Insect Vectors of Plant Viruses: Host Interactions, Their Effects, and Future Opportunities
Abstract
Plant viruses are endocellular, and they multiply inside their host plant cells. Outside of the host cells, they are acellular and cannot multiply and move to their hosts for reproduction. Plant virus use insect vectors to transmit and distribute from the infected farm to the next health plant or farms—especially the orders of Hemiptera, Thysanoptera, and Coleoptera are the vectors of plant viruses from infected to healthy plants. For instance, the hemipterans such as aphids, whiteflies, cicadas, spittle bugs, leafhoppers, assassin bugs, stink bugs, lygaeid bugs, and Thysanoptera (e.g., thrips) are the major vectors of plant viruses. Furthermore, the Aleyrodidae, Aphididae, Cicadellidae, and Delphacidae families of Hemiptera, the Chrysomelidae family of Coleoptera, and the Thripidae family of Thysanoptera were the most intriguing families of insects that vector plant viruses due to their behavior, short life cycles, easy interactions with their hosts, reproduction rapidly, and their feeding habits on a wide variety of host plants. The occurrence of these insect vectors in host plants decreased yield and led to malnutrition, reduced income, and reduced the marketability of the crops. Understanding the interactions between insect vectors, plant viruses, and crops is benefiting farmers in general for managing plant viruses and by managing insect vectors at large. Therefore, the objectives of this review are to address the insect vectors of plant viruses, host interactions, their effects, and put forward future opportunities. Finally, this review concluded that managing insect vectors at desirable stages, times, and places by available methods can manage plant viruses.
1. Introduction
Plant viruses are endocellular, and they multiply inside the cells of infected host plants [1]. Its multiplication mostly taken place in mesophyll, epidermis, parenchyma, phloem companion, and bundle sheaths of host plants [2].
The infected host plant by virus showed a variety of symptoms, such as yellowed foliage, stunted growth, mosaics, and dead patterns. Its medium of transmission from infected to healthy plants takes place via insect vectors, contaminated farm equipment, nematodes, mites, fungus, infected seed, and mechanical injury of crops during mowing, harvesting, pruning, trimming, and foraging [3]. But the main plant virus vectors were herbivorous insects [4]. About 70%–80% of plant viruses were vectored by insects [5–9]. Globally, this accounted for more than 47 plant diseases [6]. Majorly, the insect head that is oriented for piercing–sucking (opisthognathous), and biting and chewing (hypognathous) are highly vectors of plant viruses to crops [10–15]. This resulted in substantial crop production losses [3].
More than one-third of plant virus species (1200) contain single-stranded deoxyribonucleic acid (ssDNA). ssDNA plant viruses are divided into two major families: Geminiviridae and Nanoviridae [16]. The Geminiviridae plant virus is the largest group of ssDNA viruses. They are characterized by their distinct twin (geminate) particle arrangement and typically infect dicotyledonous plants. And nanoviridae viruses have small, circular ssDNA genomes and typically infect monocotyledonous plants.
Environmental factors such as temperature, moisture, and drought have a substantial impact on plant virus transmission by insects [17]. For instance, warm temperatures boost the reproduction and flying activity of vector insects, increasing virus transmission [18]. Drought stress also caused plants to feed more frequently and for longer durations, making them more vulnerable to viruses due to poor defenses [17]. This makes viruses easier to establish and spread. This can raise viral loads in plants, making them more susceptible to transmission by insects [17, 18].
Morphologically, viruses are also tiny to see under a light microscope; they are obligate parasites that multiply exclusively after entering their host cells, noncellularly, lack protein-synthesizing and energy-producing apparatuses; are immobile outside the infected host; and are inactive outside of their hosts [19]. But once viruses transmitted to host plants, they were difficult to cure because they lacked cellular machinery structures and complex plant immune responses [2, 20]; rapid evolution [21]; and lack of antiviral treatments when compared with fungicides (used to treat fungi infection) and bactericides (used to treat bacterial infection) [22].
Generally, insect pests were the most important biotic factor in plant virus transmission than any others [23, 24]. As a result, managing insect vector infestations helps to mitigate the detrimental impacts of plant viruses. These were accomplished if the insect vectors of plant viruses were identified and well-known. Therefore, the objectives of this paper are to review insect vectors of plant viruses: host interactions, their effects, and future opportunities. Thus, understanding these issues will be used to manage insect vectors and plant viruses.
2. Insect Vectors of Plant Viruses and Host Interactions
2.1. Vectors of Plant Viruses
The most well-known plant viruses, such as mosaic viruses of tobacco, cucumber, cauliflower, African cassava, brome and tomato spotted wilt and yellow leaf curl virus, plum pox virus, potato virus X, citrus tristeza virus, barley yellow dwarf virus, potato leaf roll virus, and tomato bushy stunt virus [25], were vectored and transmitted by aphids [26, 27], thrips [28, 29], whiteflies, cicadas, spittlebugs, plant hoppers, assassin bugs, plant bugs, stink bugs, lygaeid bugs, and mealy bugs [11, 12, 30–36] (Table 1). Some species of Orthoptera (e.g., grasshoppers and leafhoppers) [57], Dermaptera (e.g., earwigs) [100, 101], and Coleoptera (e.g., beetle families such as Chrysomellidae, Coccinellidae, Curculionidae, and Meloidae) [102–106], Lepidoptera, and Diptera [100] were also considered as the vectors of plant viruses. Particularly, those insect vectors that have piercing–sucking mouth parts were quite active in vectoring and spreading plant viruses. Some of which are listed in Table 1.
| Insect vectors | Host crops | Target viruses | References |
|---|---|---|---|
| Aphids | Cauliflower | Cauliflower mosaic virus | Blanc, Drucker, and Uzest [37]; Moreno et al. [38]; Hoh et al. [39]; Plisson et al. [40] |
| Cowpea | Cowpea mosaic virus | James et al. [41]; Moreno et al. [38] | |
| Cucumber | Cucumber mosaic virus | Pirone and Megahed [42] | |
| Bean | Bean common mosaic virus | Flores-Estévez, Acosta-Gallegos, and Silva-Rosales [43] | |
| Brassicas | Turnip mosaic virus | Kyrychenko et al. [44]; Hao et al. [45] | |
| Capsicum | Cucumber mosaic virus, potato virus y | Waweru et al. [46]; Raccah, Gal-On, and Eastop [47] | |
| Carrot | Carrot virus y | https://www.daf.qld.gov.au/__data/assets | |
| Celery | Celery mosaic virus | ||
| Cucurbitaceae | Papaya ringspot virus | ||
| (w strain), watermelon | |||
| Mosaic virus, zucchini | |||
| Yellow mosaic virus | |||
| Lettuce | Lettuce mosaic virus | ||
| Plum | Plum pox virus | Rimbaud et al. [48] | |
| Solanaceae | Potato virus | MacKenzie et al. [49] | |
| Sweet corn | Johnsongrass mosaic virus | https://www.daf.qld.gov.au/__data/assets | |
| Sweet potato | Sweet potato feathery | https://www.daf.qld.gov.au/__data/assets | |
| Mottle virus | — | ||
| Tobacco | Tobacco rattle virus | Mulot et al. [50] | |
| Potato | Potato virus y | Bhoi et al. [51]; Yang et al. [52] | |
| Banana | Banana bunchy top virus | Hidayat, Sartiami, and Hidayat [53], De Clerck et al. [54] | |
| Citrus plants | Citrus tristeza virus | Herron et al. [55] | |
| Green peach and cucumber | Cucumber mosaic virus | Liang et al. [56] | |
| Grasshoppers | Rice | Rice yellow mottle virus | Koudamiloro et al. [57] |
| Leafhopper | Maize | Maize chlorotic dwarf virus | Cassone et al. [58] |
| Rice | Rice yellow mottle virus | Koudamiloro et al. [57] | |
| Rice dwarf virus | Fang et al. [59] | ||
| Family Poaceae (such as rice) | Tenuiviruses, for example, Rice stripe virus | Zhao et al. [60]; Zheng et al. [61]; Nault and Ammar [62] | |
| Thrips | Tomato | Tomato spotted wilt virus | Zhang et al. [63]; Nilon et al. [64]; Nachappa et al. [65] |
| Tomato spotted wilt virus | Montero-Astua, Ullman, and Whitfield [66]; Whitfield, Falk, and Rotenberg [12]; Moritz, Kumm, and Mound [67] | ||
| Chrysanthemum, groundnut, tomato, pelargonium flower break virus, and maize | Orthotospovirus | He et al. [68]; Liu et al. [69]; Achon et al. [70]; Chen et al. [71]; Kusia et al. [72]; Wei et al. [73]; Batuman et al. [74]; Reitz, Gao, and Lei [75]; Hassani-Mehraban et al. [76]; Nagata et al. [77] | |
| Capsicum and onion | Tospoviruses | Jones [78] | |
| Soybean | Soybean vein necrosis virus | Lagos-Kutz et al. [79] | |
| Tobacco | Tobacco streak ilarvirus | Jones [78] | |
| Watermelons, cucumbers, melons, squash, and pumpkin | Melon necrotic virus | Radouane et al. [80]. | |
| Maize | Maize lethal necrosis | Regassa et al. [81] | |
| Tomato | Tomato spotted wilt orthotospovirus | Medeiros, Resende, and de Avila [82] | |
| Capsicum | Capsicum chlorosis orthotospovirus | Widana Gamage et al. [83] | |
| Whiteflies | Tomato | Tomato chlorosis virus and Tomato severe rugose virus | Shi et al. [84]; Fereres et al. [85]; Liu et al. [86]; Gottlieb et al. [87] |
| Yellow leaf curl virus | Kollenberg et al. [88]; Czosnek and Rubinstein [89] | ||
| Watermelon | Tomato Yellow Leaf Curl Virus | Zhao et al. [60]; Pan et al. [90]; Kollenberg et al. [88] | |
| Watermelon Chlorotic Stunt Virus | Kollenberg et al. [88] | ||
| Banana | Banana streak virus | Watson and Kubiriba [91] | |
| Cotton | Cotton leaf curl Multan virus | Zhao et al. [60]; Pan et al. [90] | |
| Generally has wide range of host plant species (>420) | Geminiviridae, for example, begomoviruses | Nigam [92]; Rosen et al. [93]; Ghanim [94] | |
| Mealy bugs | Sweet potato, tomato | Cucurbit yellow stunting disorder virus | Kaur et al. [95] |
| Maize | Maize chlorotic dwarf virus | Cassone et al. [58] | |
| Grapevine leaf | Grapevine leaf roll virus/ampeloviruses | Herrbach et al. [96]; Gutha et al. [97]; Tsai et al. [98] | |
| Banana | Banana streak virus | Kubiriba et al. [99] | |
The plant viruses that cause tomato yellow leaf curl and chlorosis (Image A) were vectored by B. tabaci (Hemiptera: Aleyrodidae, Image D); tomato spotted wilt Orthotospovirus and tomato zonate spot virus (Image B) were vectored by F. occidentalis (Thysanoptera: Thripidae, Image E); and tobacco and pepper (Image C) were co-infected by tobacco mosaic virus and cucumber mosaic virus that were vectored by M. persicae (Hemiptera: Aphididae, Image F) [30] (Figure 1).






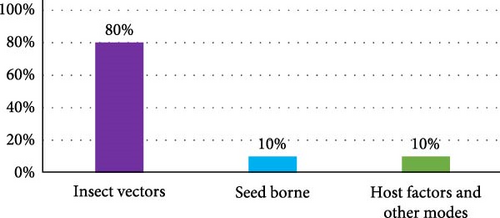
Thysanoptera and Hemiptera [4, 107] vectors up to 70% of plant viruses [3, 9], followed by Coleoptera (11%) [108–110], and the other order vectors (19%). Furthermore, the Aleyrodidae, Aphididae, Cicadellidae, and Delphacidae families of Hemiptera, the Chrysomelidae family of Coleoptera, and the Thripidae family of Thysanoptera were the most intriguing families ([111]; Figure 2) due to their behavior, short life cycles, interactions with hosts easily, their capacity to reproduce rapidly, migration, and feeding habits on a wide variety of plants. Their roles as vectors place them in the center of the plant virus transmission ecology, with far-reaching ramifications for agriculture and food security [112]. To sum up, insect vectors transmit plant viruses up to 80% [113], followed by seed-borne (10%) and host and other factors (10%) were used to transmit plant viruses (Figure 3). Figures 1 and 2 illustrate some of the key species of insect vectors of plant viruses.
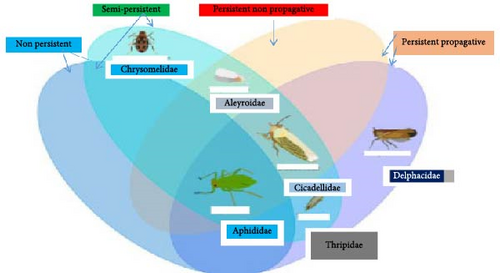
2.2. Host Interactions
2.2.1. Plant Viruses and Insect Vectors Interactions
An interaction between host plants, insect vectors, and plant viruses has an extensive history and occurs naturally. Many plant viruses were vectored and reserved in insect bodies to elongate their lifespan [103, 114, 115] to get stable environmental conditions to shield it from external circumstances and enhance its reproduction and circulation in insect guts or other tissues. This ensures the plant virus remains viable until it is transmitted to a new plant or for a constant supply of virus for transmission. In line with this, Wu et al. [116] reported that more than 600 insect species were vectored over 1,213 RNA viruses in the ecosystem.
An interaction between plant viruses and insect vectors was not simple, and they were co-evolved over time [35, 117]. These insects were feeding on the host plant parts for their survival and to continue their generations [118]. These situations made them interact with a wide host range of plants, which could be beneficial or harmful. The interaction between plant viruses and insect vectors is interspecific, meaning different groups of species. Plant viruses exhibited genetic, morphological, and anatomical variations while circulating in insect vectors [32, 119–121] due to mutation, replication, and the influence of environmental factors such as elevation and isolation. And due to morphological and anatomical variation that occurs in the digestive and reproductive systems. For transmission, their molecular contact requires specialized protein recognition [119, 122, 123]. Most plant viruses entered into plants via injured or damaged host plants by insect vectors during nutrient content feedings by piercing and sap sucking, biting, and chewing [10, 124], and they travel from cell to cell via plasmodesmata [125].
The proteins of plant virus–insect vector interactions were categorized into viral helper and capsid components [126]. The viral helper protein components served as an adaptor between the plant virus (capsid protein) and the receptor in the insect vectors. The receptors are used to assemble their interactions [126]. The components of the capsid proteins are infectious virions that are used to protect their genomes during entry and exit from the host cells to interact with their vectors. Majorly, plant viruses possess RNA along with the capsid proteins, while a few virus species contain DNA [127]. Thus, understanding the physiological and ecological interactions between insect vectors and plant viruses was critical for many purposes [128, 129].
2.2.2. Circulating and Noncirculating Plant Virus in the Bodies of Insect Vectors
- 1.
Noncirculative virus: Its constituent part attaches to the cuticle of insect vectors [130], as described in Figure 4. They did not circulate inside the insect body, but they were discharged or secreted to the feeding sites via saliva of vector insects [34, 132]. The insect carried it on its exterior cuticle, mouth lining, and foregut [133]. This group was categorized into nonpersistent and semipersistent based on the time spent in the vector’s bodies [130].
-
The nonpersistent viruses are transmitted within a few minutes or hours without entering a dormant period. It is transmitted to host plants during insect vectors feeding on the sap contents by piercing and sucking or when they regurgitate it [134, 135]. But they do not persist longer in the epidermis and mesophyll of the host plant cells [136–138] and are retained in the stylets (piercing and sucking organs) of insect vectors for a while [30, 119].
-
The semi-persistent virus lives in the chitin of insects for an hour after contacting their bodies [139, 140], then enters and spreads to salivary glands [30]. This type of virus is bound to the internal body of vector insects via the chitin lining of the gut [139]. The typical examples of these viruses are tomato chlorosis and yellow crinivirus in the genus Crinivirus, which are transmitted by the whitefly (Bemisia tabaci). Their transmission ranges were also increased as the climate changed [141].
- 2.
Circulative viruses: They are also known as persistent plant viruses. These types of viruses were retained in the guts and tissues of insect vector for many hours to days after contact with their bodies before entering to the body for vector and transmission. This type of virus was transferred from parents to the next generation, progeny, or offspring of insect vectors. It would find and propagate in their next offspring [34]. They use hemolymph, or blood, to spread to the hemocoel and accessory salivary glands of vector insects. They were able to spread and invade the salivary glands of insect vectors [9, 30]. It was replicated and invaded the tissues of insect vectors before transmitted to host plants via salivary glands or during regurgitation [34, 142]. It can also circulate through the alimentary canal (e.g., midgut and hindgut) to reach the hemocoel, the body cavity of the hemolymph, for instance, begomoviruses. However, it did not replicate in insect vector tissues, stomach, hemolymph, and salivary tissue membranes for transmission [34, 132].
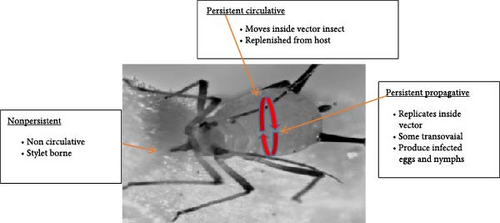
Generally, nonpersistent and semi-persistent viruses persist for a short time and cannot enter the hemolymph of insect vectors [30]. However, the persistent plant virus was retained in the hemolymph of insect vectors for a long time [30]. But both non- and semi-persistent viruses lost their transmissibility when insect vectors shed external cuticles.
The host plant uses physical barriers such as cellulose, pectins, thick waxy cuticles, and cell walls against the entrance of plant viruses. Additionally, healthy host plants also resisted the entry of plant viruses using inactive and active mechanisms, such as gene silencing [143], producing innate immunity and tolerating their infection [143, 144] and using pathogenesis-related biochemicals [145]. These were sourced from metabolites and proteins. The dominant resistance genes and corresponding avirulence protein (Avr) were derived from plant proteins [146, 147]. The host plant is diseased when a plant virus has specific molecular interaction with host plants, commonly by using proteins [119]. This protein positively affects phytoviral replication and leads to minimizing host plant immunity against it. Because immunity triggered the molecular patterns used for recognition between them. The Avr protein caused the silencing of the host plant’s RNA [148]. But to overcome these barriers, plant viruses can also influence their physiology and behavior [149, 150]. Knowing the feeding habits, behavior, physiology, and population dynamics of insect vectors and plant viruses is crucial for understanding their evolutionary relationships [35] and for planning to treat them. Lipid-based phytohormones such as jasmonate were used to confer plant defenses to various biotic challenges [151–154]. Jasmonate repairs the damaged bodies of host plants by ordering metabolism to produce defensive molecules [153, 154] and interferes with their preference, growth rate, and performance of virus vector of insects [155]. Furthermore, jasmonate stimulates the defensive mechanism of host plants by altering the qualitative and quantitative composition of plant volatile compounds. It also initiated the host plants to attract natural enemies and help them to repel herbivorous insects [156–158].
Indirectly, the mouthparts of insects modified for biting and chewing can promote and induce jasmonic acid in the host plants. This might be used to inhibit their defenses linked to salicylic acid [159–161]. Salicylic acid is used as a biocidal agrochemical against pathogens and regulates plant growth and development hormones, immunities, and signals for local defense responses and systemic acquired resistance. It also facilitated the development of disease-resistant crops during genetic manipulation [162]. Additionally, host plants defend against insect pests by secreting antibiosis (which inhibits aphid development, survival, and fecundity) and deterring or antixenosis due to their unattractive physical structures (which affect insects, for instance, aphid behavior such as the capacity to identify host plants and feed on sieve components) [163].
2.3. Host Effects of Plant Viruses
Plant viruses alter the behavior of their vector insects. It also created a suitable condition to search, manipulate, and transmit easily to their host plants [10, 164–166]. It was also stated that nonpropagative plant viruses (e.g., Turnip Yellows Virus) increased their movement speed toward host plants (e.g., Montia perfoliata) and had effects on the number of fecundity and activity levels of virulent aphids (e.g., Myzus persicae spp.). In contrast, if aphids (M. persica) feed on host plants infected by plant viruses, it decreases the movement of turnip yellow viruses [167]. Furthermore, this would have limited the dispersal rates and locomotor activity of it [165]. However, the insect vectors prefer to settle and feed on the sap contents of the diseased or infected plants by virus strains than healthy plants. For example, the turnip infected with the cauliflower mosaic virus strain W260 attracts more aphids than healthy cauliflower (Arabidopsis thaliana) due to the production of more volatile compounds that attract aphids [168, 169]. Aphids pierce the tissues of plants with their stylets to ingest copious volumes of phloem sap and deprive the photoassimilates of plants [163].
Plant viruses also changed the organic compounds and volatile profiles of host plants to settle their insect vectors [170, 171]. Furthermore, plant viruses modify the volatility compounds emitted from host plants to attract or repel insects [172–175]. By altering these cues, plant viruses influence insect vector behavior [128] and the chemistry of infected plants [176], this includes secondary metabolites, hormones, and defense-related compounds. These alterations would reduce the attractiveness of insect vectors and then virus transmissions. Some plant viruses could also make host plants more susceptible to insect vectors by suppressing plant defense mechanisms.
Plant viruses caused physiological and biochemical disorders in host plants. These disorders including altering of host plant cell structures, injuries to chloroplasts, lower photosynthetic activity, carotenoid, and nutrient uptake capacity, and retardation of growth and development levels of infected host plants [177, 178]. Regardless of pathogenicity, the plant viruses increased the ability to spread and transmit to healthy plants using vector insects [179, 180]. They also destroyed the contents of carbohydrates, polyphenols, and oxidative enzymes (e.g., peroxidase, catalase, ascorbate peroxidase, and superoxide dismutase) in the host plants [181]. Nevertheless, under regular conditions, plant viruses, without help, do not cause plant death [14]. Plant viruses require entry sites to enter into epidermal cells of host plants through mechanically wounded or injured by insect vectors or other means.
Plant viruses can also cause health problems to their insect vectors for their transmission enhancement. These problems would occur in the external parts and intestinal tracts of vector insects. The plant virus affects the life cycle, population genetics, and evolution of insect vectors [35]. As well as endophagous insects, or those that live and eat inside plant tissues, they can inflict bore, roll, tie, mine, and gall host plants. During these lifestyles, evolutionary routes, host selection processes, and rivalries with natural enemies existed [182]. But insect vectors fight against viral invaders by forming physical and immunological barriers [183]. For example, they used an intrinsic cell of antiviral immunity, a peritrophic matrix, a mucin layer, and local symbiotic microorganisms [183].
When an insect feed on an infected plant, it acquires the virus. This virus modifies the typical behavior and physiology of insects, including their overall health, lifespan, and ability to reproduce. Plant viruses also diminish the nutritional value and amount of infected host plants available for consumption by insect vectors in their various life stages, such as nymphs, larvae, or adults [184]. Generally, the interactions of plant viruses, insect vectors, and host plants have positive and negative impacts. Thus, an interaction between insect vectors and plant viruses could be mutualism, commensalism, or antagonism [185–187].
Martinière et al. [188] and Zhang et al. [189] also reported that cauliflower mosaic virus attached to aphid stylets or piercing and sucking mouthparts during feeding on diseased plant cells. This virus was recognized by aphid mouthparts and transmitted to host plants by altering their chemistry, immunity, and plant-virus interactions. In achieving this goal, the host plant protein played a vital role in plant-virus and insect–vector interactions. For instance, the proteome (set of proteins) is found in the host plant cells and used for mediating plant viruses’ transmission by insect vectors [35, 190, 191]. For example, semi-persistent beet yellow viruses that cause disease in Beta vulgaris increased its sugar contents by decreasing the total amino acid content and the aphid’s parasitoid (Lysiphlebus fabarum) attraction toward Aphis fabae [192].
3. Future Opportunities
Insect vectors transmit plant viruses from infected to healthy plants. Therefore, managing vector insect pests will be used to manage the plant viruses and their negative impacts. Consequently, it is crucial to have knowledge of the insect vectors in order to understand the mechanisms of plant virus transmission and to educate and inform stakeholders in the future. It is also utilized for developing strategic management of viruses by employing resistant breeding, plant transformation, cross-protection, and prophylaxis techniques. These methods aimed to minimize the spread of plant viruses through quarantine, certification, and removal of contaminated plants. To effectively manage insect vectors of plant viruses, it is necessary to implement cultural practices, employ biological, pesticides, adopt integrated pest management strategies, and enforce quarantine measures. These activities could reduce the cost of plant virus management at the producer level. However, it requires further investigation to accurately identify the type of plant virus and their insect vectors, their ecologically suitable conditions to occur, and records (surveys), information on economic importance, resistance variety identification, and crop systems to manage them using Eco Safe methods.
Therefore, research-based recommendations for insect vectors and plant viruses should be developed integrally by diagnosing and identifying them carefully, including their co-host plants and the co-evolution of each pest. It is important to improve different management technologies to suppress plant viruses and insect vectors simultaneously. To implement effective management options, farmers should be aware of using locally available extension services to minimize the damage caused by plant viruses and their insect vectors. Thus, the sustainable management of insect vectors can halt the transmission of plant viruses. Hence, the cooperation efforts of plant virologists and entomologists are crucial to overcome these overwhelming challenges because they increase poverty, malnutrition, food waste, and production expenses. Understanding theoretical pest management strategies is insufficient if not professional training is given to build skills, improve knowledge, and share practical experience with each other by organizing with relevant bodies. These activities have required an understanding of biology, agroecological distribution, and management methods of insect vectors to solve the problems practically at the right time with the right tools and inputs at the right place by experts, farmers, and other considered bodies.
4. Conclusion
During herbivory, insect pests caused injury, spoilage, damage, parasitization, and a reduction in yield and marketability and transmitted viruses to host plants. Insect orders such as Hemiptera, Thysanoptera, Coleoptera, Orthoptera, and Dermaptera largely vector and transmit different plant virus species to healthy plants. Among the Hemipteran order was the best at vectoring, spreading, and dispersing plant viruses. The new control approaches for plant viruses should start with their vector insects. Managing insect pests alone may have helped to reduce the spread of insect-borne plant viruses. To understand the biology, ecology, taxonomy, and associated viruses, various disciplines, including virology and entomology, must be integrated into their sustainable management. Finally, it is concluded that regulating insect vectors at appropriate stages, periods, and places by available methods can manage plant viruses.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors did not receive any money from any institutions or individuals for this study.
Open Research
Data Availability Statement
The authors did not involve primary data collection. Hence, this review has no source of primary data to share it, and the authors have no data in their hands for the report.




