Mental Fatigue After Aneurysmal Subarachnoid Hemorrhage: A Prospective 5-Year Follow-Up Study
Abstract
Objectives: Mental fatigue is a common and debilitating symptom following an aneurysmal subarachnoid hemorrhage (aSAH). However, its long-term prevalence and consequences remain unclear. In this longitudinal pilot study, the Mental Fatigue Scale (MFS) was used to evaluate the prevalence, severity, and dynamics of mental fatigue for up to 5 years and to correlate patient demographics and early complications to the development of mental fatigue.
Method: Functional outcomes were scored using the Glasgow Outcome Scale-Extended (GOSE) during telephone interviews 1, 3, and 5 years after aSAH. The MFS questionnaires (maximum score 42, ≥ 10.5 points indicating mental fatigue) were subsequently sent by mail. Patient admission data and events during the acute phase were recorded.
Results: Of 64 included patients, 31 could be assessed at all time points. Mental fatigue (MFS score ≥ 10.5) was present in 58%, 48%, and 52% of the patients at 1, 3, and 5 years, respectively. A significant decrease in the total MFS score was observed between 1 and 5 years (p = 0.025). The proportion of patients experiencing severe mental fatigue halved from 1 to 3 years. The median (range) MFS scores were significantly higher for women (14.5, 0–29.5) than for men (3, 0–17.5) at 1 year (p = 0.043). Compared with patients experiencing loss of consciousness at ictus (LOCi), those without LOCi exhibited a progressive decline in the total MFS score (p = 0.003).
Conclusions: Although total MFS scores significantly improved, mental fatigue was a common and persistent symptom affecting half of the patients up to 5 years following aSAH. Mental fatigue was more prevalent in women than men; further, patients who experienced LOCi during the acute phase improved less over time. Our results highlight the importance of evaluating mental fatigue when assessing patient recovery and long-term outcomes.
Trial Registration: Clinical Trial NCT06239142
1. Introduction
Fatigue is among the most frequently reported sequelae in stroke survivors and affects both short- and long-term outcomes [1–5]. Although fatigue is a multidimensional and complex condition comprising mental, physical, emotional, and stress fatigue, it is often studied as a singular entity [6]. Particularly mental fatigue has been identified as an important and debilitating symptom during patients’ recovery and rehabilitation following various neurological disorders and injuries, including traumatic brain injury and stroke [7–11]. It has also been identified as a significant determinant of patients’ difficulties with returning to life as before the injury [12–14]. No consensus on the full definition of mental fatigue has been established. However, it can be described as an inability to initiate and endure cognitive tasks with symptoms not relieved by normal rest [15, 16].
Aneurysmal subarachnoid hemorrhage (aSAH) is a type of stroke where fatigue, in general, is reported to be present in 30% to 90% of the patients [17, 18]. Notably, it is the mental fatigue component that significantly contributes to difficulties that patients with aSAH face when returning to normal life, as shown by Buunk et al. [7, 14]. However, there is substantial variation in the reported incidence of mental fatigue (25%–60%), which may be attributed to several methodological factors, such as differences in the follow-up periods and instruments used [5, 7, 13, 19, 20]. Consequently, a complete understanding of how mental fatigue influences long-term recovery remains elusive.
This study is aimed at using the Mental Fatigue Scale (MFS) questionnaire to determine the long-term prevalence, severity, and dynamics of mental fatigue at 1, 3, and 5 years after an aSAH. The MFS instrument includes 15 items focusing on the mental aspects of fatigue, including cognitive and sensory symptoms. The symptoms are graded by intensity, duration, and frequency on a 7-step scale [15]. Furthermore, the MFS questionnaire was used to explore the most significant problems that patients with aSAH face. Lastly, the study is aimed at identifying whether demographic characteristics and secondary complications during the acute phase after aSAH can be associated with an increased risk of developing mental fatigue.
We hypothesized that there are a significant prevalence and severity of mental fatigue in patients after aSAH that continues to develop and change during the first few years postictus. Moreover, we hypothesized that early complications are associated with the development of mental fatigue.
2. Materials and Methods
2.1. Study Population
Patients aged ≥ 18 years with a confirmed diagnosis of aneurysm rupture on digital subtraction angiography treated at the neurointensive care unit of Sahlgrenska University Hospital, Gothenburg, Sweden, were consecutively screened for inclusion by a research nurse during the study period between May 2015 and October 2016. We excluded patients with a history of stroke or other brain injuries, as well as those with atrial fibrillation and pacemakers since heart rate variability was assessed in a previous study [21]. Due to logistical reasons (interruptions in screening were made during holiday periods), 64 out of the 105 admitted patients could be included in the final study group. Written consent was obtained from all patients or their families before study participation upon arrival at the neurointensive care unit.
Patients were treated according to local guidelines coherent with international guidelines [22]. Aneurysms were promptly treated, usually within 24 h and nimodipine (Nimotop, Bayer) was administered prophylactically to all patients. Patients with hydrocephalus were treated through extraventricular drainage (EVD) during the acute phase and subsequently with a shunt if needed. Based on the recommended clinical and radiological definition from Vergouwen et al., delayed cerebral ischemia (DCI) was defined as the presence of focal or global neurological impairment that lasted ≥ 1 h or cerebral infarction detected by computed tomography or magnetic resonance imaging present within 6 weeks after ictus that cannot be attributed to other causes [23]. Patients who developed DCI underwent fluid optimization and induced hypertension.
2.2. Data Collection
Demographic characteristics, admission status, and imaging data were recorded. Clinical admission status was scored using the Glasgow Coma Scale (GCS) [24] and the World Federation of Neurological Surgeons (WFNS) scale [25]. The amount and location of subarachnoid blood on initial imaging were evaluated and scored according to the modified Fisher scale [26]. The aneurysm location was dichotomized into anterior or posterior circulation and aneurysmal treatment into surgical clipping or endovascular coiling. Early brain injury (EBI) was indicated by loss of consciousness at ictus (LOCi).
Follow-up was performed at 1, 3, and 5 years after aSAH through a structured telephone interview, where patients were scored using the Glasgow Outcome Scale-Extended (GOSE) [27]. Additionally, the patients received three self-assessment questionnaires by mail, including the MFS, at the three time points [15]. Patients were reminded to return the questionnaires three times when necessary. In the present study, only data from the MFS are presented.
2.3. The GOSE
The GOSE evaluates the patient’s functional outcome and level of independence in eight different domains of daily living, including physical, cognitive, emotional, and social functioning [27]. It was developed to classify patients after a traumatic or nontraumatic brain injury, allowing for comparisons of outcomes between patients. The scale ranges from death (1 point) to good recovery (8 points), with higher scores indicating better outcomes and higher levels of independence. A GOSE score of 8 indicates full recovery with only minor symptoms that do not affect activities of daily living (ADL). A GOSE score of ≥ 5 indicates that patients can independently manage their ADL and is used in studies to describe a favorable outcome [5, 28].
2.4. The MFS
The MFS is a multidimensional self-reporting questionnaire developed for assessing different aspects of mental fatigue in patients with brain injuries [15, 29]. It comprises 15 items regarding fatigue, including lack of initiative, mental recovery, concentration difficulties, memory problems, slowness of thinking, sensitivity to stress, increased mental fatigue, increased tendency to become emotional, irritability, sensitivity to light and noise, and decreased or increased sleep duration. Each item has four response options ranging from 0 (normal function) to 3 (maximal problems); further, one can select response points in between (i.e., 0.5, 1.5, and 2.5), which yields a total of seven options. Items 1–14 are used to calculate the total score, with a maximum score of 42. We did not include item 15, which assesses 24-h variations, in the total score, but it can be used in clinical practice. An MFS score ≥ 10.5 indicates mental fatigue. A higher MFS score indicates more severe problems. The total MFS score can be classified into levels, as shown in Table 1 [16].
| MFS score (total sum) | The severity of mental fatigue |
|---|---|
| < 10.5 | No mental fatigue |
| 10.5–14.5 | Mild mental fatigue |
| 15–20 | Moderate mental fatigue |
| ≥ 20.5 | Severe mental fatigue |
- Note: The classification of mental fatigue severity levels is based on the total MFS score. The maximum possible total score on the MFS is 42. A cut-off score of ≥ 10.5 indicates the presence of mental fatigue.
- Abbreviation: MFS, Mental Fatigue Scale.
2.5. Ethics Approval
This study adhered to the Declaration of Helsinki and was approved by the Ethical Regional Board of Gothenburg, Sweden (053-15, T 213-18). Written informed consent was obtained from all patients or their next of kin before they participated in the study.
3. Results
3.1. Patients
Out of the 105 patients admitted during the study period, 64 were included. Of these, 44, 41, and 36 patients returned the MFS questionnaire after 1, 3, and 5 years, respectively. However, only 31 patients responded to the questionnaire at all three time points, with the remaining 33 patients not responding to the questionnaire at all or at only one or two time points, Figure 1. Table 2 presents the demographic and clinical characteristics of all enrolled patients, those included in the MFS cohort, and those with incomplete responses. The results reported in the subsequent analyses are specific to the MFS cohort.
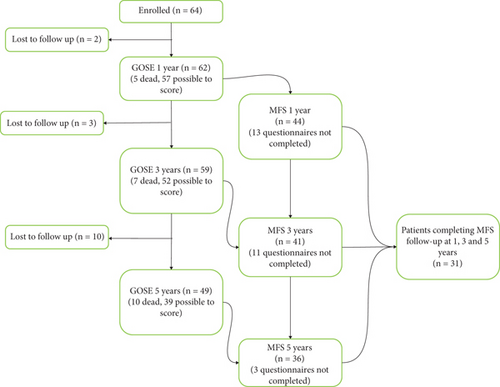
| Characteristic | All patients | MFS cohort | Incomplete MFS | |
|---|---|---|---|---|
| n = 64 | n = 31 | n = 33 | ||
| Age (years) | ||||
| Median (range) | 58 (27–75) | 57 (36–75) | 58 (27–78) | ns |
| Female n (%) | 48 (75) | 24 (77) | 24 (73) | ns |
| 1-year mortality | 5 (8) | 0 (0) | 5 (15) | 0.024 |
| Medical history | ||||
| Cardiovascular disease | 5 (8) | 1 (3) | 4 (12) | ns |
| Hypertension | 23 (36) | 14 (45) | 9 (27) | ns |
| Diabetes | 3 (5) | 1 (3) | 2 (6) | ns |
| Smoking | 9 (14) | 4 (13) | 5 (15) | ns |
| GCS median (range) | 13 (4–15) | 14 (4–15) | 13 (5–15) | ns |
| Hunt and Hess scale median (range) | 3 (1–5) | 2 (1–5) | 3 (2–5) | ns |
| WFNS median (range) | 2 (1–5) | 2 (1–5) | 2 (1–5) | ns |
| Modified Fisher scale median (range) | 4 (0–4) | 3 (0–4) | 4 (0–4) | ns |
| LOCi | 24/60 (40)a | 10/30 (32)a | 14/30 (47)a | ns |
| Aneurysm location n (%) | ||||
| Anterior circulation | 47 (73) | 21 (68) | 26 (79) | ns |
| Posterior circulation | 17 (27) | 10 (32) | 7 (21) | ns |
| Aneurysm treatment n (%) | ||||
| Surgical clipping | 17 (27) | 5 (16) | 12 (36) | ns |
| Endovascular coiling | 47 (73) | 26 (84) | 21 (64) | ns |
| DCI n (%) | 16 (25) | 10 (32) | 6 (18) | ns |
| EVD n (%) | 32 (50) | 13 (42) | 19 (58) | ns |
| GOSE median (range) | ||||
| 1 year | 5 (1–8) | 6 (3–8) | 5 (1–8) (31) | 0.002 |
| 3 years | 6 (1–8) (59) | 6 (3–8) | 5 (1–8) (28) | < 0.001 |
| 5 years | 6 (1–8) (49) | 7 (3–8) | 1 (1–8) (18) | < 0.001 |
- Note: The patient demographics for the initial 64 patients; the 31 patients from the MFS cohort who responded to the MFS questionnaire at 1, 3, and 5 years; and the 33 patients who did not respond at all time points. p value indicates the comparison between the MFS cohort and patients who did not return the MFS questionnaire at all years.
- Abbreviations: DCI, delayed ischemic ischemia; EVD, extraventricular drainage; GCS, Glasgow Coma Scale; GOSE, Glasgow Outcome Scale-Extended; LOCi, loss of consciousness at ictus; ns, nonsignificant; WFNS, World Federation of Neurosurgical Societies.
- aMissing values; the total number of patients possible to analyze is stated.
3.2. GOSE Score at 1, 3, and 5 Years
The median (range) GOSE scores were 6 (3–8), 6 (3–8), and 7 (3–8) at 1, 3, and 5 years, respectively. Further, the GOSE score significantly differed between 1 and 5 years (p = 0.006) and between 3 and 5 years (p = 0.017), but not between 1 and 3 years.
3.3. Mental Fatigue at 1, 3, and 5 Years
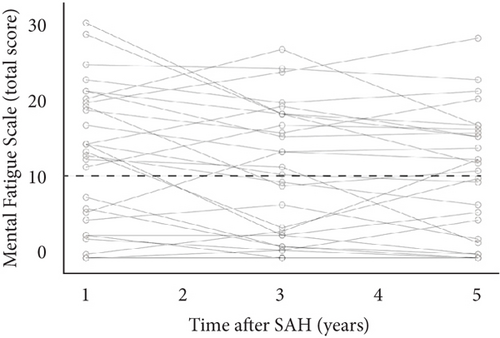
Mental fatigue (MFS score ≥ 10.5) was observed in 58, 48, and 52% of the patients at 1, 3, and 5 years, respectively. The median MFS scores (range) were 13.5 (0–31), 10 (0–27.5), and 10.5 (0–29) at 1, 3, and 5 years, respectively. Further, the MFS score significantly decreased between 1 and 5 years (p = 0.025) but not between 1 and 3 years or 3 and 5 years. Figure 2 shows the total MFS scores for each patient at 1, 3, and 5 years.
3.4. Severity of Mental Fatigue
When classifying the total score of MFS into severity levels (no < 10.5, mild 10.5–14.5, moderate 15–20, and severe > 20.5), the proportion of patients experiencing severe mental fatigue was halved from 26% to 13% between 1 and 3 years, with this change persisting at 5 years. Table 3 summarizes the distribution of patients at each MFS level.
| The severity of mental fatigue | 1 year | 3 years | 5 years |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| No mental fatigue | 13 (42) | 16 (52) | 15 (48) |
| Mild mental fatigue | 4 (13) | 4 (13) | 6 (19) |
| Moderate mental fatigue | 6 (19) | 7 (23) | 6 (19) |
| Severe mental fatigue | 8 (26) | 4 (13) | 4 (13) |
- Note: The total number and percentage of patients classified as having no, mild, moderate, and severe mental fatigue according to the total MFS score at 1, 3, and 5 years. The maximum total MFS score is 42, and the severity levels are categorized as follows: no mental fatigue (< 10.5), mild mental fatigue (≥ 10.5–14.5), moderate mental fatigue (≥ 15–20), and severe mental fatigue (≥ 20.5). Notably, the proportion of patients experiencing severe mental fatigue significantly decreased over time. Between 1 and 3 years, the percentage of patients with severe mental fatigue was halved, declining from 26% to 13%. This reduction persisted at the 5-year follow-up.
- Abbreviation: MFS, Mental Fatigue Scale.
3.5. MFS Scores and GOSE Scores at 1, 3, and 5 Years
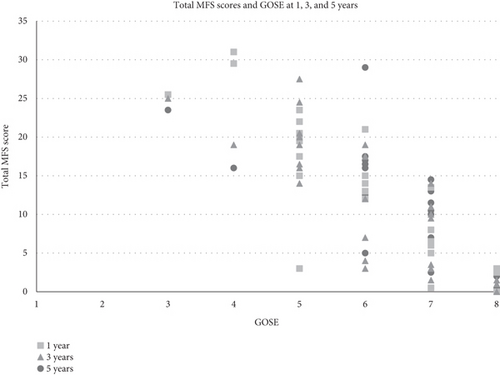
There was a significant negative correlation between total MFS scores and GOSE over time for the 31 patients assessed at 1, 3, and 5 years and also at all individual time points (1 year: n = 44, 3 years: n = 41, and 5 years: n = 36, p < 0.001 for all).
Figure 3 Patients with mental fatigue were represented in all GOSE categories except GOSE 8. All patients with unfavorable GOSE scores (3–4) had MFS scores ≥ 10.5.
3.6. Items Included in the MFS
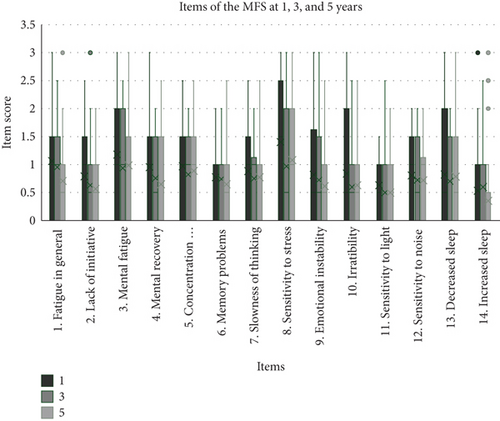
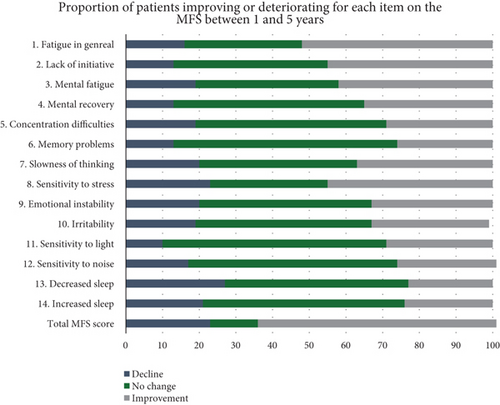
Items 1–14 included in the MFS are presented in Figure 4. At 1 year, the items with the highest scores were mental fatigue, sensitivity to stress, irritability, and decreased sleep. Further, we observed a significant improvement in sensitivity to stress between 1 and 3 years (p = 0.004), general fatigue between 3 and 5 years (p = 0.028), and lack of initiative and irritability between 1 and 5 years (p = 0.046 and p = 0.048, respectively). All other items did not significantly change with time. Figure 5 presents the percentage changes between 1 and 5 years for the included MFS items.
3.7. Mental Fatigue in Relation to Demographics and Events During the Acute Phase
3.7.1. Sex
Regarding sex, 67% and 29% of women and men, respectively, reported a total MFS score of ≥ 10.5 at 1 year. Furthermore, women had a significantly higher median MFS (range) score (14.5 [0–29.5]) than men (3 [0–17.5]; p = 0.043). There was no significant difference between the sexes in the temporal changes in MFS scores, even after adjusting for age. The between-sex difference in the total MFS score remained significant at 3 years (women vs. men: 13 [0–27.5] vs. 3 [3–14]; p = 0.014). At 5 years, the between-sex difference in the total MFS score was no longer significant (women vs. men: 12 [0–23.5] vs. 0.5 [0–13]).
3.7.2. LOCi
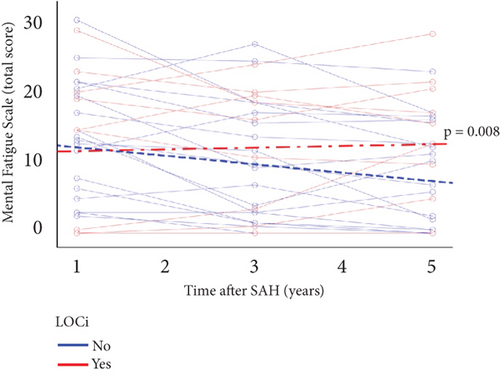
The median MFS score at 1 year did not significantly differ between patients experiencing LOCi or not (median (range): 15 [0–29.5] vs. 13.25 [0–31]). However, it significantly differed over time (1, 3, and 5 years), where patients not experiencing LOCi had a decreasing total MFS score compared with patients experiencing LOCi (p = 0.003) Figure 6.
The median MFS scores at 1 year or MFS change over time did not significantly differ according to age (< 60 vs. ≥ 60 years), WFNS score (1–3 vs. 4–5), Hunt and Hess scores (1–2 vs. 3–5), aneurysm location (anterior vs. posterior), aneurysm treatment (coil vs. clip), EVD (yes/no), or DCI (yes/no).
3.8. Statistical Analysis
Patient characteristics are presented as the median and range for continuous variables and the count and percentage for categorical variables. The Mann–Whitney test was employed to evaluate distribution differences in continuous data, while the Fisher exact test and Pearson’s chi-square test were used for categorical data. For analyzing differences between the years, the Wilcoxon signed-rank test was utilized. The development over time (1 to 5 years) was analyzed by the Friedman test. Correlations between the MFS and GOSE scores were analyzed using Spearman’s rho test. For analysis of MFS over time, a linear mixed model with an autoregressive covariance matrix was used. MFS was the dependent variable, and time was used in interaction with patient characteristics (e.g., sex and age) or clinical events (e.g., EBI and DCI) as independent variables. All tests were two-sided, with the significance level set at p < 0.05. Analyses were performed using existing data without imputation or missing values. All statistical analyses were performed using IBM SPSS Statistics (version 29.0; IBM Corp., Armonk, New York, United States). Since this was an exploratory study, no adjustments were made for multiple testing.
4. Discussion
The main finding of this prospective observational longitudinal study after aSAH is that more than 50% of the patients experienced mental fatigue to varying degrees still after 5 years. However, the total MFS scores significantly decreased from 1 to 5 years after subarachnoid hemorrhage (SAH); accordingly, there was a reduced proportion of patients with severe mental fatigue. Women had significantly higher MFS scores than men after 1 year, a difference that persisted after 3 years. When other patients improved over time, those who experienced LOCi did not.
4.1. Prevalence and Severity of Mental Fatigue
In the present study, we assessed mental fatigue using a self-assessment instrument, the MFS questionnaire, prospectively following the same patient cohort for 5 years. We identified that 58%, 49%, and 52% of the patients experienced mental fatigue 1, 3, and 5 years after an aSAH. These results are comparable to the study by Buunk et al., although they used a different instrument to evaluate mental fatigue and included patients up to 10 years after aSAH. In 133 patients assessed 3 to 10 years after SAH, they found a prevalence of mental fatigue of 48.4% [7]. In another study, Samuelsson et al. reported that moderate-to-severe mental fatigue measured by MFS was present in up to 38% of the patients 15–21 years after aSAH [20]. Taking into account patients with mild mental fatigue, they identified that about 50% of the patients suffer from mental fatigue to some extent. Western et al. reported a mean MFS score of 18.1 in good outcome patients (modified Rankin scale (mRS) 0–2) followed up 12–83 months after aSAH [13]. In this cohort, surgical treatment of the aneurysm, development of cerebral infarction, and acute hydrocephalus were more frequently reported.
While the studies by Buunk et al., Samuelsson et al., and Western et al. highlight the prevalence of mental fatigue long after an aSAH, they do not provide insights into its progression over time. A recent study by de Vries et al. [30] emphasizes the importance of repeated assessments of mental fatigue with results indicating fluctuation of symptoms daily. Evaluating the same cohort of patients prospectively at multiple assessment points provided the opportunity to describe the dynamics of recovery concerning mental fatigue. Our findings are based on data collected from the identical cohort of patients at 1, 3, and 5 years post-aSAH. We observed an overall improvement in mental fatigue over time, although the proportion of patients experiencing any level of mental fatigue did not show a significant decrease. However, as the total reported MFS score decreased, the severity of mental fatigue also declined. Notably, the percentage of patients experiencing severe mental fatigue decreased by half after 3 and 5 years compared to the first year (26%). Additionally, only two patients who did not experience mental fatigue at 1 year reported its presence at the 5-year mark, suggesting a relatively low risk of developing mental fatigue later if it is absent early on during the recovery process.
4.2. Mental Fatigue and Outcome Measures
The follow-up of patients surviving an aSAH does not regularly include specific instruments to detect mental fatigue; instead, functional outcome scales such as the GOSE and mRS are most often used to assess outcomes. Although these scales are practical and straightforward, there is a risk of missing more subtle impairments affecting patients’ recovery and quality of life since fatigue following aSAH may exist without significant functional or cognitive deficits. Although patients with GOSE ranging from 3 to 8 were represented, a median GOSE score of > 6 remained throughout the study period rendering a favorable outcome for our cohort. Mental fatigue was present in patients in all functional outcome groups apart from those fully recovered (GOSE 8). This contrasts with what Ghafaji et al. found, where three out of five patients with no limitations and symptoms after aSAH, categorized as mRS 0 (corresponding to GOSE 8), reported experiencing mental fatigue [31]. Furthermore, they found no difference in MFS score between patients with mRS 0, 1, or 2. There was in our study a relation between MFS score and GOSE, where higher MFS scores were associated with lower functional outcome scores. This is in line with previous studies showing that unfavorable functional outcome is associated with an increased risk of mental fatigue [7, 32]. This suggests that although there may be a relationship between mental fatigue and functional outcome, both GOSE and mRS may be too simplistic instruments in detecting problems related to mental fatigue, even in patients with good outcomes.
4.3. Subaspects Within the MFS
To separately analyze each item of the MFS may add knowledge to which areas contribute the most to the total MFS score. In the present study, all 15 items comprising the MFS instrument were separately analyzed. At the 1-year follow-up, “mental fatigue,” “sensitivity to stress,” “irritability,” and “decreased sleep” were the items where patients experienced the most problems. This is consistent with other studies identifying “mental fatigue” and “sensitivity to stress” as the areas where patients indicate the most problems [13, 33]. Our longitudinal design allowed the examination of the items over time. When the overall MFS score improved, it was for items “fatigue in general” and “sensitivity to stress” patients improved the most. Whether the improvements in these areas depended on patients recovering, adapting coping strategies, or avoiding stressful and tiring situations was beyond the scope of this study. However, a detailed assessment of the items separately allows for more individualized rehabilitation and provides vital information, even in patients who do not reach the cut-off score for mental fatigue.
4.4. The Impact of Sex
Between-sex differences have previously been described regarding fatigue after stroke [34]. In the present study, a significant difference in the prevalence of mental fatigue was observed 1 year after aSAH between women and men, where women reported mental fatigue to a greater extent than men (67% vs. 29%). Women also had significantly higher median MFS scores at 1 and 3 years. Since men only represented less than a quarter of the studied cohort, the results should be interpreted cautiously. However, our findings are consistent with those of Norland et al., where women were more likely to report mental fatigue 1 year after a stroke (46% vs. 28%, respectively) [33]. Similarly, Palm, Rönnbäck, and Johansson found that women had significantly higher MFS scores than men after brain injury [35]. Contrastingly, Jonasson et al. reported no significant sex differences in the MFS scores after brain injury, including traumatic brain injury, stroke, and encephalitis [8]. These inconsistent findings highlight the complexity of sex differences, which are possibly influenced by various social, cultural, and environmental factors.
4.5. The Impact of EBI
Previous studies have established a correlation between acute-phase events and patient outcomes after aSAH [5, 36, 37]. EBI is a complex pathophysiological process that occurs during the first few days after aSAH and is closely linked to LOCi. LOCi develops secondary to global ischemia and increased intracranial pressure, which leads to neuronal cell death and endothelial damage with blood–brain barrier breakdown, cerebral edema, and neuroinflammation [38–41]. LOCi has been shown to be a predictor of fatigue development [18]. In our cohort, the prevalence of mental fatigue at 1 year did not significantly differ between patients experiencing LOCi or not. However, patients without, but not with, LOCi showed significant improvement in mental fatigue between 1 and 5 years.
4.6. Limitations of the Study
Our study has several limitations that should be acknowledged. Firstly, the sample size was relatively small, comprising only 31 patients who completed all follow-up assessments over a 5-year period. Furthermore, the patients who completed the assessments had more favorable outcomes than the initial cohort included in the study. As unfavorable outcomes are associated with higher prevalence and severity of mental fatigue, this study may have underestimated the extent of the problem. However, since some responses were missing also from patients in the higher GOSE groups probably with less mental fatigue, the interpretation of the results requires caution.
Furthermore, our findings indicate an association between lower GOSE scores and higher MFS scores. However, while this relationship may hold true, it is essential to consider that in patients with lower functional outcomes, mental fatigue might still be perceived as a symptom of lesser importance than other sequelae that may significantly impact their daily life and rehabilitation. Another limitation is that we did not assess the presence of comorbidities such as anxiety or depression, which are known to be associated with fatigue after SAH [42, 43]. Furthermore, we did not investigate if patients developed additional diagnoses during the follow-up period that could have contributed to the development of mental fatigue. Self-assessment instruments, like the MFS, may also be more suitable for patients with higher-grade outcomes. Nonetheless, from a clinical standpoint, it provides valuable insights into patients’ subjective experiences of their recovery and daily symptoms.
5. Conclusions
The proportion of patients still suffering from mental fatigue 5 years after an aSAH remains high, around 50%, although there is a decrease in severity over time. Women are at increased risk of developing mental fatigue, and patients experiencing LOCi have reduced potential to recover. Conventional functional outcome scales are possibly too blunt to detect more subtle deficits, and specific instruments are necessary. Self-assessment scales such as the MFS could be easily integrated into the follow-up of aSAH patients, especially in patients with otherwise favorable outcomes.
Ethics Statement
The study adheres to the Declaration of Helsinki, and the protocol was approved by the Ethical Regional Board of Gothenburg, Sweden (053-15, T 213-18). Written informed consent was obtained from all patients or their next of kin before they participated in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-772521, ALFGBG-720511, and ALFGBG-964972), and the Healthcare Board, Region Västra Götaland (VGFOUREG-939556 and VGFOUREG-856851).
Acknowledgments
We would like to thank Editage (http://www.editage.com) for English language editing.
Open Research
Data Availability Statement
Deidentified data may be shared upon reasonable request.




