Development of Probiotics for Male Reproductive Tract Health: In Vitro Experiments and Clinical Trial on Healthy Volunteers
Abstract
Background: Health of the male urogenital tract is closely related to the microbial communities in this anatomical area. Disturbed microbial communities can be balanced with the help of carefully selected strains in which the presence of both general and specific probiotic effects has been described.
Objectives: We aimed to develop novel probiotic that could be used to support male reproductive health.
Materials and Methods: Suitable lactobacilli strains were screened from culture collection in order to find the most suitable strains for male genital tract. Clinical trial included 10 volunteer men who considered themselves healthy. They consumed enteric-coated oral capsules containing four lactobacilli strains. Blood markers and questionnaire data were recorded during the consumption period.
Results: The selected lactobacilli strains (two L. crispatus, one L. gasseri, one L. ruminis) expressed good tolerance to high pH and antagonistic activity against prostatitis-associated opportunistic bacteria. Metabolic profiling of the selected strains revealed beneficial properties for both reproductive and general health. The probiotic capsules appeared to be generally well tolerated during 1-week period. No serious adverse events and no significant changes in haemogram were observed.
Conclusions: The study confirmed that certain probiotic properties in lactobacilli are highly strain-specific, and we succeeded to select a set of strains that can be suitable for supporting male reproductive health. Our study suggested that daily oral administration of the selected strains at the dose level of billion CFU/dose during 1 week is well tolerated by healthy middle-aged men. Therefore, these strains seem to be promising candidates for the development of novel evidence-based well-focused probiotics to target male urogenital tract disorders. Their effectiveness must be confirmed in clinical trials on diseased patients.
1. Introduction
Health of the male urogenital tract is closely related to the microbial communities in this anatomical area [1]. Microbiota of sperm affects its quality [2], and associations have been found between the microbiota of the male reproductive system and infertility [3]. Moreover, disturbed microbiota of the urogenital tract can influence the development of tumors in the same region [4]. One of the most frequent diagnoses of the genitourinary tract in men under the age of 50 is prostatitis [5, 6]. Prostatitis syndrome can be caused by several opportunistic pathogens (like coliforms and enterococci), but more often the prostatitis is associated with imbalanced microbial community in the male genital organs. In that case, individual microbes with low virulence can form a more virulent community that may lead to infection [5, 7].
Disturbed microbial communities can be balanced with the help of probiotics. In vitro experiments have shown that probiotic lactobacilli can improve sperm motility and viability [8], positively affect their immune markers [9], and reduce inflammatory response of prostatic epithelium induced by bacterial infection [10]. Animal experiments have proven that probiotics can improve sperm quality [11–14], affect hormonal balance [15, 16], and prevent testicular damage [17, 18] and stress-induced male reproductive deficits [19]. Probiotics have also been tested in scarce clinical trials. Probiotic administration significantly improved sperm quality in infertile men [20–22], increased pregnancy success in couples undergoing ART [23], and even mitigated stress-induced male reproductive dysfunction [24]. They have been applied to chronic prostatitis patients in order to balance the urogenital tract microbiota, alleviate the prostatitis symptoms, and prevent new episodes [25–30]. The probiotic strains used in these trials were mostly nonspecific with respect to reproductive health.
We aimed to develop a novel probiotic that could be used to support male reproductive health. To achieve this goal, we carried out several laboratory experiments and a preliminary clinical trial in order to obtain information about safety and tolerability in healthy men.
2. Material and Methods
2.1. Lactobacilli Strains and Laboratory Methods
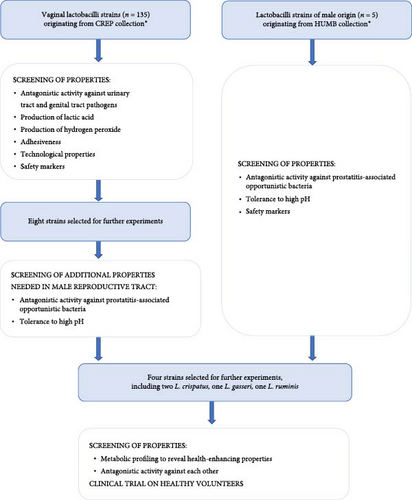
There is a culture collection of bacteria originating from the reproductive tract (CREP) in Celvia CC AS (former Competence Centre of Health Technologies, Tartu, Estonia). This collection forms a basis for the development of novel probiotics for balancing reproductive tract microbiota. During the previous studies, properties of 135 vaginal lactobacilli strains were screened [31]. The strains were tested for several functional properties (lactic acid and hydrogen peroxide production, antagonistic activity against urinary tract and genital tract pathogens, autoaggregative and adhesive ability), technological properties (lyophilisation, shelf, life, growth curves), and safety (absence of hemolytic activity and transferrable antibiotic resistance).
In the frame of this study, additional properties in eight strains of the same set (including six strains of Lactobacillus crispatus, one strain of L. gasseri and one strain of L. jensenii) were tested in order to find suitable strains for male genital tract—tolerance to high pH and antagonistic activity against prostatitis-associated opportunistic bacteria (Table 1). The properties of these eight strains were compared with that of five strains originating from male organisms (including three strains of L. ruminis and two strains of L. otakiensis) that were obtained from Human Microbiota Biobank (HUMB). Both collections can be found at https://eemb.ut.ee/eng/.
| Species | Strain | Tolerance to pH (OD 660 nm)1 |
Score | Antagonistic activity against E. coli2 |
Score | Antagonistic activity against P. bivia2 |
Score | Total score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 7.3 | pH 7.7 | pH 8.1 | ||||||||||||
| L. otakiensis | HUMB 9487 | 0.207 | 0.088 | 0.084 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L. otakiensis | HUMB 8799 | 0.229 | 0.104 | 0.086 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L. ruminis | HUMB 8194 | 0.225 | 0.095 | 0.078 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 6 |
| L. ruminis | HUMB 8337 | 0.696 | 0.600 | 0.335 | 3 | 3 | 5 | 4 | 4 | 2 | 2 | 2 | 2 | 9 |
| L. ruminis | HUMB 9667 | 0.566 | 0.189 | 0.084 | 2 | 2 | 1 | 2 | 1.7 | 2 | 2 | 2 | 2 | 5.7 |
| L. crispatus | EST-1 | 0.801 | 0.709 | 0.214 | 3 | 5 | 3 | 3 | 3.7 | 2 | 2 | 2 | 2 | 8.7 |
| L. crispatus | EST-2 | 0.610 | 0.777 | 0.775 | 4 | 5 | 4 | 4 | 4.3 | 2 | 2 | 2 | 2 | 10.3 |
| L. crispatus | EST-3 | 0.654 | 0.147 | 0.074 | 2 | 5 | 4 | 3 | 4 | 3 | 3 | 3 | 3 | 9 |
| L. crispatus | EST-4 | 0.314 | 0.094 | 0.084 | 0 | 5 | 5 | 7 | 5.7 | 0 | 0 | 0 | 0 | 5.7 |
| L. gasseri | 83 | 0.606 | 0.442 | 0.309 | 2 | 3 | 4 | 5 | 4 | 2 | 2 | 2 | 2 | 8 |
| L. jensenii | 100 | 0.774 | 0.512 | 0.109 | 3 | 4 | 4 | 5 | 4.3 | 0 | 0 | 0 | 0 | 7.3 |
| L. crispatus | EST-5 | 0.790 | 0.823 | 0.654 | 4 | 4 | 4 | 4 | 4 | 0 | 0 | 0 | 0 | 8 |
| L. crispatus | EST-6 | 0.984 | 1.040 | 0.722 | 4 | 4 | 3 | 4 | 3.7 | 4 | 4 | 4 | 4 | 11.7 |
- Note: The selected strains that were used in capsules are indicated in bold.
- 1Initial density of bacteria was 107 CFU/ml (colony forming units per milliliter). Positive growth after 24 h was considered >0.15 OD (optical density) 660 nm. Mean results of three parallels are given.
- 2The inhibition zones were measured in millimeters. Experiments were done in three parallels.
Differently from the vaginal environment, the pH is slightly basic in male genital tract, and therefore, the growth of the strains in liquid medium was assessed as optical density (OD 660 nm) at pH 7.3, 7.7, and 8.1 as described previously [32]. Antagonistic activity against prostatitis-associated Escherichia coli (three clinical strains) and Prevotella bivia (three clinical strains) was measured using agar spot method as described previously [31]. As a result, a set of the most promising strains was selected for clinical studies (Figure 1).
Metabolic profiling of lactobacilli strains grown in liquid De Man–Rogosa–Sharpe medium (MRS) medium for 24 h was carried out with a Xevo TQ-XS mass spectrometer (Waters Corporation, Milford, CT, USA) coupled with ACQUITY ultraperformance liquid chromatography (Waters Corporation, Milford, CT, USA) using MxP Quant 500 kit (Biocrates Life Sciences AG, Austria) and in-house protocols for vitamins and short-chain fatty acids (SCFA), as described previously [33]. The Biocrates MxP500 kit allows determination of over 630 different metabolites from one sample, including essential amino acids, amino acid-related compounds, fatty acids, and bile acids. The samples were prepared and analyzed according to the manufacturer’s instructions. In brief, the samples were transferred onto filter papers preloaded with internal standards. Upon drying, all compounds containing amino groups were derivatised with phenyl isothiocyanate and dried again. Finally, the metabolites were extracted with 5 mM ammonium acetate in methanol. The vitamins were analyzed according to Waters online notes [34]. SCFA were derivatized with excessive 2-nitrophenylhydrazine in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and separated on ACQUITY Premier BEH C18 Column with VanGuard FIT, 1.7 µm, 2.1 × 100 mm column.
2.2. Study Subjects of Clinical Trial
The clinical trial was carried out in collaboration with Celvia CC AS (Tartu, Estonia) and MediTA Clinic (Tartu, Estonia) from September 20 to December 20, 2022. The study group included 10 volunteer men who considered themselves healthy. Inclusion criteria were as follows: age 18–65 years, desire to participate in the clinical trial, and no known health problems. Exclusion criteria included inflammations of urogenital tract, history of chronic diseases (including diabetes, cardiovascular diseases, and allergies), acute current infections, use of antimicrobial agent within the preceding month, use of any regular concomitant medication including nonsteroidal anti-inflammatory drugs, and blood donation within the last month. Absence of chronic diseases was confirmed with the help of a questionnaire and blood analysis.
2.3. Ethical Considerations
Participation in the study was voluntary. Written informed consent was obtained from all subjects, and all men were informed that they could withdraw from the study at any time. The study protocol was approved by the Research Ethics Committee of the University of Tartu (Permission No. 368/M-5, 19.09.2022).
2.4. Study Material and Protocol
The study material included enteric-coated oral capsules that contained four different strains—three strains of female origin (two L. crispatus, one L. gasseri) and one strain of male origin (L. ruminis). The strains were selected during previous in vitro experiments as described in the first section of Results (Table 1). In addition, the strains did not have antagonistic activity to each other, and their antibiotic susceptibility profile met the requirements of the European Food Safety Authority. Probiotic bacteria were lyophilized and packaged in enteric-coated capsules (300 mg, size 2).
The eligible participants were recruited into the trial that was registered in BioMed Central (ISRCTN54942056). The subjects were asked to complete a general questionnaire about their lifestyle, sexual behavior, and medical history (Table S1). The absence of chronic diseases was confirmed with the help of blood tests that included haemogram, high-sensitive C-reactive protein (hs-CRP), markers of liver and kidney function (ASAT, ALAT, GGT, creatinine, EGFR), blood glucose, glycated haemoglobin (HbA1c), cholesterol and its fractions, prostate-specific antigen (PSA), and hormonal markers, using certified assays at Synlab Eesti OÜ (Table 2).
| Variable | Reference interval | Before consumption1 | After consumption1 | p-Value |
|---|---|---|---|---|
| Hemoglobin (g/L) | 134–170 |
|
|
0.76 |
| Hematocrit (%) | 40–49 |
|
|
0.88 |
| WBC (10E9/L) | 4.1–9.7 |
|
|
0.06 |
| RBC (10E12/L) | 4.5–5.7 |
|
|
0.57 |
| Platelets (10E9/L) | 157–372 |
|
|
0.38 |
| Neutrophils (10E9/L) | 1.9–6.7 |
|
|
0.34 |
| Eosinophils (10E9/L) | 0.02–0.4 |
|
|
0.57 |
| Basophils (10E9/L) | 0.01–0.08 |
|
|
0.59 |
| Monocytes (10E9/L) | 0.24–0.8 |
|
|
0.86 |
| Lymphocytes (10E9/L) | 1.3–3.1 |
|
|
0.44 |
| hs-CRP (mg/L) | <1.0 |
|
|
0.61 |
| HbA1c (mmol/mol) | <42 |
|
|
0.91 |
| Glucose (mmol/L) | 4.1–6.0 |
|
|
0.87 |
| Cholesterol (mmol/L) | <5.0 |
|
|
0.85 |
| LDL (mmol/L) | <3.0 |
|
|
0.54 |
| HDL (mmol/L) | >1.0 |
|
|
1 |
| GGT (U/L) | <60 |
|
|
0.97 |
| ALAT (U/L) | <50 |
|
|
0.85 |
| ASAT (U/L) | <50 |
|
|
0.64 |
| Creatinine (µmol/L) | 59–104 |
|
|
0.05 |
| EGFR (mL/min/1.73 m2) | >90 |
|
|
0.14 |
| Estradiol (pmol/L) | <161 |
|
|
0.48 |
| Testosterone (nmol/L) | 8–30 |
|
|
0.90 |
| SHBG (nmol/L) | 14–71 |
|
|
0.59 |
| FAI (%) | 24–72 |
|
|
0.65 |
| PSA (µg/L) | <1.8 |
|
|
0.67 |
- Abbreviations: ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; CRP, C-reactive protein; EGFR, estimated glomerular filtration rate; FAI, index of free androgen; GGT, gamma-glutamyl transferase; HbA1c, glycohaemoglobin; HDL, high-density lipoprotein; Ig, immune globulin; LDL, low-density lipoprotein; PSA, prostate-specific antigen; RBC, red blood cells; SHBG, sex hormone-binding globulin; WBC, white blood cells.
- 1Data are presented as mean ± SD [median (quartiles)].
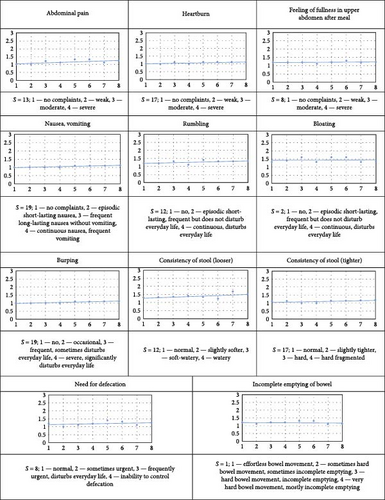
Study participants consumed 1 capsule per day (daily dose of probiotic bacteria 10E9 colony-forming units [CFUs]) for 1 week. Blood markers were recorded at the beginning and at the end of the trial. The participants filled out a short daily questionnaire during the consumption period about their general and gastrointestinal health (Figure 2). Primary outcome measure included the safety and tolerability of the probiotic capsules. Secondary outcome measures included possible beneficial effects on blood markers and/or self-evaluated health.
2.5. Statistical Methods
Statistical analysis was performed using Past 4.03 (www.uio.no). All measurements of blood markers were given as means and standard deviation, and median and quartiles. A statistical evaluation of the significance of the differences before and after capsule consumption was performed by using the Mann–Whitney rank sum test (in case of nonparametric distribution) or t-test (in case of parametric distribution). Mann–Kendall trend test was used to reveal time trends. Differences were considered statistically significant if the p-value was <0.05.
3. Results
3.1. Laboratory Experiments for the Development of Novel Probiotics
Main results of the in vitro experiments are presented in Table 1. Lactobacilli are adapted to multiply in acidic environments, and their growth rate in the case of alkaline pH appeared to be strain-specific. Both strains of L. otakiensis, one strain of L. ruminis and one strain of L. crispatus, did not tolerate the tested pH values (7.3, 7.7, and 8.1). Antagonistic activity against E. coli was missing in both L. otakiensis strains. Antagonistic activity against P. bivia was also missing in these L. otakiensis strains, but also in two L. crispatus strains and a L. jensenii strain. According to total scores, four strains belonging to three species were selected for clinical trial (two L. crispatus, one L. gasseri, and one L. ruminis).
3.2. Metabolic Profiling of Lactobacilli
All strains belonging to probiotic mixture significantly increased the amount of caprylic acid (C8) and lactate in the environment in comparison with its initial composition (Table S2). Some strains produced other short-chain fatty acids, like butyric (C4), propionic (C3), and acetic (C2) acids. Production of some vitamins and related substances was also noted among the strains, including choline (B4), pyridoxamine, pyridoxine and pyridoxal (B6), nicotinic acid (B3), and thiamine pyrophosphate (B1). Two of the strains enriched the environment with omega-3 long-chain polysaturated fatty acids (eicosapentaenoic acid [EPA], docosahexaenoic acid [DHA]). Two strains decreased the homocysteine level. All strains decreased the levels of histamine, glucose, and cortisol in the environment. The levels of oxidative stress (OxS)-related methionine sulfoxide (Met-SO), asymmetric dimethyl arginine (ADMA), and symmetric dimethyl arginine (SDMA) were lowered while that of an antioxidant cysteine was increased in environment.
3.3. Population Data of Clinical Trial
Ten healthy volunteer men were initially screened and enrolled in the study and all 10 men completed the study. The subjects were 38.6 ± 10.4 years old. Eight men self-assessed their general health as good or very good, while two men assessed their health as fair. Their background data are presented in Table S1.
3.4. Safety and Tolerability of Probiotic Mixture
The probiotic capsules appeared to be generally well tolerated during the 1-week trial. No serious adverse events were observed (Figure 2). Weak abdominal pain (score 2) during the second half of the consumption week was reported by two men. One of them also reported some other weak complaints during the same period—heartburn, nausea, rumbling, and burping and increased feeling of fullness in upper abdomen after a meal that was accompanied by slightly tighter stool consistency. On the other side, one man reported a decrease of feeling of fullness in the upper abdomen after a meal and two men reported a decrease of rumbling.
Blood markers detected before and after probiotic consumption are presented in Table 2. Probiotic consumption did not cause significant changes in haemogram. Mild statistically insignificant decrease in white blood cell (WBC) count appeared, but the values in all men remained in the frame of the reference interval. Borderline increase of creatinine level was noted, but the values in all men remained in the frame of the reference interval. Total cholesterol and low-density lipoprotein (LDL) were slightly over the reference value in half of the study subjects. Probiotic consumption did not change it. No significant impact was revealed on hs-CRP, glucose, liver markers, hormones, and PSA level.
4. Discussion
Microbial communities of the human body are significantly associated with health, including reproductive health. Imbalance of these communities may lead to a variety of diseases, including bacterial vaginosis in women and prostatitis in men. Lactobacilli form an important part of microbiota in the reproductive tract, comprising the highest proportion in a healthy vagina and being the gatekeepers of the vaginal ecosystem [35]. Proportion of lactobacilli is lower in the male reproductive tract, but they also perform a significant role in this biotope—higher concentrations of seminal lactobacilli are associated with better semen quality [2]. It creates the opportunity to use lactobacillar probiotics for balancing the microbial communities in order to improve reproductive health.
Probiotics are beneficial microorganisms that provide health benefits when consumed in suitable amounts [36]. Most probiotic bacteria are lactic acid-producing Gram-positive bacteria of the genera Lactobacillus and Bifidobacterium that belong to the microbiota of gastrointestinal and reproductive tract. The use of probiotics has shown a potentially beneficial effect in terms of semen characteristics and quality parameters in animal models, including rats, mice, chickens, and zebrafish (reviewed by [37]). Human data are more limited; however, semen quality and prostatitis have been targeted in some studies. Valcarce et al. [20] showed that L. rhamnosus and B. longum supplementation over a 6-week period improved sperm motility and decreased sperm DNA fragmentation in asthenozoospermic males. In another study of men with oligoasthenoteratospermia, a symbiotic mixture (L. paracasei with arabinogalactan, fructo-oligosaccharides, and L-glutamine) had a positive impact on sperm count, progressive motility, and percentage of typical forms of sperms [21]. In a study by Helli et al. [22], the men with idiopathic oligoasthenoteratozoospermia received a probiotic mixture of seven strains for 10 weeks that significantly increased ejaculate volume, number, concentration, and the percentage of motile sperm, and total antioxidant capacity of plasma while decreasing the concentration of plasma malondialdehyde and inflammatory markers. As concerns prostatitis, two studies [25, 26] have indicated the usefulness of the probiotic mixture VSL#3 that contains eight strains of lactic acid bacteria (S. salivarius subsp. thermophilus, L. casei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, B. longum, B. infantis, and B. breve). This mixture lowered both prostatitis symptom score and WBC count in semen. Combination of two lactobacilli (L. plantarum LP01 and L. paracasei LPC09) and a plant Serenoa Repens prevented the episodes of prostatitis [27]. Manfredi et al. [28] tested the nonpathogenic E. coli Nissle 1917 strain and found that it lowered both symptom score and recurrence rate of prostatitis. In a study of Pacifici et al. [29], consumption of probiotic formulation PRO-Men Hyperbiotics (L. plantarum 6595, L. reuteri, L. fermentum, L. casei, L. acidop h ilus, B. longum, and Meriva Curcumin Phytosome) resulted in a reduction of the bacterial load of E. coli and E. faecalis in urine.
There are some probiotics-containing food supplements for male health on the market, but in most of the cases, they do not have the strain designation behind the species name or references to scientific research on their properties and effects. We attempted to create a novel evidence-based probiotic mixture for men by performing several experiments, as the properties and impact on health are significantly strain-specific among lactic acid bacteria. Since lactobacilli in the male reproductive tract are frequently unculturable, the collection of lactobacilli originating from the female reproductive tract was included in the study, in addition to lactobacilli originating from male organism. The four-strain set of three species was selected for clinical trial. L. ruminis belongs to obligately homofermentative lactobacilli, similarly to L. crispatus and L. gasseri, and also previous studies have suggested that this species is suitable for probiotic development [38]. The set also included three vaginal strains [31] that raises a question about their suitability for the male reproductive tract. A previous in vitro study revealed that pretreatment with vaginal probiotics prevented the detrimental effect of Fe2+ on sperm motility and viability [8], thus pointing the possible beneficial impact of vaginal lactobacilli on semen. We tested the tolerance of vaginal strains to high pH that is present in the male reproductive tract and selected only the strains that were able to multiply in these conditions. We also tested their antagonistic activity against bacteria that are commonly associated with prostatitis.
According to the metabolome profile, the selected strains had several beneficial properties that support both reproductive and general health. All four strains significantly increased the amount of caprylic acid in the environment. This metabolite has numerous beneficial effects, including energetic, digestion-supporting, anti-inflammatory, antimicrobial (antibacterial, -viral, and -fungal), and it has been associated with the prevention of urinary tract and other infections [39–41]. All strains were powerful producers of lactate, that is an important antimicrobial substance in the reproductive tract. Prostatitis is associated with OxS, both in the reproductive tract and the whole body [42]. The selected strains appeared to be able to reduce OxS by increasing the level of cysteine and lowering the levels of Met-SO, ADMA, and SDMA in the environment. Moreover, since these probiotic strains are consumed orally then general health-supporting properties give additional value. In addition to caprylic acid, some strains produced other short-chain fatty acids, like anti-inflammatory butyric, propionic, and acetic acids, as well as several vitamins. Two strains enriched the environment with EPA and DHA that possess anti-inflammatory, antihypertensive, antiarythmic, and antithrombotic effects [43]. Two strains decreased the homocysteine level that is associated with decreased stroke risk [44]. All strains decreased the glucose and cortisol levels in the environment, an excess of both substances in the body being unwanted. All strains decreased histamine levels, that is beneficial for preventing allergies and histamine-related headaches.
European Food Safety Association (EFSA) has placed the lactobacilli in the list of bacteria with qualified presumption of safety (QPS). However, safety and tolerability of novel probiotics are generally tested with the help of blood markers and questionnaire data on healthy volunteers, and thereafter, the trials on the patients will be conducted. Participants of our trial consumed the probiotic mixture at 10E9 CFU per day. Previous studies have indicated suitability of daily doses of 109–1011 CFU [45, 46]. The safety of two L. crispatus strains was previously tested in healthy volunteers [47], but they were not tested together with novel L. gasseri and L. ruminis strains.
Since it is not easy to apply the probiotics directly into the male reproductive tract, we used oral administration mode that has been used also in previous trials. The capsules were enteric-coated, to ensure the viability of lactobacilli until the distal part of intestinal tract. Moreover, it has been shown that gut microbiota supports male reproduction via nutrition, immunity, and signaling [48]. Hence, modulation of gut microbiota during probiotic treatment may be considered as a positive side effect.
Limitations of the study include the small sample size in the clinical trial and its short duration. According to our previous experience with clinical trials (mostly with L. fermentum ME-3), the willingness to participate and the compliance are higher in female than male participants. Therefore, the present trial was designed to be shorter and with a smaller sample size than the trial on healthy volunteer women that we performed previously [47]. Participation was offered to several dozen men to obtain 10 participants. The small sample size limited the statistical power and increased the risk of false negativity, so these results should be considered preliminary. In addition, the long-term safety and tolerability could not be observed. On a positive note, all 10 who agreed to participate also completed the trial.
In conclusion, the study confirmed that certain probiotic properties in lactobacilli are highly strain-specific, and we succeeded in selecting a set of strains that can be suitable for supporting male reproductive health. Our study suggested that daily oral administration of the selected strains at the dose level of billion CFU/dose during 1 week is well tolerated by healthy middle-aged men. Therefore, these strains seem to be promising candidates for the development of novel, evidence-based well-focused probiotics to target male urogenital tract disorders. Their effectiveness must be confirmed in clinical trials on diseased patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Reet Mändar: designed the research study and wrote the paper. Imbi Smidt: performed the microbiological analyses, analyzed, and interpreted the data of microbiological analyses and approved the paper. Tiiu Rööp: performed the microbiological analyses, analyzed, and interpreted the data of microbiological analyses and approved the paper. Dagmar Hoidmets: performed the microbiological analyses, analyzed the data of microbiological analyses, and approved the paper. Kalle Kilk: performed the metabolome analyses, analyzed, and interpreted the data of metabolome analyses and approved the paper. Mihkel Zilmer: interpreted the data of metabolome analyses and revised the paper critically. Kristo Ausmees: performed the clinical study, analyzed, and interpreted the clinical data and approved the paper. Andres Salumets: contributed to the data analyses and revised the paper critically.
Funding
This study was supported by Enterprise Estonia (grants no. EU48695); Estonian Research Council (grant nos. IUT34-19, TEM-TA28, and PRG1076), Horizon 2020 innovation grant (ERIN, grant no. EU952516) and Estonian Ministry of Education and Research (grant no. KOGU-HUMB).
Acknowledgments
This study was supported by Enterprise Estonia (grant no. EU48695); Estonian Research Council (grant no. IUT34-19, TEM-TA28, and PRG1076), Horizon 2020 innovation grant (ERIN, grant no. EU952516) and Estonian Ministry of Education and Research (grant no. KOGU-HUMB). The design, conduct, and analysis of the study were completed independently by the investigators.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data used to support the findings of this study are included within the manuscript.