Disconnective Approach Leads to Superior Seizure Outcome Compared to Other Hemispheric Procedures—A Meta-Analysis
Abstract
Hemispherectomy is the most promising treatment for patients with severe hemispheric intractable epilepsy. Several techniques for this surgical intervention have been established, but the choice of technique is currently mostly dependent on the surgeon’s experience with a specific approach. We aim to demonstrate whether the choice of the surgical technique moderates surgical outcome in patients with severe hemispheric intractable epilepsy, as measured by seizure freedom and the incidence of death after surgery. We extracted 2382 articles from PubMed and Cochrane. Two independent experts selected 555 articles. We performed a meta-analysis for all studies and a pooled data analysis for studies where information on individual patients was available. None of the retrieved studies was randomized. Disconnective surgery yielded significantly higher rates of seizure freedom (0.83) than resective (0.70, p < 0.001) or combined surgery (0.64, p < 0.001) for patients with at least 1 year follow–up (N cases = 1165). For death (N cases = 1197), resective surgery had the highest rate of death within a year (0.07), significantly higher than disconnective surgery (0.012, p = 0.001) and combined surgical techniques (0.006, p < 0.001). The assessed techniques did not systematically differ in rate of acute complications, but in their type, for example, acute neurological complications were most common after disconnective surgery (p < 0.001), unspecific symptoms after resective surgery (p < 0.004). Chronic neurological complications were most common after resective surgery (p < 0.001). Seizure freedom is more likely following disconnective surgery as compared to resective or combined techniques. Disconnective and combined surgical techniques lead to fewer chronic complications and death than resective approaches.
1. Introduction
Hemispheric surgery is the most promising treatment for patients with severe drug-resistant epilepsies associated with large unihemispheric lesions [1]. Different surgical approaches have been developed over time (Figure 1).
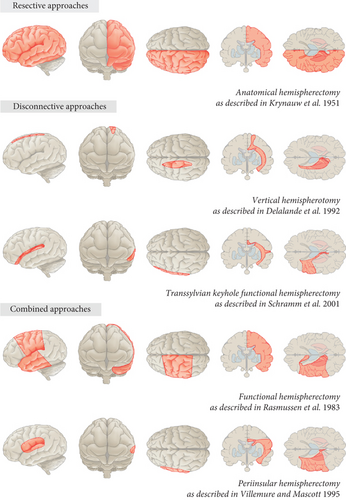
The primary approach was the anatomical hemispherectomy, the use of which was first described in epilepsy patients by Krynauw [2]. This intervention comprises the en bloc resection of the affected hemisphere, with or without sparing the basal ganglia, hypothalamus, and diencephalon. Although showing favorable seizure control, a high rate of surgical complications, including hydrocephalus, and a high mortality rate became evident over time [3–6]. This is likely caused by the large proportion of denuded subcortical tissue and a high volume of parenchyma-free intracranial space resulting in microhemorrhage and subdural membrane formation, which is referred to as superficial cerebral hemosiderosis [3]. One solution to avoid superficial hemosiderosis was the development of hemidecortication, removing all of the cortex while minimizing exposure of the ventricular system by maintaining surrounding white matter [7, 8]. Besides these two purely resecting approaches, further surgical alternatives have been developed. These are based on the principle of leaving vascularized brain behind that is disconnected from healthy tissue. Disconnecting techniques can be distinguished by the approach (lateral or vertical) and by the amount of resected tissue. Rasmussen et al. [9] introduced functional hemispherectomy including resection of the central (frontoparietal junction) cortex and the anterior temporal lobe, complete corpus callosotomy, and disconnection of the residual hemisphere from within the lateral ventricle. Since the 1990s, Rasmussen’s technique has been further modified: Villemure and coauthors [10–12] described the lateral peri-insular hemispherectomy providing a considerably reduced but still significant resection of peri-insular parenchyma (opercular frontal, parietal, and temporal), accompanied by deafferentation of the frontal, parietal, occipital, and temporal lobes. Delalande and coauthors [13–15] introduced a mainly disconnective, vertical parasagittal hemispherotomy, which includes a small resection of the posterior part of the gyrus rectus, a callosotomy, a disconnection of the hippocampus, and a disconnection of white matter tracts from the rectus gyrus to the anterior temporal horn through the caudate nucleus. Schramm and coauthors [16] promoted another mainly disconnective approach, the transsylvian functional hemispherotomy, which comprises an unco-amygdalo-hippocampectomy, the transection of the long fibers of the corona radiata, the disconnection of the corpus callosum, and the occipital and parietal white matter fibers.
Current evidence suggests that the surgical technique might affect the rate of postoperative complications [17] but that seizure outcome is mainly influenced by etiology and not by surgical approach [13, 14]. However, since retrospective studies and recent meta-analyses only compare particular techniques or focus on predictors of seizure outcome in general [13, 18, 19], the actual impact of the surgical approach on outcome and morbidity is unclear. Because there are no high-quality studies providing direct comparisons between techniques, the choice of technique is currently mostly dependent on the surgeon’s experience with a specific approach. This meta-analysis aims to demonstrate whether the choice of surgical technique influences surgical outcome in terms of seizure freedom, risk of death, and occurrence of acute and chronic complications.
2. Methods
We followed PRISMA reporting guidelines [20].
2.1. Eligibility Criteria, Information Sources, and Search Strategy
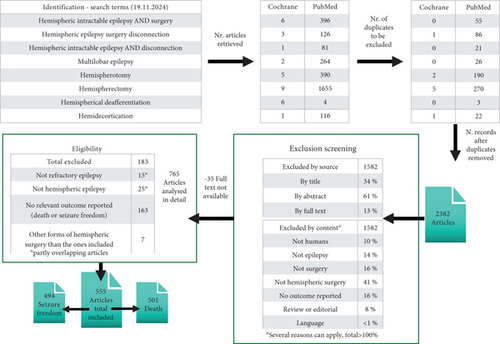
We defined the search strategy based on eight key word combinations (see Figure 2). These key words were used to search both the databases of PubMed and Cochrane on November 19, 2024.
2.2. Selection and Data Collection Process
After exclusion of double entries, 2382 articles were selected to be screened independently by two experts (AK and BPS). Next, consensus was reached on exclusion using the following criteria: The study was not conducted in humans, did not concern epilepsy, and did not involve surgery and specifically hemispheric surgery; no outcome was reported; or the article was a review without additional data (for details, see Figure 2).
Reviews were manually searched for additional articles. No articles had to be excluded because of language (articles in English, German, Italian, Spanish, and French could be included). This screening led to exclusion of 1582 articles, and a further 35 had to be excluded because full text was not available even after contacting the authors and accessing purchase and library services of the coauthors’ institutions of this project. The remaining 765 articles were analyzed in detail by the same two experts independently. Of these, 183 were excluded in consensus after reading the full text, because the article did not concern refractory or hemispheric epilepsy, because no relevant outcome was reported, or because another form of hemispheric surgery than the ones included were applied, for example, hemispheric thermocoagulation (number per exclusion criterion is detailed in Figure 2). Eventually, a total of 555 articles was included in this meta-analysis, among which 501 reported the outcome death and 494 reported the outcome seizure freedom.
2.3. Extraction of Data Items and Effect Measures
- •
Resective: anatomical hemispherectomy and hemidecortication
- •
Mainly disconnective: transsylvian hemispherotomy and vertical hemispherotomy
- •
Combination of resective and disconnective (hereafter “combined”): Rasmussen’s functional hemispherectomy and peri-insular hemispherectomy.
For evaluation of outcome, we extracted the number of patients that were seizure-free among all patients included in a study and the number of deaths reported among all patients included in a study.
2.4. Study of Risk of Bias Assessment
To assess quality of studies and risk of bias, we extracted study design (retrospective, prospective, and randomized), surgical approaches used (one or several), and reasons for the choice (preference of the surgeon and patient-specific reasons).
2.5. Synthesis Methods
For statistical analyses, the software R was used (V 4.2.1 2022-06-23). The analysis was based on the surgical categories: resective, disconnective, and combined. Studies that could not be clearly assigned to a single surgical category (or were assigned to more than one category) were excluded from the meta-analysis but were kept for the pooled data analysis if the surgical method was clear for individual cases. We combined case reports and case series (sample size < 5) such that they would virtually form one study to enter the overall analysis, separately by surgical method.
Meta-analysis was conducted with the metaprop function (package “meta” V5.2-0). We used a random intercept logistic regression model, maximum likelihood estimator for tau2, logit transformation, and continuity correction of 0.5 in studies with zero cell frequencies (only used to calculate individual study results). In line with the Cochrane Handbook recommendations [21], we reported an estimate of the between-study variance as tau2 for the random-effects model. This measure can be understood such that the square root of it (i.e., tau) is the estimate of the standard deviation of effects across studies. For the common-effect model, the alternative measure to tau2 was obtained via the Q-test and the respective test statistic Q. For all analyses, we reported the results of the more conservative random-effects model. For analyses of death, we added the results of the common-effect model which results in slightly higher estimates. For visualization of results, we created forest plots (using the forest function in R).
To analyze the effect of age (0–48 months, 49–72 months, and older than 72 months), we performed a pooled data analysis by age groups for those studies that reported age information of individual patients. Since follow-up times varied across studies, we reported results for (i) all studies, (ii) studies with information on individual patients’ ages, and (iii) cases with a minimum follow-up of 1 year. Pooled data analysis is inferior to meta-analysis [22], but is more appropriate in this case since meta-analysis would result in overlaps because age groups are not clearly distinct between studies. For all analyses that involved proportions based on single cases, we applied the test for equal proportions that can be used for pairwise comparisons or several groups, yielding a Pearson’s χ2 test statistic. We used the pro.test function from the statistical package Version 4.3.0 in R for this purpose and the same test for post hoc comparisons. Multiple comparisons were interpreted at the Bonferroni-corrected level of significance.
2.6. Reporting Bias and Certainty Assessment
The risk of reporting bias was statistically analyzed via funnel plots and tests for heterogeneity among studies. Furthermore, we examined the effect of the year of publication via logistic regression, to rule out that medical advances over the years would moderate the results rather than the different surgical procedures.
3. Results
3.1. Study Selection
The process of study identification and exclusion is outlined in the flow diagram in Figure 2.
3.2. Study Characteristics
A full list of all citations and extracted outcome data is given in the File S1 (seizure freedom) and File S2 (death).
3.3. Bias Assessment
Among the studies in which the study design could be defined, 484 had a retrospective design, while nine qualified for a prospective one. Surgical strategies were clearly not compared in 470 studies, whereas 24 studies did so to some extent. However, none of the studies was randomized.
No reason was given for the surgical approach chosen in 447 studies; in 37 studies, the type of surgery was the choice of the surgeon; in 3 cases, disadvantages of other approaches were mentioned; and in 7 cases, patient-specific reasons were decisive.
Since the studies included in this meta-analysis did not primarily aim at comparing specific surgical techniques and since none of them was randomized, there is a considerable risk of bias for nonsystematic reporting of certain outcomes, specifically with respect to acute and chronic complications as well as deaths.
3.4. Seizure Freedom
From studies with available information on seizure freedom, studies in which sample size was unclear were excluded, resulting in 483 usable studies. Among them, there were 235 case reports or studies with sample sizes smaller than five. We further excluded all studies with mixed approaches. Finally, a total of 167 studies and three sets of case studies merged by surgical category were included in the meta-analysis. Overall, of 4445 patients, 3202 were seizure-free after surgical intervention, which resulted in an overall ratio of 0.72 (95% CI: [0.69; 0.75]) according to the random-effects model. Results for subgroups to surgical categories are shown in Table 1 and forest plots in Figures 3, 4, and 5.
| Subgroup | N studies | N patients | Proportion 95% CI | Tau2 | Tau |
|---|---|---|---|---|---|
| Resective | 53 | 1298 | 0.67 [0.61; 0.73] | 0.75 | 0.86 |
| Disconnective | 35 | 1234 | 0.80 [0.76; 0.83] | 0.22 | 0.47 |
| Combined | 79 | 1912 | 0.70 [0.66; 0.74] | 0.59 | 0.47 |
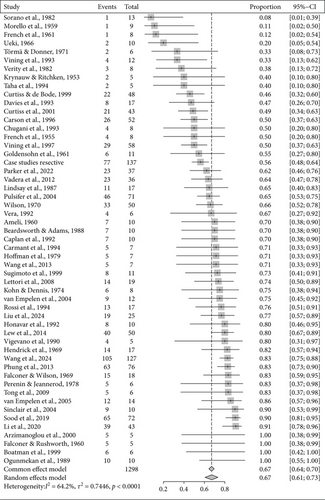
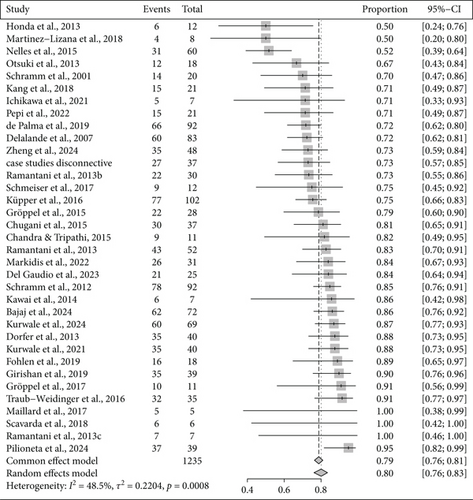
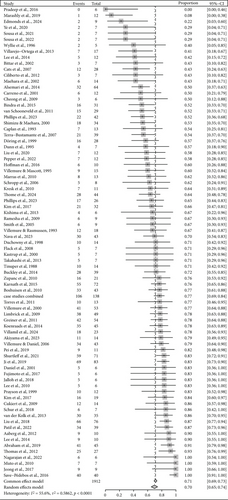
We found significantly different rates of seizure freedom (Q (2) = 17.19; p = 0.0002) between surgical categories. Seizure freedom was significantly less frequent after resective as compared to disconnective surgery (Q (1) = 12.71; p = 0.0004) and significantly less frequent after combined as compared to disconnective surgery (Q (1) = 12.34; p = 0.0004).
There were 2501 patients with individual information available for the pooled data analysis. Among those with information on the surgical approach (N = 1681), seizure-free outcome differed significantly (X2(2) = 26.25; p < 0.001). Seizure freedom was less common after resective as compared to disconnective surgery (X2(1) = 24.52; p < 0.001) and more common after disconnective than combined surgery (X2(1) = 17.25; p < 0.001), but did not differ between resective and combined surgery.
We furthermore reduced the cohort to those 1165 patients where follow-up period was at least 1 year (see Table 2 for the proportions resulting from pooled data analysis separately for the whole cohort and the cohort with follow-up periods of at least 1 year).
| Subgroup | N patients | Proportion | 95% CI |
|---|---|---|---|
| All patients with information on surgical approach(N = 1681) | |||
| Resective | 536 | 0.65 | 0.61–0.69 |
| Disconnective | 373 | 0.80 | 0.76–0.84 |
| Combined | 772 | 0.69 | 0.65–0.72 |
| Thereof with follow-up period of at least 1 year (N = 1165) | |||
| Resective | 356 | 0.70 | 0.65–0.74 |
| Disconnective | 240 | 0.83 | 0.77–0.87 |
| Combined | 569 | 0.64 | 0.60–0.68 |
| All patients with information on age (N = 2467) | |||
| ≤ 48 months | 1467 | 0.72 | 0.70–0.74 |
| 49–72 months | 381 | 0.73 | 0.68–0.77 |
| > 72 months | 378 | 0.69 | 0.64–0.73 |
| Thereof with follow-up period of at least 1 year (N = 1887) | |||
| ≤ 48 months | 1235 | 0.71 | 0.69–0.74 |
| 49–72 months | 258 | 0.72 | 0.66–0.77 |
| > 72 months | 260 | 0.67 | 0.61–0.73 |
Also in this reduced cohort, significantly different seizure-free outcomes were obtained for the three surgical categories (X2(2) = 26.41; p < 0.001). Disconnective surgery yielded higher rates of seizure freedom than resective surgery (X2(1) = 11.87; p < 0.001) and as compared to combined surgery (X2(1) = 25.56; p < 0.001).
Analysis of age (see Table 2) among patients with individual information on age (N = 2467) showed that the proportion of seizure freedom was not different between age groups. Similarly, in the cohort of patients with a minimum follow-up period of at least 1 year (N = 1887), there was no significant difference between age groups.
3.5. Deaths
For analysis of mortality, assumed that no data on deaths means that no patient died. Studies in which cohort size was missing or unclear were excluded, resulting in 497 complete data entries. Among them, there were 241 case studies or studies with sample sizes smaller than five. We excluded mixed studies where different surgical approaches were used such that the number of studies used in the meta-analysis was 171 (168 plus the three sets of case studies merged by surgical category).
Among 4505 patients, death was noted in 120 cases resulting in a ratio of 0.027 according to the common-effect model (95% CI: [0.022; 0.032]), while the random-effects model yielded a ratio of 0.003 (95% CI: [0.001; 0.008]). Results for subgroups according to the surgical categories are shown in Table 3 and the respective forest plots in Figures S1–S3.
| Common-effect model | |||||
|---|---|---|---|---|---|
| Subgroup | N studies | N patients | Proportion 95% CI | Q | |
| Resective | 55 | 1343 | 0.0566 [0.0454; 0.0703] | 51.62 | |
| Disconnective | 34 | 1224 | 0.0065 [0.0033; 0.0130] | 6.10 | |
| Combined | 82 | 1938 | 0.0188 [0.0136; 0.0259] | 33.05 | |
| Random-effects model | |||||
| Subgroup | N studies | N patients | Proportion 95% CI | Tau2 | Tau |
| Resective | 55 | 1343 | 0.0203 [0.0089; 0.0454] | 3.24 | 1.80 |
| Disconnective | 34 | 1224 | 0.002 [0.0019; 0.0021] | 3.00 | 1.73 |
| Combined | 82 | 1938 | 0.0009 [0.0001; 0.0079] | 7.43 | 2.73 |
Significantly different rates of death were noted between the surgical categories, both according to the common-effect model (Q (2) = 55.81; p < 0.001) and the random-effects model (Q (2) = 30.61; p < 0.001). The death rate was significantly higher for resective than for disconnective surgery, according to the common-effect model (Q (1) = 34.95; p < 0.001) and according to the random-effects model (Q (1) = 30.11; p < 0.001). In addition, death rate was significantly higher for resective than for combined surgery according to the common-effect model (Q (1) = 31.01; p < 0.001) and the random-effects model (Q (1) = 6.97; p = 0.008). Finally, death rates were higher for combined surgery as compared to disconnective surgery, but this difference was significant only according to the common-effect model (X2(1) = 7.46; p = 0.006).
There were 2463 patients with individual information available for the pooled data analysis of death. Among the 1694 patients who could clearly be assigned to one of the three surgical categories, the three surgical categories yielded significantly different death rates (X2(2) = 48.22; p < 0.001). Resective surgery lead to higher rates of death as compared to disconnective surgery (X2(1) = 12.52; p < 0.001) and as compared to combined surgery (X2(1) = 38.37; p < 0.001).
Again, we reduced this cohort to those 1197 patients with a follow-up period of at least 1 year (see Table 4 regarding the proportions of the groups according to surgical categories, separately for the whole cohort and the cohort with follow-up periods of at least 1 year).
| Subgroup | N patients | Proportion | 95% CI |
|---|---|---|---|
| All patients with information on surgical approach(N = 1694) | |||
| Resective | 573 | 0.0681 | 0.0501–0.0916 |
| Disconnective | 376 | 0.0160 | 0.0073–0.0344 |
| Combined | 745 | 0.0054 | 0.0021–0.0137 |
| Thereof with follow-up period of at least 1 year (N = 1197) | |||
| Resective | 408 | 0.0711 | 0.0499–0.1002 |
| Disconnective | 241 | 0.0124 | 0.0042–0.0360 |
| Combined | 548 | 0.0055 | 0.0019–0.0160 |
| All patients with information on age (N = 2429) | |||
| ≤ 48 months | 1676 | 0.0233 | 0.0171–0.0317 |
| 49–72 months | 385 | 0.0182 | 0.0088–0.0370 |
| > 72 months | 368 | 0.0217 | 0.0110–0.0423 |
| Thereof with follow-up period of at least 1 year (N = 1801) | |||
| ≤ 48 months | 1263 | 0.0223 | 0.0154–0.0319 |
| 49–72 months | 275 | 0.0182 | 0.0078–0.0418 |
| > 72 months | 263 | 0.0228 | 0.0105–0.0489 |
Also in this reduced cohort, significantly different death rates were found between the three surgical categories (X2(2) = 38.46; p < 0.001). The death rate was significantly higher after resective as compared to disconnective surgery (X2(1) = 9.89; p = 0.001) and as compared to combined surgery (X2(1) = 29.12; p < 0.001).
Death rates did not differ by age groups (see Table 4) among all patients with individual information on age (N = 2429), and neither did they differ in the cohort of patients with a minimum follow-up period of at least 1 year (N = 1801).
3.6. Acute and Chronic Complications
We investigated acute and chronic complications reported in the studies. Chronic complications were defined as those that persisted longer than 2 weeks. There were 304 studies including 2920 patients reporting on acute complications (Table 5).
| Acute complications | Overall N = 2920 |
Resective N = 906 |
Disconn. N = 893 | Comb. N = 1121 | χ2 | p |
|---|---|---|---|---|---|---|
| Surgical | ||||||
| CNS infections | 0.031% | 0.020% | 0.037% | 0.035% | 5.35 | 0.069 |
| Other infections | 0.007% | 0.010% | 0.004% | 0.006% | 2.06 | 0.356 |
| Hemorrhage/infarction | 0.021% | 0.034% | 0.016% | 0.015% | 10.66 | 0.004 |
| Hydrocephalus | 0.046% | 0.042% | 0.046% | 0.048% | 0.451 | 0.798 |
| Other surgical | 0.002% | 0% | 0.001% | 0.004% | 4.00 | 0.136 |
| Neurological | 0.030% | 0.031% | 0.046 | 0.017% | 14.29 | < 0.001 |
| Internal | 0.011% | 0.011% | 0.018% | 0.004% | 8.59 | 0.014 |
| Unspecific symptoms | 0.022% | 0.050% | 0.013% | 0.006% | 48.39 | < 0.001 |
| Any acute complicationa | N = 497 | N = 181 | N = 162 | N = 154 | ||
| Chronic complications | Total N = 2536 | Resective N = 756 | Disconn. N = 742 | Comb. N = 1038 | χ2 | p -val |
| Surgical | ||||||
| CNS infections | 0.005% | 0.013% | 0.003% | 14.57 | 0.001 | |
| Other infections | 0.004% | 0.011% | 0.003% | 10.56 | 0.005 | |
| Hemorrhage/infarction | 0.007% | 0.016% | 0.001% | 0.004% | 14.01 | < 0.001 |
| Hydrocephalus | 0.027% | 0.030% | 0.022% | 0.027% | 1.16 | 0.559 |
| Other surgical | 0.002% | 0.004% | 0.001% | 4.41 | 0.110 | |
| Neurological | 0.048% | 0.089% | 0.039% | 0.026% | 36.34 | < 0.001 |
| Internal | 0.003% | 0.007% | 0.002% | 6.39 | 0.041 | |
| Unspecific symptoms | 0.008% | 0.028% | 0.002% | 35.11 | < 0.001 | |
| Any chronic complicationa | 271 | 149 | 49 | 73 |
- aNote that a patient can have more than one complication and can be counted more than once in these numbers.
More details on types and frequencies of acute complications are summarized in Table S1. Neurological acute complications were significantly more frequent after disconnective as compared to combined surgery (χ2(1) = 13.22; p < 0.001). Acute unspecific symptoms were significantly more frequent after resective as compared to disconnective surgery (χ2(1) = 18.08; p < 0.001) and as compared to combined surgery (χ2(1) = 36.08; p < 0.001).
Table 5 also lists the number of chronic complications found in 275 studies reporting on chronic complications, including 2536 patients. More details on types and frequencies of complications are summarized in Table S2. CNS infections lasting longer than 2 weeks were significantly more common after resective as compared to disconnective surgery (χ2(1) = 7.99; p = 0.005) and as compared to combined surgery (χ2(1) = 5.14; p = 0.023). Similarly, hemorrhage/infarction > 2 weeks was more common after resective as compared to disconnective surgery (χ2(1) = 7.57; p = 0.006) and as compared to combined surgery (χ2(1) = 5.85; p = 0.016). Chronic neurological complications were significantly more common after resective as compared to disconnective surgery (χ2(1) = 13.22; p < 0.001) and as compared to combined surgery (χ2(1) = 31.11; p < 0.001). Finally, also, chronic unspecific complications were significantly more common after resective as compared to disconnective surgery (χ2(1) = 15.93; p < 0.001) and as compared to combined surgery (χ2(1) = 17.07; p < 0.001).
3.7. Reporting Biases and Certainty of Evidence
For seizure freedom, the funnel plot showed some asymmetry, with a slight bias towards larger studies publishing better outcomes (Figure 6).
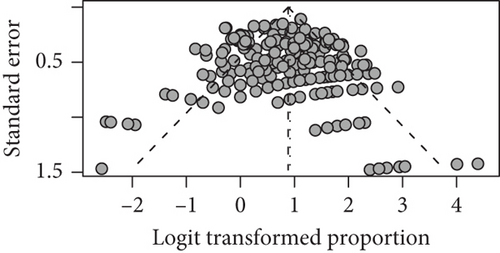
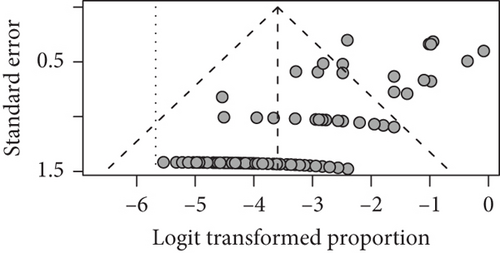
For seizure freedom, heterogeneity of studies was quantified (tau2 = 0.618; tau = 0.786; I2 = 61.0% [53.9%; 67.0%]; H = 1.60 [1.47; 1.74]), and we found a significant heterogeneity via the Wald-type test (Q(166) = 425.82; p = <0.001) as well as a significant heterogeneity via the likelihood ratio test (Q(166) = 602.45; p < 0.001). Since there is a significant heterogeneity in our studies, this might have contributed to the relation between study size and outcome which caused the asymmetrical funnel plot [19]. Also, for mortality, the funnel plot showed considerable asymmetry with a bias towards small death rates (Figure 6). This may be partly due to our policy of assuming for all the studies which did not report any mortality that there were no patients who died after the intervention. Furthermore, there was again considerable heterogeneity among included studies (tau2 = 5.70; tau = 2.39; I2 = 0.0% [0.0%; 19.2%]; H = 1.00 [1.00; 1.11]). Heterogeneity was not significant according to the Wald-type test but significant according to the likelihood ratio test (Q(170) = 452.27; p < 0.001).
Since our meta-analysis included studies back to the 50s and since the resective category was the oldest one included in our analysis, we cannot exclude that the relative disadvantage of the resective category was biased by the fact that articles on disconnective surgery were published at a time when surgical aids and techniques had improved overall. To analyze the bias of time, we used the samples from our pooled data analysis for only those patients with a follow-up period of at least 12 months to conduct a logistic regression. We analyzed the impact of the following factors: year of surgery, follow-up period in months, age at surgery, and surgical category on the selected outcomes seizure freedom and death. Surgical approach was included by comparing the relative advantage/disadvantage of disconnective and combined surgery with resective surgery. The results of this analysis for deaths and seizure freedom are shown in Table 6.
| Estimate | Std. error | z-value | p-value | |
|---|---|---|---|---|
| Seizure freedom (N = 1159) | ||||
| Year of study | −0.005 | 0.01 | −0.91 | 0.362 |
| Resective vs. disconnective | 0.88 | 0.27 | 3.23 | 0.001 ∗ |
| Resective vs. combined | −0.13 | 0.20 | −0.68 | 0.504 |
| Age at surgery | −0.001 | 0.001 | −0.77 | 0.443 |
| Risk of death (N = 1191) | ||||
| Year of study | −0.03 | 0.01 | −2.72 | 0.007 ∗ |
| Resective vs. disconnective | −0.55 | 0.79 | −0.69 | 0.489 |
| Resective vs. combined | −1.65 | 0.72 | −2.30 | 0.021 ∗ |
| Age at surgery | 0.003 | 0.001 | 1.95 | 0.052 |
- ∗Significant effect.
There was a significant effect of the year of study such that the younger the year of study, the lower the risk of deaths. A higher patient age at surgery was only by tendency related to risk of death. The analysis confirmed the relative disadvantage of the resective approach as compared to the combined approach. However, no significant disadvantage of resective versus disconnective surgery when controlling for the year of study was found.
Regarding seizure freedom, the year of study had no effect, nor had patients’ age at surgery. There was only a significant advantage of disconnective surgery as compared to the resective surgery.
4. Discussion
4.1. Surgical Strategies
In contrast to previous studies comparing results of surgical approaches [23, 24], this is the first meta-analysis that examines systematically the influence of the two surgical principles of hemispheric procedures, resection and disconnection, on seizure outcome and complications. Resective surgery includes anatomical hemispherectomy, that is, complete removal of the hemisphere including white matter, and hemidecortication or hemicorticectomy, that is, complete removal of the cortex sparing white matter. Mainly, disconnective surgery comprises transsylvian hemispherotomy, that is, complete disconnection of the hemisphere by the transsylvian-transventricular approach, and vertical hemispherotomy, aiming at the disconnection of the hemisphere using a parasagittal vertical approach. Additionally, a combined surgical approach was examined in which both surgical strategies of resection and disconnection are used in the same patient. Combined resective/disconnective procedures include Rasmussen’s functional hemispherectomy consisting of the removal of the temporal lobe and the central area, while the residual frontal and parieto-occipital lobes are disconnected, and its modifications: (i) hemispherical deafferentation, that is, removal of the temporal lobe, while disconnecting the rest of the hemisphere, and (ii) peri-insular hemispherectomy which consists of removing the frontal and temporal opercula creating access to the ventricular system through which the complete hemisphere is disconnected [25].
Today, resective techniques such as anatomical hemispherectomy and hemidecortication have been largely replaced by disconnective procedures and combined resective/disconnective strategies. Combined approaches have been most frequently used during the last decades and can be applied in cases with normal as well as enlarged hemispheres. Variations of combined strategies mainly refer to the approach (lateral vs. vertical), and the proportions of resection and disconnection, and thus the extent of exposure necessary which depends on the proportion of resection. The choice of a surgical technique is largely determined not only by the individual experience of the neurosurgeon but also by the individually given pathology of the patient. This meta-analysis aimed to find out whether and to what extent the principles resection and disconnection used in hemispheric procedures have an impact on the results and should therefore influence the choice of the surgical strategy.
4.2. Seizure Freedom
Rates of freedom of disabling seizures (Engel I) after hemispheric surgery have been reported to range from 54% to 90%, and even in patients who lacked complete postoperative seizure freedom, seizure burden could be improved [12, 14, 26–30]. Our pooled data analyses revealed the rate of seizure freedom after a minimum follow-up period of 12 months to be 71%, which is slightly lower compared to previous reports [13] with an overall seizure-free rate of 79% at 6 months, 75% at one, and 71% at 2 years following surgery [27]. Moreover, our study showed that seizure freedom was more frequent after a disconnective surgical approach as compared to resective or combined surgery. We did not find differences in seizure outcome between age groups in our meta-analysis which stands in contrast with some previous reports [31, 32] but is in line with others [33, 34].
Modified hemispherotomy and anatomical procedures in which the basal ganglia and thalamus were routinely resected are associated with increased seizure-free rates (67–100%) compared to functional procedures (60–67%) in which those structures are not removed [27]. The single-center, retrospective, nonrandomized study by Kwan and coauthors [28] is one of the few comparing surgical techniques, and the results match our findings: the authors reported significantly more favorable seizure outcome (Engel I/II) following peri-insular hemispherotomies as compared to hemidecortication. Recent studies comparing seizure outcome following disconnective techniques show conflicting results of vertical versus lateral hemispherotomy [35, 36]. Unfortunately, with our methodological approach, we were unable to perform a subsequent analysis of vertical versus lateral hemispherotomy. Nevertheless, the surgical procedure is not the only factor determining surgical outcome in terms of seizure control. In addition, underlying pathology [36, 37], contralateral MRI findings, prior resective surgery, and side of hemispherotomy [16, 36] play a role. Particularly, in patients with bilateral pathology, hemispheric surgery might be applied with a purely noncurative intent [38, 39].
4.3. Morbidity
Overall, acute complications such as CNS infections and other infections were found to be less frequent than in previous literature [40], whereas cranial nerve deficits and other neurological deficits occurred in comparable frequency in previous studies [30]. The lower rates can be explained by the inclusion of all studies, regardless of whether complications were systematically documented or not. Therefore, only relations between complications should be interpreted. We found postoperative fever to be the most commonly reported symptom, similar to previous research [41]. Temperature elevation after hemispherectomy is a common phenomenon and has been attributed aseptic meningitis. Further observed unspecific complications were postoperative headache and nausea/vomiting as well as superficial cerebral hemosiderosis, as reported previously [42]. Chronic complications such as postoperative hydrocephalus were found more frequently than in previous reports [43]. In line with earlier research [42], hemianopia was seen as an expected side effect as for the resection extent.
Among different surgical categories, we observed comparable rates of acute complications without clear advantage of one particular technique. The rate for chronic complications and death was highest for resective approaches, while no significant differences between disconnective and combined techniques were found. This is in line with the previous literature stating overall fewer adverse outcomes following disconnective as compared to resective procedures, specifically comparing anatomical hemispherectomy and functional hemispherectomy [40, 44]. In particular, the previous literature reported lateral hemispherotomy to be associated with the least intraoperative blood loss, shortest intensive care unit stay, and lowest complication rate, whereas anatomical hemispherectomy was stated to cause the longest hospital stay with delayed oral food intake, the highest postsurgical fevers, and the highest incidence of shunt requirement [27]. Additionally, following peri-insular hemispherotomy, fewer major complications were observed compared to hemidecortication [28].
We found CNS infections to be more commonly reported following resective surgery. Di Rocco et al. [40] reported superficial skin infections as well as bone flap and CNS infections in 18% of patients with anatomical hemispherectomy, compared to none after functional hemispherectomy. Other authors stated 6.8% overall postoperative infections following lateral hemispherotomy [39]. Our data is not perfectly in line with these prior reports, but, again, these conflicting results might result from reporting bias due to inclusion of studies that are not considering all possible complications.
According to our data, patients experienced intraoperative hemorrhage or infarction more commonly after resective than after disconnective and combined surgical approaches. The most common adverse outcome reported after hemispherectomy surgeries is hydrocephalus occurring within months or years after of the procedure, ranging from 9% to 81% [1, 27, 39, 40, 42, 45]. We can speculate that the reason for this wide range is the fact that these prior studies tended to combine surgical techniques in their analyses. In particular, anatomical hemispherectomy has been blamed to be burdened with a higher risk of secondary hydrocephalus [40]. One multicenter retrospective review revealed a 50% higher rate of hydrocephalus following anatomic hemispherectomy [43]. In our analysis, we did not find any significant difference in rates of hydrocephalus between surgical techniques. Previous authors reported a higher shunting rate following hemidecortication compared to peri-insular hemispherotomy [28]. The etiology for the development of hydrocephalus is discussed to be a defect of cerebrospinal fluid resorption secondary to the huge removal of the subarachnoid space [9]. Lew et al. [43] proposed that this might be due to a greater exposure of the intraventricular and subarachnoid spaces to blood products and inflammatory changes after extensive removal of brain tissue leading to an environment similar to that seen in patients with intraventricular hemorrhage, subarachnoid hemorrhage, or meningitis. Besides, anatomical characteristics of hemimegalencephaly are also discussed to be a potential cause for postoperative hydrocephalus [40].
4.4. Mortality
Whereas, in the past, mortality rates in hemispherectomy series varied between 2% and 5% [1, 14], it is rarely reported in modern era series (0% or well below 1%) [27, 46]. Especially more recent studies demonstrate that hemispherectomy can be conducted safely in adults [47]. This is comparable to the data in our meta-analysis. We found significantly higher rates of death for resective compared to either combined or disconnective surgery. Considering all patients with individual information on age, the occurrence of death did not significantly differ between age groups. This is in contrast with previous findings where mortality is closely related to the small blood volume and severe cortical malformations sometimes requiring larger resection in infants [48]. However, studies included in the meta-analysis were not selected based on their aim for reporting mortality, which might result in a lower mortality rate in general, and can lead to age-specific biases, as well. Even if early surgery is discussed for better neurocognitive and psychosocial development because of its advantages for developmental plasticity, that is, the transfer of motor functions in one hemisphere or language capability in the right hemisphere [26, 42, 49], surgical consideration for younger children requires careful analysis of several age-related issues in comparison with adults.
4.5. Time of Publication
The risk of death after surgery decreased over the years. Interestingly, the relative disadvantage of the resective versus the disconnective method was not present anymore when controlling for the year of study, well in line with more recent publications [47]. However, for seizure freedom, the year of study did not bias the relation between surgical approaches. The influence of the year of study can likely be attributed to medical technology advances, for example, in electrophysiological techniques and high-resolution MR imaging.
4.6. Limitations
Including all patients into the calculation of rates of death, acute and chronic complications led to overall lower rates of death and complications as compared to restricting the analysis to studies that would include the rate of death or complications explicitly into their outcome measures. Therefore, the rates reported here were overall lower than what was found in the literature, especially for complications. We assumed that this bias is comparable across surgical techniques, such that the comparison of these still allows for valid conclusions. However, a bias with respect to surgical technique cannot be ruled out, for example, if reporting practices changed over time. Hemidecortication is a technique that does not fit well into any of the predefined categories. To avoid dismissing the studies that included hemidecortication, we included them in the resective group. Since there were only five studies with predominantly very small numbers of patients, a subanalysis of these studies was statistically not feasible.
One recent publication underlines the importance of etiology for seizure outcome after surgery [36]. However, in older studies, etiology is not reported in agreement with current standards. Therefore, one limitation of our study is the fact that we could not consider the underlying pathology in outcome analyses of articles spanning a wide range of time. More specifically, complex cases such as patients with hemimegalencephaly typically undergo a larger resection and consequently suffer from a higher complication and mortality risk.
One previous meta-analysis included only studies that were published after 2000, excluding older studies for various reasons and analyzing only 25 studies in total [23]. In contrast, by including a large number of studies, considering also the year of study as well as the effect of age at surgery, our work adds relevant information to the present knowledge.
5. Conclusion
There is a clear superiority of disconnective approaches over resective as well as combined techniques regarding seizure freedom. Different acute complications occur depending on the surgery type, without clear superiority of one technique. The rate of chronic complications and death is highest for resective approaches without significant differences between disconnective and combined techniques.
Disclosure
The results presented in this article were previously presented in the form of a poster and the abstract is published in the abstract of the 35th international Epilepsy Congress (ILAE epilepsy congress) [50].
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Barbara Puhahn-Schmeiser: investigation (equal), writing–original draft (equal), writing–review and editing (equal). Yvonne Höller: software (lead), methodology (lead), visualization (lead), formal analysis (lead), writing–original draft (equal), writing–review and editing (equal). Franziska vom Hofe: investigation (equal), data curation (equal). Josef Zentner: writing–review and editing (equal), conceptualization (supporting). Julia Jacobs: conceptualization (lead), writing–review and editing (equal). Kerstin Alexandra Klotz: resources (lead), investigation (equal), visualization (supporting), writing–original draft (equal), writing–review and editing (equal). Barbara Puhahn-Schmeiser and Yvonne Höller contributed equally and share the first authorship. Julia Jacobs and Kerstin Alexandra Klotz contributed equally and share the last authorship.
Funding
Franziska vom Hofe got funded by Erasmus+. The other researchers did not receive specific funding for this work but conducted the research on the basis of their academic commitment at the respective universities.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supporting information (File 1 and File 2) of this article.




