Hereditary Ataxias: A Genetic Epidemiological Study of a Danish Clinical Cohort
Abstract
Background: Ataxia, characterized by incoordination of movement, presents a diverse etiological spectrum, including genetic forms such as spinocerebellar ataxias (SCAs), Friedreich’s ataxia (FRDA), and other hereditary ataxias. Identifying and understanding the distribution of the genetic subtypes in specific populations are crucial for clinical management, genetic counseling, and prognostication.
Objective: This study is aimed at investigating the genetic epidemiology of hereditary ataxias in a clinical cohort from Eastern Denmark, focusing on the prevalence and distribution of the genetic ataxias.
Methods: We conducted a chart review of 297 patients diagnosed with ataxia from the two major referral centers in Eastern Denmark between 2018 and 2023. Diagnoses were divided into groups: confirmed genetic ataxia, presumed genetic ataxia (no genetic variant identified, positive family history) and possible genetic ataxia (debut before the age of 40, no family history), sporadic adult-onset ataxia (SAOA) (debut after the age of 40, no family history), and multiple system atrophy–cerebellar type (MSA-C)). Data collected included demographics, clinical features, age of onset, and results of genetic testing.
Results: Of the 297 patients, 144 (48.5%) had a confirmed genetic ataxia, 26 (8.8%) were classified as presumed genetic ataxia, and 19 (6.4%) were categorized as possible genetic ataxia. The most common subtypes were SCA6, SCA2, and SCA3. The study revealed notable differences in the prevalence of specific ataxia subtypes compared to global patterns.
Conclusion: This study provides an overview of the epidemiology and genetic landscape of hereditary ataxias in Denmark. The high prevalence of SCA6 and unique distribution patterns emphasizes the need for population-specific data to guide clinical practice. Ongoing trials for SCA1 and SCA3 highlight the importance of understanding the epidemiology of ataxias across different countries to establish trial-ready cohorts and address future treatment needs.
1. Background
Ataxia means “absence of order,” which clinically appears as incoordination of movement. Ataxia as a clinical symptom is characterized by substantial etiological heterogeneity. The spectrum of possible etiologies includes brain lesions (e.g., strokes, tumors, traumatic injuries, and infections), metabolic or toxic factors (e.g., vitamin deficiencies and alcohol abuse), genetic ataxias, and sporadic ataxias. According to the mode of inheritance, the hereditary ataxias can be divided into autosomal dominant, autosomal recessive, mitochondrial, and X-linked ataxias. Our primary objective is to investigate the epidemiology of hereditary ataxias in a clinical cohort representing the eastern part of Denmark.
Spinocerebellar ataxias (SCAs) encompass a diverse range of autosomal dominantly inherited ataxias, with over 50 genetically distinct subtypes, including SCA1 (ATXN1), SCA2 (ATXN2), SCA3 (ATXN3), SCA6 (CACNA1A repeat expansion), SCA7 (ATXN7), and SCA17 (TBP), characterized by a glutamine-encoding CAG repeat expansion in the respective genes (SCA17 with a CAG/CAA repeat). These disorders typically present with progressive impairment of coordination leading to gait disturbances, dysarthria, and oculomotor abnormalities [1–4]. The worldwide prevalence of the SCAs is estimated at 1–5:100,000 individuals, and SCA3 seems to be the most frequent in most populations, followed by SCA2 and SCA6 [5]. There is a large variation between different regions of the world, where SCA3, SCA6, and SCA7 are the most frequent in Northern Europe and specifically SCA7 in Sweden and Finland, representing approximately 50% of families with genetic confirmed SCAs in Scandinavia. However, SCA3 accounts for 20%–50% of families with SCA and is particularly frequent in Portugal, Brazil, China, the Netherlands, Germany, and Japan. It is less frequent in the United States, Canada, France, Mexico, Australia, and India, and very rare in South Africa and Italy [6, 7]. SCA2 accounts for 13%–18% and is particularly frequent in Mexico, Korea, India, Italy, Spain, and Cuba, but rarely seen in Japan [1, 8]. SCA6 is the third most common SCA and accounts for 13%–15% worldwide. It is the most frequent in Germany, The Netherlands, United Kingdom, Taiwan, Australia, the United States, and Japan [9, 10].
Hereditary ataxias are often caused by the expansion of specific trinucleotide sequences within certain genes. When the number of these repeats exceeds a particular threshold, they become pathogenic, leading to disease manifestation. Table 1 shows the genes of SCA1, 2, 3, 6, 7, and 17 with their normal and pathogenic CAG repeats.
| Gene | Normal CAG repeat range | Pathogenic CAG repeat range |
|---|---|---|
| ATXN1 | 6–38 | 39–91 |
| ATXN2 | 13–31 | 32–200 |
| ATXN3 | 12–44 | 55–86 |
| CACNA1A (repeat expansion) | 4–19 | 21–33 |
| ATXN7 | 4–19 | 37–460 |
| TBP | 25–42 | 43–66+ |
Friedreich’s ataxia (FRDA) is an autosomal recessive ataxia, with typical onset in childhood, attributed to a GAA repeat expansion in the FXN gene. FRDA is affecting 1:40,000 individuals [11] and stands as the most prevalent ataxia globally but is rarely seen in Northern Europe [12].
Cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (CANVAS), a relatively newly discovered and potentially more frequent autosomal recessive ataxia, is associated with repeat expansions in the RFC1 gene, presenting with a combination of cerebellar ataxia, sensory neuronopathy, and vestibular dysfunction [5, 13–15]. The prevalence of CANVAS across various countries remains unexplored, although the estimated frequency of biallelic expansion in RFC1 is approximately 1 in 20,000 individuals (18). However, the prevalence of symptomatic CANVAS is anticipated to be lower due to the onset of symptoms typically occurring in the sixth decade of life [16].
Primary mitochondrial diseases (PMDs) are common metabolic disorders featuring ataxia as a cardinal symptom, often due to pathogenic variants in mitochondrial or nuclear DNA, encoding mitochondrial proteins. PMDs are the most frequent metabolic disorders in humans, with a prevalence of approximately 1 in 4300 adults in the United Kingdom. However, little is known of the true epidemiological burden of mitochondrial ataxias [17].
Fragile X-associated tremor/ataxia syndrome (FXTAS) primarily affects males and results from a premutation (55-200 CGG) in FMR1 on the X chromosome, leading to ataxic symptoms and other neurological manifestations [18, 19].
Novel genetic causes of ataxia continue to be discovered.
The treatment options for hereditary ataxias remain limited, but recently omaveloxolone, an NRF2 activator, has been approved in the United States (2023) and EU (2024) as the first drug for Friedreich’s ataxia (FRDA) [20]. Additionally, a clinical trial is currently underway, testing intrathecal administration of VO659 for SCA1 and SCA3 (Phase 1/2a) [21]. The approval of treatment for FRDA and ongoing trials for SCA1 and SCA3 highlight the importance of understanding the epidemiology of ataxias across different countries to better address future treatment needs. To support this, we have conducted an epidemiological study of frequencies of hereditary ataxias in Eastern Denmark.
2. Methods
A chart review was conducted on patient records from two outpatient clinics covering the eastern part of Denmark, between January 2018 and December 2023. The two clinics were the Danish Dementia Research Centre, Rigshospitalet, Copenhagen University Hospital, and the Movement Disorders Clinic, Bispebjerg Hospital, Copenhagen University Hospital, serving as a tertiary referral center for genetic movement disorders and atypical Parkinson’s disease, respectively. Diagnoses adhered to the ICD-10 classification, and Table 2 shows the codes encompassing the different diagnoses used. Patient charts with any of these diagnoses or indicating suspicion of slowly progressive ataxia during the 5-year study period were included in the evaluation, with documented clinical data such as symptoms and age at onset. Also, demographic data such as sex, educational level, partnership status, offspring, diagnostic investigations, way of referral, and any hospital ward referrals were recorded. In cases where the patient belonged to a family with a specific known genetic etiology, testing for the specific variant was initiated. In other cases, screening for SCA repeats disorders (SCA1, 2, 3, 6, 7, 17, and DRPLA), and, in relevant cases, FMR1, FRDA, and RFC1 testing (from 2020) was performed. Subsequently WES- or WGS-based panel testing of genes involved in ataxia was performed selectively. We have divided the diagnoses in groups inspired by the SPORTAX [22]: Confirmed genetic ataxia, presumed genetic ataxia (no genetic variant identified, positive family history) and possible genetic ataxia (onset before the age of 40, no family history), sporadic adult-onset ataxia (SAOA) (onset after the age of 40, no family history), and multiple system atrophy–cerebellar type (MSA-C)).
| ICD-10 code | Disease name |
|---|---|
| DG111 | Early-onset cerebellar ataxia |
| DG112 | Late-onset cerebellar ataxia |
| DG114 | Hereditary spastic paraplegia |
| DG118 | Other hereditary ataxias |
| DG119 | Hereditary ataxias, unspecified |
| DG233 | Multiple system atrophy, cerebellar type (MSA-C) |
| DG239 | Degenerative disease of basal ganglia, unspecified |
| DG312 | Degeneration of nervous system due to alcohol |
| DG319 | Degenerative disease of nervous system, unspecified |
| DR270 | Ataxia, unspecified |
3. Results
A total of 297 patients were registered with ataxia across the two centers. Of these, 144 (48.5%) patients had a confirmed genetic ataxia, 26 (8.8%) were classified as presumed genetic ataxias, and 19 (6.4%) were classified as possible genetic ataxias. The remaining patients did not fall into these categories: 69 patients were diagnosed with a presumed neurodegenerative disease such as MSA-C or SAOA, 9 patients had an acquired ataxia, and 30 patients did not receive a final diagnosis of ataxia.
Figure 1 shows the relative frequency of genetic ataxia subtypes. The six most common causes of hereditary ataxias in this clinical cohort were 26 patients with a pathogenic repeat expansion in CACNA1A followed by 22 with ATXN2, 17 with ATXN3, 14 with CACNA1A-associated ataxia caused by pathogenic single nucleotide variants (SNVs), 11 with PRKCG, and 9 with ATXN1. Four patients had FRDA, all harboring a biallelic GAA repeat expansion in Intron 1 of the FXN gene. Five patients had FXTAS. Four patients had myoclonic epilepsy associated with ragged-red fiber (MERRF syndrome).
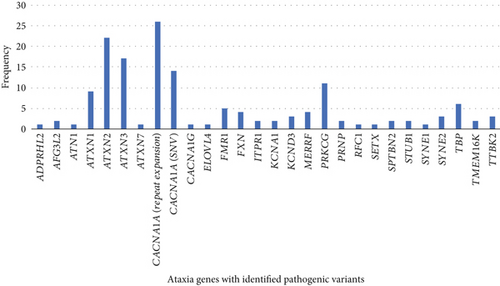
Approximately 2.6 million people live in Eastern Denmark, which is divided into the Capital Region of Denmark and the Region Zealand, representing 44% of the Danish population. Based on this data, approximately 0.01% will have a hereditary ataxia in the Capital Region of Denmark and the Region Zealand. The population in Denmark is relatively homogeneous, and projecting this prevalence to the entire Danish population seems credible. The Danish population is 5.9 million. Therefore, it can be extrapolated from the data that a minimum of 590 patients in Denmark are living with a hereditary ataxia.
Figure 2 shows the age of onset of hereditary ataxias in the cohort. Of the six most frequent inherited ataxias, the age of onset for CACNA1A (repeat expansion) was 59 years old; for ATXN2, 42 years old; for ATXN3, 41 years old; for CACNA1A-associated ataxia caused by pathogenic SNVs, 16 years old; for PRKCG, 41 years old; and for ATXN1, 45 years old.
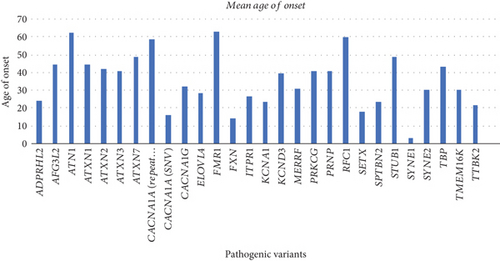
Table 3 shows the variant frequencies and the frequencies of symptoms/signs noted by patient or physician of the six most common hereditary ataxias in the cohort. The average SARA (Scale for the Assessment and Rating of Ataxia) score was calculated based on the first medical examination upon referral. Numbers in parenthesis are percentage of symptoms/signs associated to the pathogenic variant. We had a few missing data from ATXN1, ATXN2, and ATXN3 patients where a symptom/sign did not appear in the record. The missing data are not shown in the table but can be acquired by inquiry.
| Pathogenic variant (n) | Diplopia | Dysartria | Dysphagia | Ataxia in upper extremities | Ataxia lower extremities | Gait problems | Unsteadiness | Cognitive problems | Psychiatric problems | SARA |
|---|---|---|---|---|---|---|---|---|---|---|
| CACNA1A (repeat expansion) (26) | 14 (54%) | 22 (85%) | 7 (27%) | 19 (73%) | 23 (88%) | 23 (88%) | 26 (100%) | 3 (12%) | 4 (15%) | 12 |
| ATXN2 (22) | 2 (9%) | 20 (91%) | 6 (27%) | 14 (64%) | 21 (95%) | 21 (95%) | 22 (100%) | 7 (32%) | 7 (32%) | 11 |
| ATXN3 (17) | 12 (71%) | 14 (82%) | 11 (65%) | 13 (76%) | 15 (88%) | 16 (94%) | 17 (100%) | 4 (24%) | 4 (24%) | 15 |
| CACNA1A (SNV) (14) | 1 (7%) | 7 (50%) | 1 (7%) | 5 (36%) | 6 (43%) | 11 (76%) | 13 (93%) | 1 (7%) | 0 (0%) | 7 |
| PRKCG (11) | 3 (27%) | 11 (100%) | 0 (0%) | 5 (45%) | 8 (73%) | 11 (100%) | 11 (100%) | 2 (18%) | 3 (27%) | 10 |
| ATXN1 (9) | 2 (22%) | 9 (100%) | 4 (44%) | 4 (44%) | 9 (100%) | 8 (89%) | 9 (100% | 2 (22%) | 3 (33%) | 11 |
4. Discussion
This study provides an overview of the epidemiology of hereditary ataxias within a Danish clinical cohort, emphasizing the prevalence and genetic diversity of SCAs. Our findings indicate that SCA6 is the most prevalent cause of hereditary ataxia in this region, followed by SCA2, SCA3, CACNA1A SNVs, PRKCG, and SCA1. This distribution aligns with other European population-based studies, where SCA1, SCA2, SCA3, and SCA6 are commonly reported as leading causes of autosomal dominant ataxias [1]. However, the high prevalence of SCA6 in Denmark is notable and contrasts with global data, where SCA6 is less common [2, 3]. The increased prevalence of SCA6 observed in our study is unlikely to be due to a founder effect. A clear family history was present, with affected individuals originating from distinct, unrelated families. Moreover, a 2025 study conducted by Waerling et al. reported comparable findings, encompassing data from molecular genetic testing for polyglutamine ataxias from the entire Denmark and demonstrated an even distribution of cases across the country [23]. Therefore, we do not have the impression that a single ancestral allele is driving this observation. However, future studies could be aimed at investigating whether the same haplotype is inherited across different Danish families or whether multiple haplotypes contribute to the high prevalence of SCA6 in Denmark. Such research could provide deeper insights into potential genetic and environmental factors influencing the observed distribution of hereditary ataxias in Denmark. Additionally, SCA3, which is the most common SCA worldwide, is less frequently seen in our study, a pattern consistent with findings from other studies in Northern Europe [4, 13].
Interestingly, our data reveal an unusually high frequency of PRKCG in Denmark, accounting for a notable proportion of cases. We do not suspect founder effect to be the reason, as several different pathogenic variants were identified. This contrasts with global reports where PRKCG is relatively rare, comprising only 1%–4% of SCAs [24].
When looking at the six most frequent inherited ataxias, we found that the correlation between CAG repeat length and age of onset in SCA patients follows an inverse relationship: The higher the number of repeats, the earlier the onset of symptoms. This phenomenon is particularly evident in SCA1, SCA2, and SCA3; however, in SCA6, the correlation is weaker, as repeat expansions are generally smaller and show a more variable onset [25]. The reported SARA score reflects the assessment conducted at a specialized neurogenetic center and may primarily indicate the severity of symptoms/signs at the time of referral. This could reflect the threshold at which both patients and physicians decide to seek or initiate specialist evaluation. SCA3 patients had the highest SARA scores at first evaluation, reflecting its known early onset and rapid progression. In contrast, SCA6 showed moderate severity despite later onset, consistent with its milder and slower course [4].
FRDA, the most prevalent autosomal recessive ataxia worldwide, remains relatively rare in our cohort, consistent with its low frequency in Northern Europe. This aligns with previous epidemiological studies, in accordance with the described geographic variability in FRDA prevalence [5, 13].
The inclusion of RFC1 in our study, despite the relatively recent implementation of clinical testing in Denmark, highlights a potential underestimation of its prevalence due to the late onset of symptoms typically in the sixth decade of life. As genetic testing becomes more widespread, it is likely that the reported frequency of RFC1 will increase, providing a more accurate epidemiological picture.
In terms of treatment, the recent approval of omaveloxolone for FRDA and ongoing clinical trials for SCAs highlight the critical need for precise epidemiological data to inform clinical practice and resource allocation [20, 21]. Understanding the distribution of hereditary ataxias in Denmark aids in anticipating healthcare needs and tailoring interventions accordingly.
5. Strengths and Limitations
The strength of this study lies in its comprehensive approach, using a well-defined clinical cohort. However, several limitations should be acknowledged. The retrospective nature of the study may introduce selection bias, and the reliance on clinical records may result in underreporting of less common ataxias or atypical presentations. Potential siblings and children are not systematically reported in the charts, which leaves a risk of underestimating the prevalence of especially the autosomal dominant ataxias. However, when suspecting a neurogenetic disease, national neurological guidelines in Denmark strongly recommend referral to tertiary highly specialized neurogenetic centers. Therefore, we do not believe that a significant number of patients are diagnosed at primary and secondary levels. Additionally, the relatively small sample size adds a risk of both underestimating and overestimating the population prevalence, which also limits the generalizability of our findings to broader populations.
Future research should be aimed at expanding the cohort size and include prospective data collection to strengthen the robustness of the epidemiology. Furthermore, exploring the genetic basis of idiopathic ataxias through advanced genomic technologies could uncover new pathogenic variants, contributing to a more nuanced understanding of hereditary ataxias (e.g., the newly discovered SCA4 and SCA27B) [26, 27].
6. Conclusion
This genetic epidemiological study provides an insight into the distribution and clinical characteristics of hereditary ataxias in Eastern Denmark. The identification of SCA6 as the most prevalent subtype and the notable frequency of PRKCG highlight the importance of regional genetic studies in uncovering unique population-specific patterns. These findings emphasize the need for continued research and genetic testing to improve diagnosis, management, and treatment of hereditary ataxias.
Ethics Statement
The study was approved by the National Ethics Committee (221244). We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent
All genetic analyses were performed in a clinical setting, after informed consent was obtained from the patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




