsivity in CH patients.
Methods: This is a cross-sectional observational study. Barratt Impulsiveness Scale (BIS-11) was administered to evaluate impulsivity.
Results: Fifty CH patients outside the bout and 60 matched controls were included. Patients were recruited from an outpatient headache unit. The percentage of episodic CH patients with a diagnosis of impulsivity (BIS-11 ≥ 73) was 14.2% compared to 1.6% in the control group (p = 0.02). The mean score on the BIS-11 was 58.5 (SD: 14.3) in the case group and 57.1 (SD: 9.2) in the control group. Although the global score on the scale did not differ between both groups, there were differences in cognitive (16.2 [SD: 4.4] vs. 14.5 [SD: 3.5]; p = 0.01) but not in motor and nonplanning impulsivity.
Conclusion: Our findings suggest that CH patients have greater cognitive impulsivity. If impulsivity plays an important role in the risk of suicide and substance use disorders, early detection and an effective multidisciplinary management could reduce CH-related burden and impact.
Summary
- •
Because of the role of the hypothalamus in the pathogenesis of cluster headache (CH), patients should be at greater risk of developing an altered emotional response.
- •
Impulsivity is associated with depression, bipolar disorders, suicide attempts, and addictive disorders, which can be frequent in CH, so we hypothesized that CH patients are more impulsive than healthy controls.
- •
Our findings suggest that CH patients have greater cognitive impulsivity, so early detection and effective multidisciplinary management could reduce CH-related burden and impact.
1. Introduction
CH, widely described as the most painful condition a human being can experience or as the “suicide headache,” is the most prevalent trigeminal-autonomic cephalalgia. It affects approximately 0.1% of the population, and it is four times more common in males. It has a typical age of onset between 20 and 40 years [1, 2].
According to the International Classification of Headache Disorders (ICHD) [3], CH is described as attacks of severe, strictly unilateral pain which is orbital, supraorbital, temporal, or in any combination of these sites, lasting 15–180 min and occurring from once every other day to eight times a day. The pain is accompanied by marked ipsilateral autonomic symptoms (conjunctival injection, lacrimation, nasal congestion, rhinorrhea, forehead and facial sweating, miosis, ptosis, and/or eyelid oedema) and/or with restlessness or agitation.
The regularity and seasonality of CH attacks are characteristic. Headache attacks occur with regular circannual (mainly in the autumn and spring) and circadian timing. They recur over periods of weeks to months (cluster period or bout) separated by attack-free remission periods lasting months to years. Based on this duration and frequency of episodes and remissions, CH is divided into episodic and chronic subtypes. In this episodic form of CH, which comprises approximately 80% of cases, cluster periods are separated by pain-free remission periods of 3 months or more [3, 4].
The pathophysiology of CH remains poorly understood. The question of whether CH pain is of peripheral or central origin remains a matter of debate, and both possibilities are considered. Research evidence supports the hypothesized involvement of the posterior hypothalamus and trigeminal-vascular system (TVS) in CH pathophysiology. During attacks, TVS and trigeminal-autonomic reflex become activated under fluctuating control from the hypothalamus and other central pain-processing regions including the occipital, cerebellar, and salience network [4, 5].
In patients with CH, there are not only biological but also psychosocial factors underlying the disorder, which have not been sufficiently investigated [6]. CH not only significantly affects the patient’s life at a professional level but also at a family and social level because psychological comorbidities such as anxiety, depression, and suicidality increase continuously with increasing delays in diagnosis. In this regard, a recent study analyzing the psychosocial aspect of CH found suicidal ideation in more than 40% of the patients included. Added to this situation is the hopelessness, rumination, and feeling of helplessness that many patients report when they cannot control their pain and, ultimately, their lives [7].
Impulsivity is associated with depression, bipolar disorders, suicide attempts, and addictive disorders, which can be frequent in CH [8–14]. Nevertheless, the prevalence of impulsivity has not been studied in this subgroup of patients. Based on all referred previously, the hypothesis of this study is that patients with episodic CH are more impulsive than healthy controls. The main objective of this study is to evaluate the prevalence of impulsivity in patients with episodic CH. The secondary objectives are to analyze the demographic and clinical variables that could be related to impulsivity and to determine if there is any correlation with comorbid anxiety-depressive syndrome.
2. Methods
2.1. Participants and Design
This is a cross-sectional observational study. Patients with the diagnosis of episodic CH from the headache unit of a tertiary care center will be included. Control subjects were recruited from local communities, randomly selected, and matched for sex and age with the case group. Recruitment of both groups was performed from September 2023 to February 2024.
Inclusion criteria were (1) age older than 18 years and (2) diagnosis of episodic CH according to the ICHD-3 criteria (3). Exclusion criteria were (1) serious psychiatric or cognitive disorder that implies the use of medication, (2) inability to communicate in Spanish or to sign an informed consent form, and (3) refusal to participate in the study. None of the patients were in an active bout or taking preventive treatment.
Demographic (sex and age) and clinical characteristics (comorbid conditions, concomitant treatments, time of evolution, laterality, and autonomic symptoms) were collected.
Barratt Impulsiveness Scale (BIS-11) Spanish version [15–17] and Hospital Anxiety and Depression Scale (HADS) [18] were administrated to evaluate impulsivity and comorbid anxiety–depression. The Headache Impact Test (HIT-6) [19] and EuroQol-5 Dimension (EQ-5D) [20] were administrated too to evaluate the impact of headache on quality of life.
2.2. Impulsivity Assessment
2.2.1. The Barratt Scale (BIS-11)
BIS-11 was developed in 1959 as an instrument to assess personality and behavioral constructs related to impulsivity [15]. It was revised in 2009 and is recommended as a reliable tracking instrument. It was designed to be a “multifaceted” measure of trait impulsivity based on anxiety and sensation/thrill-seeking research [16].
It is a self-report questionnaire. It comprises 30 questions, which cover six first-order factors: attention (having difficulty concentrating), motor impulsivity (doing things without thinking about them first), self-control (having difficulty refraining from certain actions and like spending money), perseverance (giving up on things quickly), cognitive instability (having extraneous thoughts when thinking), and cognitive complexity (having difficulty focusing on a problem). These 30 items can be grouped into three domains: attentional/cognitive (inattention and cognitive instability), motor (motor impulsiveness and lack of perseverance), and nonplanning (lack of self-control and intolerance of cognitive complexity) impulsivity. Scores are calculated by frequency attributions that range among rarely/never (1), occasionally (2), often (3), and almost always/always (4).
The higher the total BIS-11 score, the higher the impulsivity level of the patient. Its score ranges between 30 and 120, with a value ≥ 73 being indicative of impulsiveness, according to various previous studies [16, 17].
2.3. Comorbid Anxiety and Depression Assessment
2.3.1. HADS
This is a 14-item self-assessment questionnaire used to screen for symptoms of anxiety and depression in nonpsychiatric settings. It consists of a depression subscale (HADS-D, seven items) and an anxiety subscale (HADS-A, seven items). The total score on each subscale ranges from 0 to 21 points, with scores > 8 indicating clinically relevant depression or anxiety [18].
2.4. Headache Impact Assessment
2.4.1. HIT-6
The items of the HIT-6 cover several health-related quality of life domains: pain, social functioning, role functioning, vitality, cognitive functioning, and psychological distress. Each item is answered on a 5-point Likert scale (6 = never, 8 = rarely, 10 = sometimes, 11 = very often, 13 = always). The final score is obtained from the simple summation of the six items. The HIT-6 total score ranges between 36 and 78, with larger scores reflecting greater impact. Four groups have been derived to aid in the interpretation of HIT-6 scores: scores ≤ 49 represent little or no impact, scores between 50 and 55 represent some impact, scores between 56 and 59 represent substantial impact, and scores ≥ 60 indicate severe impact [19].
2.5. Quality of Life Assessment
2.5.1. EQ-5D
The EQ-5D is a standardized preference-based instrument designed to provide an indication of health-related quality of life. It is easy and simple to use and is designed for self-completion with accompanying instructions to respondents. It is based on a descriptive system that comprises five dimensions of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has five response options: no problems, slight problems, moderate problems, severe problems, and extreme problems [20].
2.6. Data Analysis
Statistical analysis will be performed using the Statistical Package for the Social Sciences (SPSS; Version 23.0). The data will be summarized using descriptive statistics. Normality was tested using the Kolmogorov–Smirnov test. Qualitative variables were expressed as frequencies and percentages, quantitative variables as mean and standard deviation (SD), or median and interquartile range (IQR) for not normally distributed data. Qualitative variables were compared using the chi-squared test, and quantitative variables were compared using Student’s t-test for independent samples or the Mann–Whitney U test, depending on the normality of the distribution. Pearson’s coefficient was applied to determine correlations. For all tests, a two-tailed p value of < 0.05 was considered statistically significant.
2.7. Ethical Considerations
The study is approved by the Local Ethics Committee of Clinical Research based at the Aragon Institute for Health Research (IIS Aragon) with Reference Number PI23/123. It will be conducted in accordance with the Declaration of Helsinki and reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement. Patient anonymity and compliance with the Data Protection Laws were maintained at all times.
3. Results
Fifty male patients with a mean age of 51.3 (SD: 11.4) years were included in the case group and 60 healthy male patients with a mean age of 48.1 (SD: 10.7) years in the control group. There were no missing data for any of the study variables.
The main demographic and clinical characteristics of participants are shown in Table 1. There were no statistically significant differences between groups in this regard except for smoking, psychiatric comorbidity, and quality of life. The mean of CH patients on the anxiety subscale was 8.4 (SD: 4.6), compared with 6.1 (SD: 3.1) for controls (p = 0.005). Regarding the score on the depression subscale, the mean in the patient group was 5.7 (SD: 4.1) compared to that of the control group (4.1, SD: 3.3) (p = 0.03).
| Variable | Cases (N = 50) | Controls (N = 60) |
|---|---|---|
| Age (years), mean ± SD | 51.3 ± 11.4 | 48.1 ± 10.7 |
| Male gender, n (%) | 50 (100) | 60 (100) |
| Smoking, n (%) | ||
| Nonsmoking | 21 (42) | 22 (36.6) |
| Past smoking | 6 (12) | 13 (21.6) |
| Current smoking | 23 (46) | 7 (11.6) |
| Education, n (%) | ||
| University | 10 (20) | 12 (20) |
| High school | 18 (36) | 25 (41.7) |
| Elementary and middle school | 22 (44) | 23 (38.3) |
| Disease duration (years), mean ± SD | 9.7 ± 7.5 | |
| Cluster free period (months), median (IQR) | 4 (3–9) | |
| Clusters per year, median (IQR) | 1 (1–2) | |
| Attacks per cluster, mean ± SD | 1.5 | |
| Attacks duration (min), median (IQR) | 30 (20–39) | |
| Intensity of pain (NRS), median (IQR) | 10 (9–10) | |
| Right-side pain, n (%) | 25 (50) | |
| Tearing, n (%) | 29 (58) | |
| Ptosis, n (%) | 27 (54) | |
| Miosis, n (%) | 21 (42) |
- Note: Continuous data is represented in median (IQR) or mean (SD), and categorical data is represented in counts (percent).
- Abbreviations: IQR, interquartile range; min, minutes; NRS, numeric rating scale; SD, standard deviation.
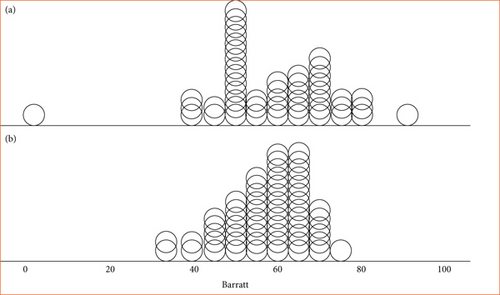
The percentage of episodic CH patients with a diagnosis of impulsivity (BIS ≥ 73) was 14.2% compared to 1.6% in the control group (p = 0.02). The mean score on the Barratt scale was 58.5 (SD: 14.3) in the case group and 57.1 (SD: 9.2) in the control group. Figure 1 shows the distribution of BIS-11 scores in the two groups. Although the global score on the scale does not differ between both groups, there are differences in cognitive impulsivity (16.2 [SD: 4.4] vs. 14.5 [SD: 3.5]; p = 0.01). Table 2 shows the comparative study of the different variables between the group of patients with CRE and the control group. There was no correlation between impulsivity, HADS score, nor any clinical variables.
| Tests | Cases (n = 50) | Control (n = 60) | p value |
|---|---|---|---|
| HADS-A, mean ± SD | 8.44 ± 4.64 | 6.18 ± 3.16 | 0.005 ∗ |
| HADS-D, mean ± SD | 5.74 ± 4.12 | 4.16 ± 3.37 | 0.03 ∗ |
| EuroQol5D, mean ± SD | 70.23 ± 22.25 | 85.71 ± 8.28 | 0.007+ |
| HIT6, mean ± SD | 57.08 ± 9.58 | ||
| BIS-11, mean ± SD | 58.51 ± 14.39 | 57.18 ± 9.29 | 0.28 ∗ |
| Motor impulsivity | 18.40 ± 4.72 | 17.96 ± 4.39 | 0.31 ∗ |
| Cognitive impulsivity | 16.28 ± 4.46 | 14.57 ± 3.55 | 0.01 ∗ |
| Nonplanning | 23.62 ± 6.49 | 24.39 ± 5.35 | 0.25 ∗ |
- Note: Continuous data is represented in mean (SD).
- Abbreviations: BIS-11, Barratt Impulsiveness Scale; EuroQol5D, European Quality of Life-5 Dimensions; HADS-A, Hospital Anxiety Scale; HADS-D, Hospital Depression Scale; HIT-6, Headache Impact Test; SD, standard deviation.
- ∗Statistical significance assessed with the unpaired t-test.
- +Statistical significance assessed with unpaired Mann–Whitney U test.
CH patients with BIS ≥ 73 did not present significant differences in anxiety-depressive comorbidity and other clinical and demographic variables. However, scores in each of the subdomains were higher in relation with those patients without impulsivity.
4. Discussion
The main objective of this study was to evaluate the prevalence of impulsivity in patients with episodic CH. Secondary objectives were to analyze the demographic and clinical variables that could be related to impulsivity and to determine if there is any correlation with comorbid anxiety-depressive syndrome. Even though we found a larger prevalence of high scores on BIS-11 in the CH patients (14.2%), the mean global score (except for the cognitive domain) did not differ in the two groups. In the subgroup of CH patients with impulsivity, we did not find a higher rate of anxiety, depression, or differences in quality of life. These patients also scored higher in all three domains.
Patients with CH frequently experience headache-related disabilities, decreased quality of life, economic burdens, and job-related issues [2]. The overall impact of CH is not limited to the pain experienced during attacks. Interictal burden, such as emotional aspects like anxiety, worry about future attacks and avoidance of triggers, significantly contribute to the overall burden of the disease [21–23]. Anxiety–mood spectrum disorders and psychopathological symptoms are usually comorbidities in CH patients [8, 9]. Personality disorders and substance abuse/addiction have showed associations with CH too. Many CH patients have a lack of sleep due to nocturnal attacks, potentially contributing to depressive symptoms [10]. In addition, CH patients are able to perceive other people’s or selves’ feelings but have more difficulties than healthy individuals at recognizing beliefs [24].
Although it could be difficult to define rigorously a personality profile for CH patients, it seems that some personality traits belonging to Cluster A are quite common among CH patients. Cluster A comprises odd or eccentric traits and reveals a rigid way of thinking and feeling. Most patients diagnosed with CH exhibit a paranoid–schizoid position that may influence their response to pain. Several authors have also described personality traits belonging to Cluster C in CH patients, with traits of dependency, inability to reveal their feelings and ambitions, hypochondriasis, hysteria, and obsession [9].
The concept of impulsivity covers a wide range of actions that are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation and that often result in undesirable outcomes. Various operational definitions of impulsivity have been proposed. It can be defined as a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions to the impulsive individuals or to others. Impulsive behavior almost always responds to three underlying mechanisms: reward-seeking personality, acting without planning, and compulsively seeking relief from tension or stress [25]. It is also associated with anxiety and depression, which are psychiatric conditions common in CH patients, and is an important risk factor for suicidality and the development of substance abuse [26, 27].
We have to differentiate cognitive (or choice) from motor (or behavioral) impulsivity. Cognitive impulsivity is considered the inability to weigh the consequences of immediate and future events and delay gratification. Lesion studies have suggested the ventromedial prefrontal cortex as the main area involved in this type of impulsivity. On the other hand, motor impulsivity is defined as the inability to inhibit immediate behavior, and it is associated with impairments to the dorsolateral prefrontal cortex [28]. The human prefrontal cortex is thought to participate in pain modulation and belong to the general pain-processing network in CH [4]. Although the prefrontal cortex has been studied for its role in impulse control, the hypothalamus plays a significant role in regulating cognitive impulsivity by impairing the brain’s ability to modulate cognitive and behavioral responses to pain and stress. This influence is mediated through neural circuits and hormonal pathways implicated in stress response and emotional regulation [4].
Specific forms of impulsivity correlate with the psychopathic trait. Therefore, we did not expect to find differences in the Barratt scale score between the two groups. However, the stressful complications and the high frequency of suicidal ideation in these patients could be justified by a higher score in the cognitive domain [29]. In this sense, the BIS has been widely applied in research related to Axis I disorders, in people with suicidal intent, as well as in those with substance abuse [16].
Sex differences are present in personality traits and behaviors, such as impulsivity. However, work investigating sex differences in impulsive action in both animals and humans has shown mixed results. In laboratory animals, males tend to be more impulsive than females, especially when sex hormones are taken into account [30]. Circulating levels of testosterone may contribute to the greater impulsivity observed in males, but ovarian hormones also play a role [31]. In this sense, females display fluctuating levels of impulsivity dependent on cycle phase and estrogen levels [32].
Even when men and women perform comparably in impulsivity tasks, different neurobiologies may underlie the behaviors. Men tend to show more activation in the lentiform nucleus, parahippocampal gyrus, posterior and anterior cingulate cortices, middle and medial frontal cortices, and thalamus compared to women, despite similar performance on stop-signal task [33].
The pathophysiology of CH is not completely clarified, but the role of areas involved in cognitive and affective responses, like the hypothalamus, insula, amygdala, and anterior cingulate and prefrontal cortex, is unquestionable [4, 29, 34, 35]. This has led to investigating intelligence, personality, and executive and memory function in these patients [9, 36]. Regarding the neurobiological substrate of impulsivity, patients show dysfunction in the pathways that mediate the generation and regulation of emotions, such as corticolimbic and corticostriatal pathways. This dysfunction is mediated by alterations in the main modulatory neurotransmitters and by changes in the white and gray matter. Impulsive patients display alterations in emotional regulation in response to external or internal stimuli, and pain is a negative one. It could be hypothesized that there is a connection between the neurobiological basis of impulsivity and that of cluster pain.
This is a single center study with a small sample size. Outcomes of the study should be re-examined with larger participants in order to strengthen the results. As all patients come from a specific population, a selection bias could exist. Scales are based on the patient’s self-report; therefore, there are always possibilities of irrelevant or misleading answers. Furthermore, other pain conditions can influence the results. Suicidal thoughts, substance use disorders, and insomnia should also be assessed in our study population; this would be an important topic for future research. In further studies, patients with chronic CH could also be included to investigate impulsivity in this subgroup.
To sum up, our findings suggest that CH patients have greater cognitive impulsivity. If impulsivity plays an important role in the risk of suicide and substance use disorders, early detection and effective multidisciplinary management could reduce CH-related burden and impact. Further studies are needed to investigate the hypothetical relationship between the neurobiological basis of impulsivity and pain, especially chronic pain, as well as its influence on the emergence of dysfunctional patterns in pain management.
Nomenclature
-
- CH
-
- cluster headache
-
- BIS-11
-
- Barratt Impulsiveness Scale
-
- ICHD
-
- International Classification of Headache Disorders
-
- HADS
-
- Hospital Anxiety and Depression Scale
-
- HIT-6
-
- the Headache Impact Test
-
- EQ-5D
-
- EuroQol-5 Dimension
-
- SD
-
- standard deviation
-
- IQR
-
- interquartile range
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




