Annona muricata Aqueous Extract Ameliorates Testicular and Epididymal Sperm Toxicity in Rats Exposed to Gasoline Fumes
Abstract
In the present study, aqueous leaf extract of Annona muricata was assessed for its capacity to mitigate testicular damages and sperm toxicity induced by gasoline fumes. Thirty-two male Wistar rats were randomized into four groups of eight animals per group: the control group was given distilled water, and the PEBI group was exposed to petrol by inhalation and petrol-exposed animals that were concomitantly treated with A. muricata at 100 and 200 mg kg−1 body weight (b.w), respectively (PEBI + AM100 and PEBI + AM200) via oral gavage. Oxidative stress markers, sperm quality variables and histological examination of the testes evaluated at the end of the 10-week exposure period showed that coadministration of A. muricata significantly reduced thiobarbituric acid reactive substances (TBARS) and hydrogen peroxide (H2O2) concentration, elevated catalase (CAT) activity, diminished DNA fragmentation and improved sperm quality, thereby attenuating testicular and epididymal sperm toxicity in rats exposed to gasoline fumes. Interestingly, the higher dose A. muricata could also lower lipid peroxidation to below the control values, demonstrating the antilipoperoxidative and antioxidant protective effects of A. muricata in a gasoline fume model of testicular and spermatoxicity in rats.
1. Introduction
Petrol (gasoline) is one of the fractions derived from the process of refining petroleum. It contains aliphatic, aromatic and numerous branched saturated and unsaturated hydrocarbons. Petrol is said to be highly volatile; human beings and animals are therefore mainly exposed to these fumes by inhalation. Pump attendant and petroleum industry workers are occupationally exposed to these fumes. Exposure to the fumes of petroleum fractions has been reported to have negative health impact, such as nephrotoxicity, reproductive toxicity, hepatotoxicity and alterations of lipid metabolism and other biochemical activities [1]. Generally, hydrocarbon components of petrol are classified as endocrine-disrupting chemicals. These chemicals adversely affect the male reproductive system and decrease the weight of the testis [2].The increase in the incidence of infertility among petrol pump attendants has generated public health attention on the antifertility effects of gasoline fumes [3].
Generally, it is believed that the metabolism of hydrocarbons found in petrol results in free radical generation. These active oxygen metabolites have the ability to compromise the activity of certain antioxidant defence molecules resulting to lipid peroxidation and enzyme inactivation via protein oxidation [2]. It is important to appreciate that most testicular problems that threaten male reproductive health are associated with free radical generations and reactive oxygen species-induced oxidative stress. Testicular tissue is highly predisposed to free radical-induced oxidative stress due to its high cell division capacity and competition for oxygen rate and decrease in oxygen pressure as well as increased levels of unsaturated fatty acid [4]. The various indices of sperm quality like sperm count, motility and morphology are also susceptible to free radical toxicity and oxidative stress and contribute to abnormal sperms and fragmenting of the sperm DNA [5]. It is believed that fragmentation of DNA is commonly reported in the spermatozoa of infertile people due to high concentration of reactive oxygen species in their sperms [4].
The male gonad is said to possess a vast array of free radical scavengers and antioxidant enzymes like the superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) peroxidases and nonantioxidant enzymes including reduced GSH. These antioxidant defence systems help ensure that the spermatogenic and steroidogenic functions of the gonads are not compromised by oxidative stress in tissues and cells [4, 5]. It is believed that environmental factors such as pollutants, diet and chemicals interfere with the actions of the antioxidant defence enzymes in the testis. As such the antioxidant system of the gonads might be supported by the intake of plant-based natural nonenzymatic antioxidant and antioxidant therapy, which help to improve male reproductive health [4, 5].
Soursop (Annona muricata L.) belongs to the family Annonaceae which is found mainly in tropical regions. It has several medicinal applications [6] in tropical African countries including treatment of parasitic infections and cancer [6]. Soursop also exhibits antiviral, anti-inflammatory, antitumor and antioxidant properties. It is suggested that the plant contains acetogenins, as the principal bioactive agent. Other constituents of the plants are alkaloids, phenols, vitamins, carotenoids, amides, cyclopeptides and megastigmanes [7]. Organic extracts of A. muricata have been found to contain steroids, alkaloids, flavonoids, phenols and saponins and have excellent antioxidant activity that is attributed to the polyphenolic constituents of the plant. Acetogenins are also considered to have an effective free radical scavenging and antioxidant property. Several phenolic constituents identified in the leaves, roots and stems of A. muricata are cinnamic acid derivatives and p-coumaric acid, which together with several other phytochemicals such as rutin, luteolin, homoorientin, tangeretin, quercetin, daidzein, epicatechin, emodin, epicatechin and ferulic acid contribute to the antioxidant health benefits of soursop [8–11].
Pharmacological activities of the aqueous extract, including hypotensive, cancer killing and hepatoprotective effects and antitumorigenic, antimetastatic and antioxidant activities, have also been established [7]. The leaves and fruits of A. muricata could mitigate caffeine induced sperm toxicity and testis weight loss [12] and exhibit antimutagenic, antilipoperoxidative and antioxidant effects [7]. It was recently reported that A. muricata could ameliorate testosterone propionate-induced prostatic hyperplasia and monosodium glutamate-induced liver and testes injury in rats, suggesting the beneficial effects of soursop intake on male reproductive health [13]. Soursop juice in animal models has also been found to enhance the spermatozoa fertilizing ability of rooster semen during processing of the ejaculates for insemination and seminal antioxidant defence and semen quality of rabbit bucks [14].
In the present study, we evaluated the protective effect of the aqueous leaf extract of A. muricata against gasoline fume-induced testicular oxidative stress and epididymal spermatotoxicity focusing on the antioxidant profiles and histological features of the testes as well as the sperm quality variables of young adult rats after 10 weeks of gasoline inhalation.
2. Materials and Method
2.1. Collection of Materials
A. muricata fresh leaves were harvested from household gardens in Udebu Community, Ahoada, Rivers State, Nigeria. The plant was identified by a botanist in the Department of Plant Science and Biotechnology, University of Port Harcourt. The voucher specimen was deposited at the herbarium under a reference UPH/V/1447.
2.2. Plant Extract Preparation
2.3. Animal Study
Thirty two young male albino rats (100 ± 15 g) were obtained from Ogive integrated farms (Osisioma, Aba, Nigeria). The animals were kept in the Department of Biochemistry animal house and allowed to acclimatize for 1 week before the study. The temperature of the room was maintained under standard conditions: temperature of 22−25°C, relative humidity of 65% and a 12 h light:12 h dark cycle. The animals also had free access to clean drinking water and rat feeds throughout the duration of the experiment. Ethical approval for use of laboratory animals was obtained from the Department of Biochemistry Research Ethics Committee and was in line with the standard best practice for handling of laboratory animals (National Institute of Health Publication Number, 85–23).
2.4. Experimental Design
The rats were randomly divided into four groups of eight rats each. The control animals were given distilled water; the PEBI group was exposed to petrol fumes by inhalation; 100 mg/kg body weight (b.w) (PEBI + AM100) and 200 mg/kg b.w (PEBI + AM 200) of A. muricata aqueous leaf extract were administered to the PEBI group respectively by oral gavage. These doses (100 and 200 mg/kg b.w) were 1/25 and 1/50 of the LD50 (5000 mg/kg b.w) of A. muricata aqueous leaf extract. Consequently, many other studies have used doses of A. muricata aqueous leaf doses that are within the range used in the present study and found them to be effective in many rodent models of tissue injury associated with oxidative damage [7, 15]. Additionally, high doses (800 mg/kg b.w) of A. muricata extract could be detrimental to sperm function, whereas modest doses up to 200 mg/kg b.w have antioxidant protective effect in rat models of testicular injury [16–18].
2.5. Exposure to Gasoline Vapour
One week was given to the animals for acclimatization before the study. The rat exposure to petrol fumes was based on the method adopted by Uboh et al. [19]. The PEBI group of animals was exposed to petroleum fumes 6 h a day and five times a week for 10 weeks in an exposure chamber [3]. Six hundred microlitres of petrol was aliquoted into a beaker and placed in the exposure chamber during the period of exposure to gasoline fumes [20]. Daily records were taken for the initial and final volumes of gasoline before and after exposure; the difference was used to calculate the relative amount of vapours released in this exposure method. The average dose of exposure was calculated to be 9.5 cm3/h, 6 h daily, 5 days in a week for 10 weeks. The control rats were housed in different room free of petroleum fumes and were given distilled water only. The animals were housed in a standard plastic cage and in a large room with windows for proper cross ventilation; they had uninterrupted access to clean drinking water and food during the study period. This experimental exposure model of rodents to gasoline fumes has been validated in other studies and mimics human work place exposure to petrol fumes [3, 19].
2.6. Preparation of Tissue Extracts for Biochemical Assays
Ice-cold mortar and pestle were used to homogenize the rat’s testis in 4 vol. of ice-cold 50 mM Tris-HCl buffer (pH 7.4) containing 1.15% KCl. Tissue homogenates were centrifuged at 10,000 revolutions per min at 4°C for 15 min, and the supernatants were carefully collected and used for the following biochemical assays.
2.6.1. Quantification of GSH Concentration by Ellman’s Reaction
Ellman’s reagent for colour development was used to evaluate the level of reduced GSH according to the method of [21]. The absorbance was read in a spectrophotometer at 412 nm. Standard curve (20–200 µg mL−1) was prepared, and the concentration was extrapolated from it. The results were expressed as µg GSH mg protein−1 mL−1.
2.6.2. Quantification of Lipid Peroxidation Levels by the Thiobarbituric Acid (TBA) Reaction
TBA was used for the formation of the pink chromophore as previously described by Ohkawa, Ohishi and Yagi [22]. The absorbance of malondialdehyde (MDA) of 1.56 105 M−1 cm−1 at 532 nm was used to calculate for lipid peroxidation in the sample, and the results were given as µmol MDA mg protein−1 mL−1.
2.6.3. Hydrogen Peroxide (H2O2) Quantification by the Wolff-FOX Assay
The concentration of H2O2 was evaluated using the method described by Wolff [23]. The sample was directly mixed with FOX reagent (100 µM xylenol orange, 250 µM ammonium ferrous sulphate and 25 mM H2SO4) and incubated for 30 min at room temperature. The solution was read at 560 nm, and the level of H2O2 in the sample was quantified using a standard curve (10–100 µM) for H2O2, and its concentration was given as μmol H2O2 mg protein−1 mL−1.
2.6.4. Quantification of CAT and SOD as Antioxidant Marker Enzymes
The activity of CAT in the tissue sample was measured based on its ability to consume H2O2 at 240 nm as previously described by Chance and Maehly [24]. The absorbance reading was taken at 10 s intervals for 1 min. The quantity of protein that causes an absorbance change of 0.01 per min−1 is equivalent to one unit of CAT. The activity was expressed as a µmol H2O2 mg protein−1 min−1. SOD activity was measured as previously adopted by Kakkar, Das and Viswanathan [25]. The reaction mixture containing phenazine methosulphate (186 μM) and nitroblue tetrazolium (300 μM), pyrophosphate buffer (0.052 M, pH 8.3) and sample was incubated for 90 s at 37°C. The reaction was stopped with glacial acetic acid, and the absorbance of the blue-coloured chromogen was measured at 560 nm.
2.6.5. Nitrite Concentration Quantification by the Griess Assay
Nitrite concentration of tissue samples was evaluated according to the method of Green et al. [26]. In brief, 5% ZnSO4 was used to precipitate the sample, after which it was centrifuged at 4000 g for 10 min at 4°C. Freshly prepared Griess reagent (1% sulphanilamide in 5% phosphoric acid and 0.1% N- (1-naphthyl) ethylenediamine hydrochloride in water) in a 1:1 ratio was mixed with the supernatant. The mixture was covered with foil and kept away from light for 20 min at room temperature. The absorbance of the reaction mixture was read at 540 nm. Sodium nitrate standard curve (0–500 µM) was prepared from which the concentration of nitrite was extrapolated and expressed as µmol NO2 mg protein−1 mL−1.
2.6.6. Quantification of DNA Fragmentation by Diphenylamine Reaction
DNA lysis buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8) containing 0.2% Triton X-100 was added to the tissues for homogenization. The homogenate was centrifuged (3000 × g, 20°C) for 10 min; the supernatant was thrown away, while the pellet was carefully collected, resuspended in the lysis buffer and mixed by shaking. It was centrifuged (5000 × g, 20°C) for 30 min; the supernatant was carefully put in new test tubes. Trichloroacetic acid (25%) was added to the supernatant and the pellet samples; they were mixed and incubated overnight at 4°C. The next day, diphenylamine reaction for DNA content in the samples was assayed at 600 nm as described by Sandau, Pfeilschifter and Brune [27].
2.7. Assay for Sperm Quality Parameters of Epididymal Sperms
Sperms were pressed out on a slide through a cut made on the cauda epididymis and mixed with normal saline prewarmed to 37°C. Motile sperms were estimated from a total of 200 sperms and expressed as percentage of the total sperms counted. Sperm viability was assessed by using the eosin-nigrosin staining technique [28]. Sperm viability was expressed as the percentage of unstained sperm of the total sperm counted. Sperm count was performed using a Neubauer’s chamber as described previously [29]. Sperm counts were expressed as million sperms per millilitre. Sperm cells that possess abnormal head and flagellum were also counted and were expressed as percentage of a total of 200 sperms counted.
2.8. Statistical Analysis
Data obtained from this work were analysed by ANOVA followed by Tukey’s post hoc test. p values less than 0.05 were statistically significant. Results were expressed as mean ± standard error (SD). Graphs were done using GraphPad Prism 6.
3. Results and Discussion
3.1. Body and Paired Testis Weight of Animal at the End of the Experiment
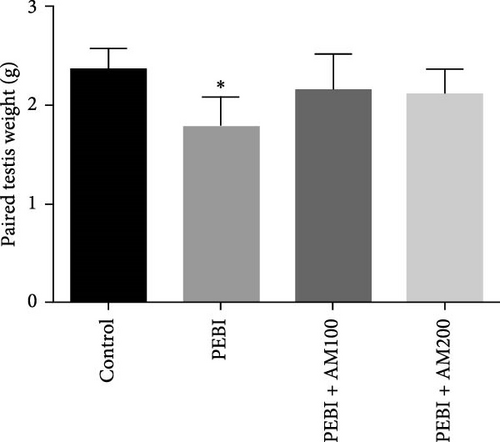
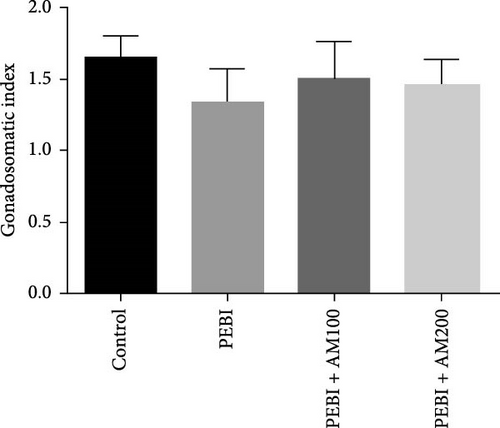
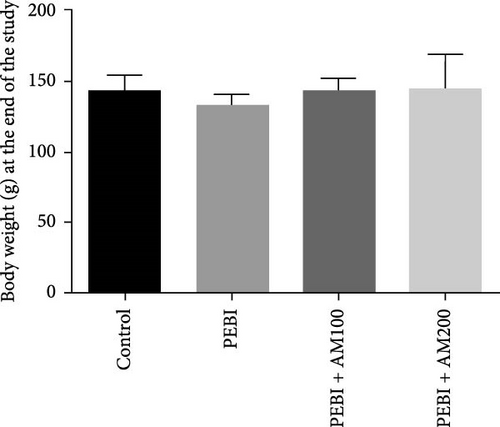
Figure 1 shows the body and paired testis weight of animals and gonadosomatic index at the end of the study. The result shows absent of effect on body weight and gonadosomatic index (testis weight relative to body weight) across all groups (Figure 1A–C). However, there was a significant (p < 0.05) decrease in paired testis weight of animals in the PEBI group (Figure 1B). There was no significant effect on paired testis weight for the animals in PEBI + AM100 and PEBI + AM200 groups relative to the control values, although the values in these groups were higher than those of the PEBI group but could not reach statistically significant thresholds (p > 0.05).The gonadosomatic index in PEBI group was slightly decreased and could not reach levels considered as statistically significant (p > 0.05) (Figure 1B). The decreased absolute testis weight in the PEBI group agrees with the findings of Ekaluo, Ikpeme and Udokpoh [30], confirming an injurious effect of gasoline fumes on the testis of rats. This observation is significant because testis weight is essential for assessing the risk of toxic effect on the male reproductive system. Although the body weight of PEBI animals was not significantly altered, the decreased GSI did not reach a statistical significant threshold. However, we consider this decrease in GSI of 19% in the PEBI animals to be physiologically relevant since its average values in this group of animals were inflated than those of the control and especially that absolute testis weight was significantly decreased. Some studies have reported that gasoline fumes could raise testosterone levels resulting in the shrinkage of the testis [31]. Since body weight remained unaltered throughout the study, the decreased testis weight and slight change in GSI confirm an unswerving adverse effect of gasoline fumes on the testis of rats. This was confirmed in the present study by the histological changes seen in the testes of the PEBI animals. It is also important to appreciate that GSI is related to spermatozoa production efficiency in males, and its decrease may result in low sperm production. Thus, the decrease in testis weight could account for the impaired sperm quality of the rats exposed to gasoline fumes [32].
3.2. GSH and MDA Concentrations, H2O2 Generation and CAT and SOD Activities in the Testes of Animals at the End of the Study
The results for GSH and MDA concentrations in the testicular tissues of the studied animal are as presented in Figure 2A,B. The concentrations of GSH and MDA in the testis of the PEBI group were higher (p < 0.05) than the control and remained higher than those of the extract cotreated groups (PEBI + AM100 and PEBI + AM200). There was no dose-dependent difference in the concentrations of GSH between the PEBI + AM100 and PEBI + AM200 groups (p > 0.05). MDA concentration was also not notably altered in the PEBI group compared with the control (p > 0.05). However, the thiobarbituric acid reactive substances (TBARS) (MDA) concentration in the testes of animals in the PEBI + AM200 group was reduced than those of the PEBI + AM100 group (p < 0.05), and both values were significantly lowered compared to the control (p < 0.05). Figure 3A–C shows the result for H2O2 concentration and enzyme activities of CAT and SOD. The PEBI group shows increased H2O2 concentration and SOD enzyme activity with significant (p < 0.05) decrease in the activity of CAT. Moreover, the extract cotreated groups (PEBI + AM100 and PEBI + AM200) reduced the concentration of H2O2 and enzyme activity of SOD with significant increase in CAT enzyme activity compared to the PEB1 group. There was no consequential difference in H2O2 levels and activities of SOD and CAT between the PEBI + AM100 and PEBI + AM200 groups.
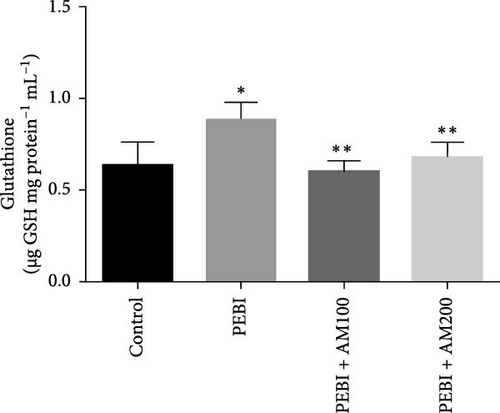
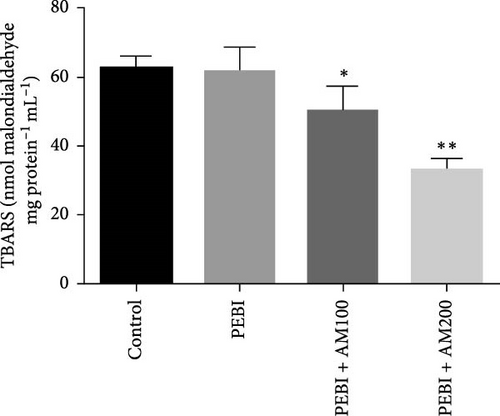
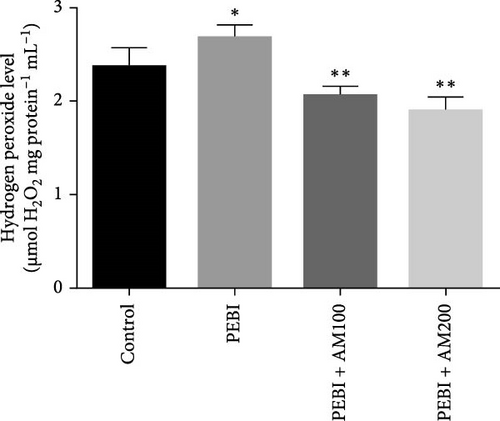
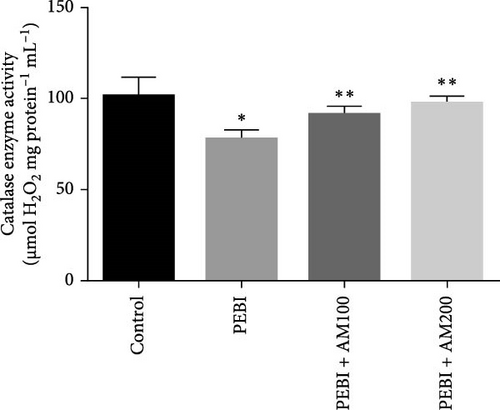
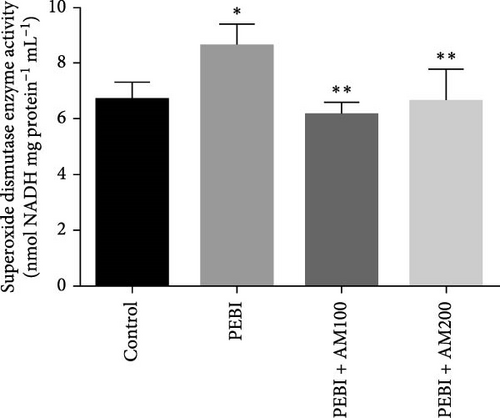
The unchanged MDA concentration, despite the increase in the levels of H2O2 and GSH and SOD activity, suggests that lipid peroxidation was not induced by gasoline fumes in the testes of the exposed animals. This observation is quite interesting because CAT activity was found to be reduced in response to the higher H2O2 concentrations in the testes of these animals. It is rationale to assume that the elevated SOD activity was responsible for the higher H2O2 concentration. This could mean that the antioxidant enzyme defence system of the testis acted cooperatively to prevent oxidative damage in testicular tissues [4, 5]. These postulations are even more evidently expressed in the fact that an increase in SOD activity and GSH concentration was compensated for by the low CAT activity and may work in concert with nonenzymatic antioxidants such as GSH in the testes of the PEBI group of animals in the present study. These sorts of fluctuations in the antioxidative defence system are an adaptive strategy to oxidative stress and provide an insight at further understanding the role oxidative stress plays in PEBI-induced testicular damage. In support to this finding, toluene, a component of petrol could also increase the activities of CAT and GSH peroxidase in rat’s tissues [33]. Overall, the increase in the defensive antioxidant enzymes could be responsible for the unchanged lipid peroxidation in the testes of PEBI animals. Also, it is believed that in response to elevated SOD activity and H2O2 levels, CAT activity is expected to decrease confirming the thought that elevated gonadal H2O2 concentrations and free radicals dampen the activity of CAT [34, 35]. Although some studies found decreased SOD and CAT activities and GSH concentrations and ultimately elevated lipid peroxidation in other experimental models [36], the adjustable SOD/CAT activities reported in the present study could be responsible for the unchanged MDA concentration in the PEBI group. It is worth considering that GSH which plays similar role as CAT in the metabolism of H2O2 predominates in the testis than CAT and could therefore play significant role in protecting the gonads from PEBI-induced lipid peroxidation [37]. Consequently, the testicular injuries observed in the testes of PEBI animals, including the abnormal histological features, may be due to the direct effect of gasoline fumes.
3.3. Nitrite Concentration in the Testis of Animals at the End of the Study
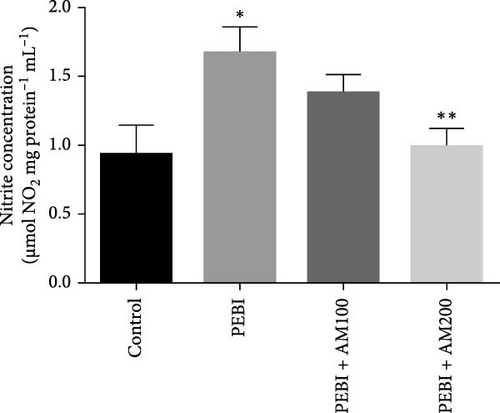
The PEBI group had the highest nitrite concentration, while the PEBI + AM200 group had a value close to the control values and significantly lower than the PEBI values (p < 0.05). The PEBI + AM100 group also shows no significant difference in nitrite concentration as against the control group, although the values are higher than those of the control and the decrease in the values as against the PEBI group did not reach a statistical significant level (Figure 4). Previous findings established a direct correlation between high nitrite levels in the testis and testicular assaults such as inflammation [38]. Furthermore, products of nitric oxide metabolism and active oxygen species including superoxide anions and peroxynitrite have been shown to exert toxic effects on gonadal tissues which could contribute to the testicular and sperm toxicity of gasoline fumes observed in this study. The spermatoxicity observed in these studies above is speculated to be due to the oxidation of the thiol group in spermatozoa which leads to the loss of sperm function including inhibited sperm motility. Thus, the elevated testicular nitrite concentration seen in PEBI animals could indirectly account for testicular damage due to inflammation [39].
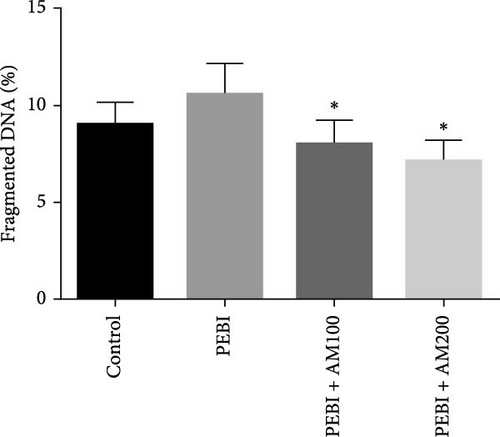
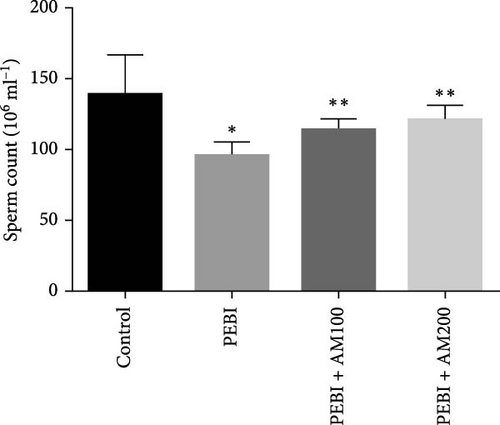
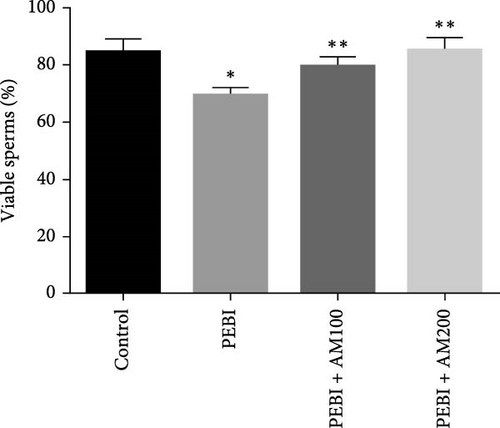
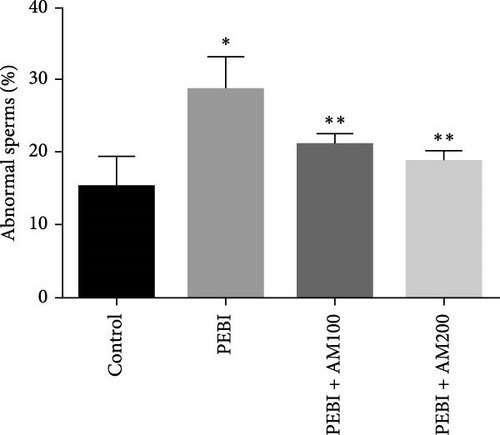
3.4. Fragmented DNA and Histology of the Cross-Sections of the Testes and Sperm Quality Indices at the End of the Study
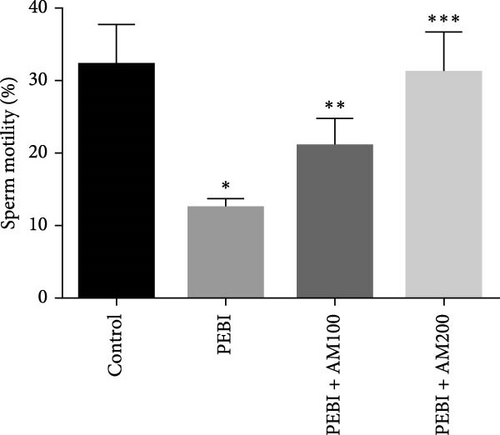
As shown in Figure 5, the PEBI group shows an increase in % fragmented DNA relative to the extract cotreated groups (PEBI + AM100 and PEBI + AM200) (p < 0.05) by 27% and 36%, respectively. But the 18% increase in the % fragmented DNA in the PEBI group compared to the control values did not reach a statistically significant threshold (p > 0.05). The PEBI + AM200 group shows a lower level of % fragmented DNA than the PEBI + AM100, and the differences are not statistically consequential (p > 0.05). Previous studies support the claim that exposure to gasoline fumes is toxic to DNA through oxidative stress mechanisms [40]. When active oxygen species including H2O2 and radicals like nitric oxide, superoxide and hydroxide radicals come in contact with DNA, they may modify DNA bases and break DNA strands [41] and consequently make sperm DNA to be highly predisposed to oxidative damage. Therefore, the increased level of fragmented DNA, although it did not reach a statistical threshold level, might be important toxicologically since the testes of the PEBI animals have high levels of reactive oxygen species such as H2O2. Coadministration of A. muricata leaf extract increased sperm motility in a dose-dependent fashion (p < 0.05). Furthermore, the sperm count and percentage viable sperms in these groups of animals were increased in comparison to the PEBI group (p < 0.05). The percentage of abnormal sperm was significantly (p < 0.05) reduced in animal cotreated with the extract (PEBI + AM200 than the PEBI + AM100) as against animals in the PEBI group. Sperm motility, counts and viability were declined, and abnormal sperms were inflated in the PEBI group as against animals in the control (p < 0.05) (Figure 6). The notably decrease in sperm count, percentage sperm motility and viable sperms with escalated abnormal sperms observed in the animals of PEBI group further attest to the susceptibility of the male gonads and sperms to oxidative stress. The initiation of oxidative stress in the testes of rats exposed to petrol fumes is therefore the cause of the diminished sperm quality variables including epididymal sperm count, sperm motility and sperm damage. It was previously established that a decrease in sperm count is linked to inflated sperm cell apoptosis resulting from oxidative stress and that DNA damage of sperm cells contributes to the decrease in sperm quality and increased sperm abnormalities [42]. Our present results are in agreement with the above findings.
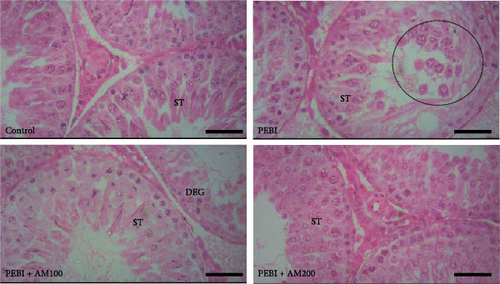
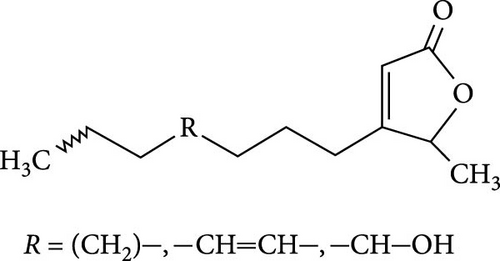
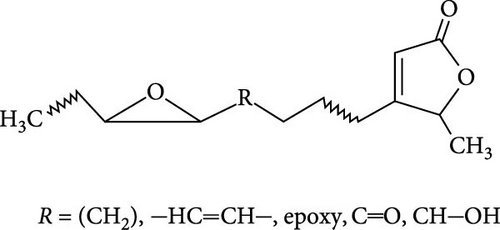
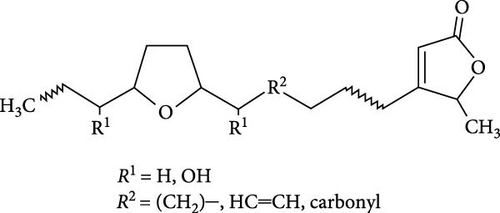
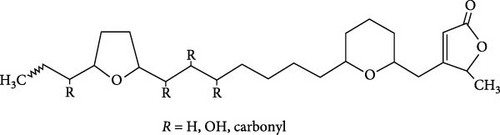
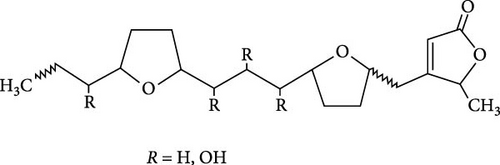
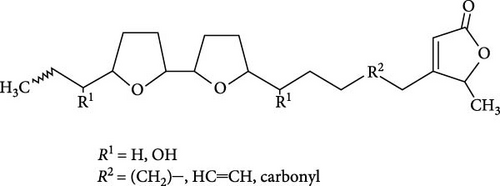
The seminiferous tubules with thin basement membrane and normal spermatogenic progression of germ cells from the basement membrane were the histological features of the testes of the control animals. However, the PEBI group showed histological changes such as shedding and derangement (spermatogenesis having a disorderly appearance and the tubular lumina are filled with desquamated immature cells), patchy spermatocytic arrest depicted by the arrest of spermatogenesis in some of the tubules, spermatogenic hypoplasia (tubules having a decreased population of germ cells and a poor state of spermatogenesis), tubules had small diameter with obviously thickened basement membrane (tubular hyalinization), simultaneous incidence of disfigured seminiferous tubules containing germ cells and tubules with only Sertoli cells among other damages to the testis. The cotreated groups showed decreased testicular changes dose dependently with the PEBI + AM 200 showing better decrease of the histological injury in the testicular tissue (Figure 7). Decreased testicular weight occurs without change in body weight and lipid peroxidation in the PEBI group is suggestive of direct gonadal effects of gasoline fumes in this study. Furthermore, the aqueous leaf extract of A. muricata was observed to protect against the increase in the concentrations of H2O2, nitrite and fragmented DNA in the testes of the extract coadministered animals. Furthermore, the fluctuated changes in the antioxidant system as previously reported in other model systems [4] were halted by extract of A. muricata confirming the antioxidant and DNA protective effects of the aqueous extract in our animal model. The testis weight of the animals was also recovered to normalcy after cotreatment of the extract at the different doses confirming the direct protective effects of the extract on the gonads against gasoline fume-induced testicular toxicity. Additionally, the sperm parameters of the animals of the extract cotreated group were better than those of the PEBI group. These outcomes are ascribed to the acetogenin components (Figure 8) of the aqueous extract which has the ability to mop-up active oxygen species and inhibits inflammatory signals that threatens the health of the testis. Other components of the extract including phenols and alkaloids [7] may also contribute to the antioxidant effects of A. muricata. Thus, later studies on the direct effect of the acetogenins derivatives from A. muricata on the testes are warranted. Some findings have also affirmed that the antioxidant potential of A. muricata was accountable for their protective effect on the testis against oxidative stress-related effects in a diabetic rat model [10, 18] and on the sperms in a caffeine model of spermatotoxicity in rats [6, 12], thereby supporting the hypothesis of the present findings. Therefore, the protective effects on gonadal tissues under various pathological and diseased conditions have been demonstrated by A. muricata, thereby supporting our present findings in a PEBI model of testicular injury. Taken as a whole, the higher dose (200 mg kg−1 b.w) of the aqueous leaf extract demonstrated to be more effectual than the lower dose (100 mg kg−1 b.w) in the protection against PEBI-induced oxidative stress and inflammatory injury in the testes of rats.
4. Conclusion
The present study demonstrated that A. muricata aqueous leaf extract attenuated testicular and epididymal sperm toxicity in rats exposed to gasoline fumes by scavenging H2O2, minimizing the fluctuation in the antioxidant defence system and DNA damage, as well as improving of epididymal sperm quality parameters. Furthermore, the direct effects of gasoline fumes on the testes that resulted in abnormal histological features and diminished testis weight are due to the enhanced nitrite concentration that was inhibited by A. muricata. Studies are ongoing to decipher the mechanisms of these protective effects of A. muricata in a PEBI model of testicular and sperm toxicity.
Ethics Statement
This prospective study was approved by the Ethical Committee for Research on Laboratory Animals of the Department of Biochemistry, University of Port Harcourt, and was carried out in accordance with the standard guide for the care and use of laboratory animals (National Institute of Health Publication Number, 85–23).
Disclosure
This study has been done as part of the MSc dissertation of Naboth E. Agabus in the Biochemistry Department, University of Port Harcourt, Choba, Nigeria.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
O.E.E. participated in the design of the study and performed the experiments. S.O.A. designed the study, supervised the experiments, interpreted the data and edited the draft manuscript. N.E.A. performed the experiments and wrote the draft manuscript. E.N.O. supervised the experiments and participated in the design of the study.
Funding
No funding was received for this research.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.