Medicinal Plants and Herbal Medicines for Managing Anxiety and Depression via Gut Microbiota Modulation
Abstract
Currently, there is a high prevalence of depression and anxiety worldwide. Recent research about psychopharmacology based on natural products has revealed promising alternatives in mental disorder’s treatment. Medicinal plants are rich in bioactive compounds with beneficial effects on many diseases, among these, mental illnesses. This manuscript presents current research on the role of the gut microbiota in mental health and the potential of some medicinal plants and herbal medicines to modulate this system for anxiety and depression management. Several studies have demonstrated a complex cross talk in the gut–brain axis, which occurs through the autonomic nervous system directly with the enteric nervous system via the vagus nerve, or even through the hypothalamic–pituitary–adrenal axis. Recently, it has been discovered that natural products and their bioactive compounds, especially flavonoids, can alter the composition and diversity of intestinal microbiota, as well as their produced metabolites. Metabolites produced by the microbiota from bioactive substances may be responsible for the anxiolytic and antidepressant effects attributed to their consumption. This interaction between natural products and the gut microbiota is bidirectional. It is necessary for further studies to determine if those compounds themselves exhibit beneficial effects on psychiatric disorders or if these effects are primarily due to the metabolites produced by the gut microbiota fermentation.
1. Introduction
Mental disorders are characterized by a clinical disturbance in cognition, behavior, or emotional regulation, being normally associated with distress/impairment in important functioning areas. There are different types of mental disorders, where anxiety and depression are the most common. The World Health Organization (WHO) presented worrying data: one in eight people had some mental illness in 2019, representing 970 million people worldwide. Among these, 280 million are diagnosed with depression, including 23 million children and adolescents, while 301 million are diagnosed with anxiety disorder, including 58 million children and adolescents [1]. Healthcare costs and the economic impact resulting from the loss of productivity associated with depression exceed $200 billion. Concurrently, this public health crisis, also regarded as a global psychological pandemic, has driven many countries to invest in promoting mental well-being [2].
Several studies have demonstrated a complex role of intestinal microbiota on the central nervous system (CNS) and vice versa. The gut microbiota (GM) corresponds to 70% of the human microbiome species and is mostly composed of anaerobic bacteria. A healthy GM is mainly composed of the bacteria phyla Bacillota (30%–52%), Bacteroidota (9%–42%), and Actinobacteria (1%–13%), in addition to fungi, viruses, and phages, and is vital for proper nutrition, immunity, and mental health [3, 4].
This microbial community in the human intestine is a highly dynamic ecosystem, and numerous physical, chemical, and biological factors can disrupt its balance (dysbiosis), leading to many diseases, such as Type 2 diabetes, obesity, cardiometabolic diseases, nonalcoholic liver disease, and gastrointestinal and mental disorders [5]. Main risk factors for dysbiosis development are disturbed eating and sleeping habits, stress, smoking, genetic susceptibility, medical treatments, and irreversible surgeries [3, 6, 7].
Herbs, spices, and herbal medicines have long been connected to traditional cuisines and are recognized for their health benefits. Since ancient times, cultures in Mesopotamia, China, India, Ancient Egypt, and Greece have utilized several herbs and spices to prevent, treat, and improve a range of diseases. Depending on the specific illness, one or more plants may be used as preventive or complementary medicine [8], helping to reduce the risk of developing various diseases, such as cardiovascular and CNS diseases, diabetes, and cancer [9].
Recently, it has been discovered that natural products and their bioactive compounds can alter the intestinal microbiota composition and diversity, as well as the metabolites they produce. Such alterations in GM can modulate mucosal immunity and gut homeostasis, thus influencing the prognosis of some diseases [8], like functional gastrointestinal conditions, inflammatory bowel diseases, obesity, and metabolic syndrome [5, 10]. Moreover, the microbiota is also linked to disorders like anxiety, depression, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and autism [11, 12]. However, only a limited number of studies have established specific mechanisms between the modulation of GM and brain function [13]. Preclinical studies, mostly in the mice model, suggest that alterations of GM by the intake of herbal medicines can be related to the mitigation of depression-like behavior [14, 15]. However, similar studies correlating the intake of herbal medicines with changes in GM and depression/anxiety symptoms in humans are still emerging. Therefore, this manuscript explores recent research on the application of some medicinal plants and herbal medicines in the management of anxiety and depression and examines their influence on mental health through the modulation of GM composition.
2. Medicinal Plants, Herbal Medicines, and Their Bioactive Compounds
The largest market for herbal medicines is the United States followed by Europe, Australia, and Canada [16]. According to a report from the British Broadcasting Corporation (BBC), the global herbal medicine market generated $153.5 billion in 2022, with a 10.6% forecast of compound annual growth rate (CAGR) between 2023 and 2028, reaching $279.8 billion [17]. This market has shown significant growth in recent years and continues to expand as more people seek alternative and natural medicines for many health issues. Increased concern about the importance of maintaining health and preventing diseases, as well as the preference for natural and plant-based products, may also contribute to this growth. Furthermore, herbal medicines are increasingly popular among patients due to the absence of unwanted side effects, good tolerability, and traditional use [18].
The WHO considers herbal medicines as preparations and products made from whole plants, parts of plants, or other plant materials and/or their extracts, with the aim of therapeutic use or other health benefits, with continuous growing demand, both in developing and developed countries [16]. The Brazilian National Health Surveillance Agency (ANVISA) defines herbal medicines as industrialized products obtained exclusively from plant raw materials. Their effectiveness must be based on clinical evidence, risks related to their use, and ability to reproduce and maintain their quality. In relation to traditional herbal medicines, also obtained exclusively from plant raw materials, their safety and effectiveness must be proven by frequent use and technical-scientific literature, without requiring clinical evidence or medical supervision for their use [19].
Recommendations for some diseases and symptoms treatment with herbal medicines, based on high levels of evidence studies such as randomized controlled trial (RCT), are presented in Table 1. However, more studies are still needed about the specific markers of such products in order to determine their safety and clinical efficacy [18]. Medicinal plants like saffron (Crocus sativus), kava (Piper methysticum), ginkgo (Ginkgo biloba), lavender (Lavandula spp.), black cohosh (Actaea racemosa), and chamomile (Matricaria chamomilla) have been shown to be effective in treating psychological and psychiatric conditions such as depression. It has been frequently documented that fibers, polyphenols, polysaccharides, and other bioactive components present in herbs and spices (Figure 1), like alkaloids, carotenoids, coumarins, lignans, saponins, terpenoids, and plant sterols, have beneficial effects on many diseases, such as cardiovascular disease, cancer, diabetes, and inflammatory conditions [8, 20].
| Herbal medicine | Common name | Recommendation |
|---|---|---|
| Hypericum perforatum | St. John’s wort | Depressive disorder |
| Vitex agnus castus | Chaste tree | Menstrual complaints |
| Cimicifuga racemosa | Black cohosh | Menopausal symptoms |
| Iberis amara, M. chamomilla, Mentha x piperita, Carum carvi, Glycyrrhiza glabra plus Melissa officinalis | Candytuft, chamomile, peppermint, caraway, liquorice plus lemon balm | Functional dyspepsia |
| Centaurium erythraea, Levisticum officinale plus Rosmarinus officinalis | Common centaury, lovage plus rosemary | Urinary tract infections |
| Pelargonium sidoides | Umckaloabo | Bronchitis and sinusitis |
- Note: Data compiled from Salm et al. [18].
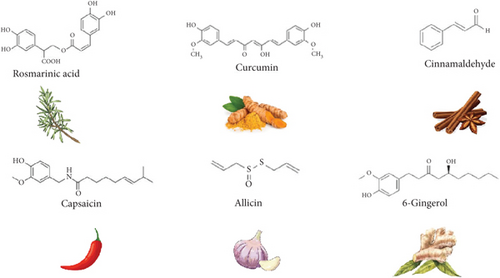
Polyphenols, also known as phenolic compounds, are a category of phytochemicals that serve as secondary metabolites. They are present in a wide range of plant-based foods, such as herbs and spices [20]. Flavonoids are one of the most important polyphenols of natural plants and foods, which have antioxidant, antibacterial, antiviral, and anti-inflammatory activities. These substances act as free radical scavengers due to the presence of hydroxyl groups, preventing oxidative damage. Such compounds have several biological properties with benefits to human health, preventing the development of many diseases, such as cancer, cardiovascular disorders, diabetes, and inflammation, as well as improving the immune system [9] and, more recently, emerging as antidepressant compounds [21].
Most used herbal medicines that are rich in flavonoids are chamomile, dandelion (Taraxacum officinale), ginkgo, hawthorn (Crataegus spp.), liquorice (Glycyrrhiza glabra), passionflower (Passiflora incarnata), milk thistle (Silybum marianum), onions (Allium cepa), rosemary (Rosmarinus officinalis), sage (Salvia officinalis), thyme (Thymus vulgaris), yarrow (Achillea millefolium), and green tea (Camellia sinensis) [9].
Despite polyphenols having been mostly reported as functional molecules in herbal medicines, the importance of other compounds has also been described. Terpenes, or terpenoids, are a diverse group of natural compounds occurring in several plant sources including tea, thyme, sage, Cannabis, and Citrus fruits. Carvone, menthol, eugenol, curcumin, piperine, carvacrol, citronellol, and α-limonene are some examples of this large group of bioactive molecules associated with diverse health benefits. Some terpenes were widely used in traditional medicine since they have anti-inflammatory, anticancer, antimicrobial, antioxidant, antiplasmodial, digestive, diuretic, and many other properties, such as cardioprotective and neuroprotective effects [22, 23].
Alkaloids constitute another class of natural compounds found in medicinal plants. Their broad spectrum of biological activities includes anti-inflammatory, anticancer, antioxidant, antimicrobial, antimalarial, immunomodulatory, antidiabetic, cardioprotective, hepatoprotective, and analgesic properties [24]. Some examples of well-known bioactive alkaloids comprise ephedrine, morphine, codeine, caffeine, capsaicin, and theobromine.
Saponins are a diverse group of plant secondary metabolites that have valuable biological activities in the pharmaceutical field, such as anti-inflammatory, antimicrobial, antiviral, antiparasitic, cholesterol-lowering, and anticancer properties. Liquorice, soapbark tree (Quillaja bark), and Yucca are among the plant sources containing the highest amounts of saponins, although milkwort, primula, and fenugreek also contain considerable quantities [25]. Moreover, several polysaccharides from medicinal plants, including glucans, fructans, and pectins, are an integral part of plant cells. Besides their importance for plant structure and metabolism, substantial benefits to human health have been associated with the immunomodulatory, hepatoprotective, and antidiabetic properties of plant polysaccharides [26].
3. Natural Products for Mental Health Disorders: Anxiety and Depression
Depression is considered a multifactorial, chronic, and life-threatening disease, being one of the main causes of death and the number one cause of health problems and disability worldwide [6]. It has several pathophysiological conditions involved, such as central dopamine levels, inflammation, stress responses through the hypothalamic–pituitary–adrenal (HPA) axis, and the autonomic nervous system (ANS), as well as brain-derived neurotrophic factor (BDNF) dysfunction. In this sense, it is considered a chronic inflammatory disease with altered levels of serum cytokines [27].
In major depressive disorder (MDD), the individual presents a depressed mood (sad, irritated, and feeling of emptiness) or loss of pleasure and interest in activities during most of the day, almost every day, or for at least 2 weeks. Other symptoms include lack of concentration, overwhelming guilt, low self-esteem, hopelessness, being tired or unmotivated, thoughts about death or suicide, disturbed sleep, low libido, and changes in appetite or weight [1, 28].
There is a higher prevalence of depression among females compared to males, with neurostructural and neurofunctional factors suggested as potential contributors to this difference. Changes in estrogen secretion, particularly during menopause, may significantly affect depression’s pathophysiology by altering levels of norepinephrine and serotonin in the brain, thereby manifesting classic depressive symptoms such as irritability, melancholy, mood swings, and emotional instability [29].
The response to antidepressant medication is slow, requiring weeks to months before any symptomatic change. Most synthetic antidepressants act on monoamines (mainly norepinephrine and serotonin), like selective serotonin reuptake inhibitors (SSRIs). However, variable responses to medication suggest that the increase in these neurotransmitters is insufficient and other mechanisms may be involved in symptom remission [30]. Several patients present multiple depressive episodes, with increasing frequency and duration, and are resistant to current pharmacological treatments, where only around 30% of patients achieve acceptable results, 40% mixed results, and 30% insufficient results. Furthermore, a significant part of patients does not experience full remission with pharmacological treatment [30, 31].
Anxiety involves intense fear, worry, and associated behavioral issues. The symptoms are severe, causing distress or substantial disruption to daily life. Various forms of anxiety disorders exist, including generalized anxiety, panic attacks, social anxiety, and separation anxiety [1]. The generalized anxiety disorder (GAD) diagnosis is made by the marked presence of worry and anxiety, accompanied by more than three somatic symptoms (such as tension and irritability), which occur frequently over a period of at least 6 months [28], being the benzodiazepines generally used as first-line treatments. Main causes can be genetic, physiological, emotional, behavioral, and cognitive [6].
In the last 10 years, there have been no relevant advances in the development of new classes of synthetic antidepressant and anxiolytic medications. Meanwhile, research about psychopharmacology based on natural products has revealed many promising alternatives in depression and anxiety disorder treatment. Although the use of synthetic medications and psychological interventions is still the main approach, natural alternatives can offer additional safe and effective treatment options [31].
Anxiety is the most thoroughly researched topic in the field of herbal medicines, with several studies highlighting their calming effects. Among these, kava has been the most investigated. However, there are safety concerns related to its potential to cause liver toxicity. Kava’s calming effects are thought to result from interactions between its active compounds, known as kavalactones, and various neural pathways. These interactions include the modulation of gamma-aminobutyric acid (GABA) voltage-gated sodium ion channels, the enhancement of binding at specific GABA-a receptor subtypes, and the reduction of excitatory neurotransmitter release through calcium ion channels block [28].
Several herbal medicines have received approval from regulatory agencies for psychiatric disorder treatment. For instance, Brazilian ANVISA has approved products derived from passionflower, valerian (Valeriana officinalis), black cohosh (Cimicifuga racemosa), and kava for anxiety or depression. Similarly, the European Medicines Agency (EMA) has recognized plants such as St. John’s wort (Hypericum perforatum), lemon balm leaf (Melissa officinalis), and valerian root as approved treatments for mental stress and mood disorders [32].
In fact, some herbal preparations for psychiatric disorders treatment have consistent clinical evidence confirmed by meta-analyses and indicated for well-established use in EMA monographs, such as St. John’s wort, valerian, and kava [33, 34]. Other medicinal plants with such activity, like passionflower, hops (Humulus lupulus), and lemon balm, are also employed due to their long traditional use supported by controlled clinical trials. Most of the herbal medicines’ clinical effects may be due to synergistic action between their bioactive compounds. Nevertheless, no studies have been carried out confirming such hypotheses for natural products applied to mood disorders to this date [31].
According to Sarris [28], there are 24 herbal medicines for 11 psychiatric conditions, with high standard evidence for kava and St. John’s wort, but also with several current research studies considering the use of saffron for depression and passionflower for anxiety. Other plants with anxiolytic activity are gotu kola (Centella asiatica), chamomile, ginkgo, ashwagandha (Withania somnifera), lavender oil, and lemon balm. For depressive disorders, the most used plants are, in addition to saffron and St. John’s wort, turmeric (Curcuma longa), Korean ginseng (Panax ginseng), and roseroot (Rhodiola rosea).
Clinical interventions with level A evidence (meta-analysis or two or more RCT) consider the following plants for treating mood disorders: St. John’s wort, saffron, roseroot, and lavender for MDD and bipolar depression, as well as lavender, kava, ashwagandha, galphimia (Galphimia glauca), and chamomile for GAD [35]. Clinician guidelines with level A evidence for herbal medicines use in MDD and GAD treatment, recommended daily dose and safety level, are presented in Table 2.
| Mental illness | Herbal medicine | Daily dose | Safety |
|---|---|---|---|
| MDD | St. John’s wort | 600–1800 mg (standardized to hypericin 0.2%–0.3% and/or 5%–6% hyperforin) | Acceptable |
| MDD | Saffron | 30 mg of stigma (standardized to safranal or crocin) | Acceptable |
| MDD | Curcumin | 500–1000 mg of curcumin | Acceptable |
| MDD | Rhodiola | 340–680 mg (standardized to rosvarin) | Acceptable |
| MDD | Lavender | 80–160 mg oil (capsule) or 500 mg–1.5 g of dried flower (standardized to linalool) | Acceptable |
| GAD | Kava | 60–250 mg of kavalactones | Fair |
| GAD | Ashwagandha | 300–600 mg (standardized to 5% withanolides) | Acceptable |
| GAD | Galphimia | 350–700 mg (standardized to galphimine-B) | Fair |
| GAD | Chamomile | 220–1500 mg (standardized to chrysin or apigenin) | Robust |
| GAD | Lavender | 80–160 mg oil (capsule) or 500 mg–1.5 g of dried flower (standardized to linalool) | Acceptable |
- Note: Data compiled from Sarris et al. [35].
Kava roots possess strong anxiety-reducing properties linked to kavalactones action on GABA receptors. Passionflower has an anxiolytic effect by antagonizing GABA-b receptors. Valerian root extracts are effective in treating anxiety, depression, sleep issues, and cognitive functions due to valepotriates’ influence. Chamomile flowers help with anxiety control by modulating GAD activity. St. John’s wort extracts act as antidepressants and anxiolytics by affecting monoamine transmission with components like hypericin. Lemon balm presents sedative effects through GABA transaminase and monoamine oxidase A (MAO-A) inhibition [36]. Some medicinal plants with anxiolytic and antidepressant effects and their active metabolites can be seen in Figure 2.
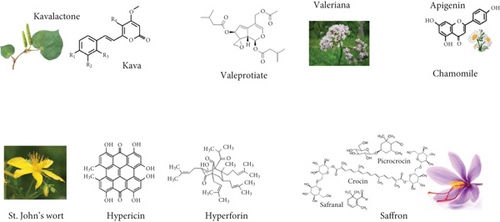
A double-blind clinical trial study on the effect of valerian on depression and anxiety symptoms of the menopause revealed that anxiety level and depressive indicators significantly decreased in valerian group as compared to placebo, suggesting that valerian could be considered an alternative treatment [37]. Researchers examined St. John’s wort effects on menopausal symptoms, focusing on hot flashes and depression. They discovered a notable reduction in both the severity of depression and the frequency of hot flashes among the participants. St. John’s wort acts by inhibiting the uptake of serotonin, dopamine, and norepinephrine [29]. It is believed that St. John’s wort’s neurochemical modulation occurs through synergy between its constituents, such as hyperforin, hypericin, and many flavonoids. Such herbal plant antidepressant mechanism of action is mediated by nonselective inhibition of the neuronal reuptake of serotonin, dopamine, norepinephrine, GABA, and l-glutamate, decreased degradation of neurochemical substances and increased binding to various receptors, like GABA, glutamate, and adenosine [28].
A systematic review and meta-analysis performed to compare the effects and safety of ginkgo on patients with depression showed that treatment with ginkgo resulted in better values of subjective and objective indicators of depression after 4, 6, and 8 weeks as compared to the control group, indicating that ginkgo might reduce the risk of depression or depressive symptoms with safe clinical efficacy [38]. The effectiveness of chamomile as an herbal medicine for anxiety treatment was evaluated through a systematic review of clinical trials. The study showed that oral consumption of chamomile was effective in reducing anxiety, although additional clinical studies are suggested to provide standard dosages for users [39]. Although the exact mechanism of anxiolytic action is not completely elucidated, evidence suggests that apigenin and other active compounds may modulate the function of the HPA axis by affecting neurotransmitter pathways [40].
Results from a systematic review and a meta-analysis of RCT showed significant efficacy of lavender in reducing depression scores compared to the control group. However, the authors recommended that additional large clinical trials should be conducted with more homogeneous populations and rigorous designs [41]. A compilation of studies conducted on diverse individuals, including preoperative, cardiovascular, hemodialysis, cancer, and dental patients, and women in prelabor, revealed that lavender was effective as an anxiolytic in various settings [42]. In addition, no major adverse events were reported, and the preferred long-term option was the oral route.
Current evidence indicates the promise of lemon balm as a calming agent exhibiting both anxiolytic and antidepressant properties, likely by modulating brain signaling pathways, such as GABAergic, cholinergic, and serotonergic systems [43]. A randomized double-blinded placebo-controlled clinical trial was conducted to investigate the effects of M. officinalis on depression and anxiety in Type 2 diabetes patients with depression. A significant reduction in depression and anxiety severity at the end of the study was observed in the intervention group as compared to the baseline [44].
Among the plants examined in clinical trials, saffron, turmeric, ginkgo, St. John’s wort, and passionflower stand out for their broad biological activity and documented history in preventing or treating depression and anxiety [33, 34]. Most reasonable options to consider in depression and anxiety treatment using natural medicines are chamomile, lavender, passionflower, and saffron, due to their minimal risk of serious side effects. Additionally, other studies have shown that green tea can effectively treat depression by modulating the GM and inhibiting the HPA axis. Theanine, an amino acid present in green tea, has been found to alleviate depressive symptoms by regulating dopamine and serotonin levels, thereby influencing the HPA axis [29]. These species continue to be central to research into their biochemical and therapeutic properties. However, more studies are still needed to elucidate the mechanisms of action and pharmacokinetics, their potential synergistic or antagonistic interactions, and the safety and efficacy of such herbs. Furthermore, large-scale clinical trials with robust methodology and use of well-characterized standardized agents should be performed [45, 46].
4. GM, Gut–Brain Axis, and Mental Health
There is a complex role of the CNS on the intestinal microbiota, and such interchange is bidirectional. Communication between them occurs through the ANS directly with the enteric nervous system via the vagus nerve, or even through the HPA neuroendocrine axis. The influence of GM on CNS occurs through diverse and complex pathways, such as the regulation of cerebral anti-inflammatory activity, the modulation of blood–brain barrier (BBB) permeability, and the transfer of neuroactive peptides to CNS through the vagus nerve [3, 27].
During periods of increased inflammation, such as stress, the intestinal barrier can become compromised, leading to bacterial infiltration and higher plasma lipopolysaccharide (LPS) levels called “leaky gut.” Research has shown that gut bacteria can trigger a neuroinflammatory response and affect the HPA axis by increasing cytokine and chemokine production, which can reach the brain through blood, lymphatic system, vagus nerve, and increased BBB permeability [47]. Disruptions in this axis are linked to numerous immune, neurological, and psychiatric disorders [6, 48].
When the balance of GM is disrupted, it can lead to damage to the intestinal mucosal barrier, resulting in reduced immune function. Many diseases, such as depression, multiple sclerosis, diabetes, autism, and cancer, are associated with imbalanced GM [6, 49]. Brain’s interactions with the intestinal microbiota, primarily through the neurological, endocrine, immune, and metabolic systems, have an indirect effect on cognition, sleep, and mood. GM not only regulates host metabolism and immunity but also plays a crucial role in the bidirectional communication between the gastrointestinal tract and the CNS [21]. The key role of immune dysregulation and dysbiosis of GM on depression has been well-established. Several herbs from traditional Chinese medicine have a significant effect on reducing inflammation and regulating GM, thus alleviating symptoms of many neurodegenerative and mental conditions [50].
Also, gut microbial populations have been found to produce many neurotransmitters. Major neurotransmitters include GABA from Lactobacillus and Bifidobacterium; norepinephrine from Escherichia, Bacillus, and Saccharomyces; dopamine from Bacillus; acetylcholine from Lactobacillus; and serotonin from Escherichia, Enterococcus, Candida, and Streptococcus. In addition, several gut bacteria are involved in tryptophan metabolism, the only precursor to serotonin, which has an essential role in the control of emotional regulation, sleep, hunger, and pain, as well as modulation of gut physiology. Neural activity is also modulated by tryptophan catabolites from the kynurenine degradation pathway [51]. Evidence that tryptophan metabolism pathways differ between healthy individuals and those with neurological diseases reinforce the role of serotonin as a key neurotransmitter in various psychiatric disorders, including anxiety and depression [47, 52].
Intestinal microbiota can also produce several bioactive chemicals, including bacteriocins, bile acids, choline, and short-chain fatty acids (SCFAs). SCFAs, like butyrate, propionate, and acetate, are produced from the fermentation of polysaccharides and can induce the synthesis of such neurotransmitters [47]. In general, propionate is primarily produced by Bacteroidota, whereas Bacillota plays a key role in butyrate production [4].
Gut microorganisms are accessible and can be influenced through many ways, including probiotics, prebiotics, and dietary interventions. Probiotics are live beneficial bacteria, and prebiotics are substances selectively used by host microorganisms to confer health benefits, such as nondigestible carbohydrates and plant polyphenols [27, 53]. Probiotics are recommended for MDD treatment, based on strong safety data, at doses of 1–10 billion units per day [35].
Lactobacillus and Bifidobacterium genera have the most researched species, but products used with different probiotic strains can vary greatly. Optimal probiotic strains for treating depression have yet to be determined, and it is believed that the effectiveness of different strains may vary depending on an individual’s genetics, diet, and microbiome composition. The examination of a total of 10 RCTs suggested psychological benefits for the consumption of certain probiotics in reducing human anxiety and depression, despite the complexity of gut–brain interactions and the methodological limitations of the trials [54].
Some studies suggest that probiotics may also reduce gastrointestinal side effects associated with antipsychotic medications. However, the lack of clinical trials in this area highlights the need for further investigation to assess the potential of probiotics as complementary treatments for psychiatric disorders [55].
Emerging evidence suggests that targeting the GM could represent a novel approach to mental health maintenance, leading to the introduction of the term “psychobiotic.” Such compounds are any substance that exerts a psychological effect mediated by the microbiome, including probiotics, prebiotics, synbiotics (combinations of probiotics and prebiotics), and postbiotics (probiotics metabolic byproducts). The term “phytopsychobiotics” can describe medicinal plants whose mental health effects are mediated through GM modulation [27, 29].
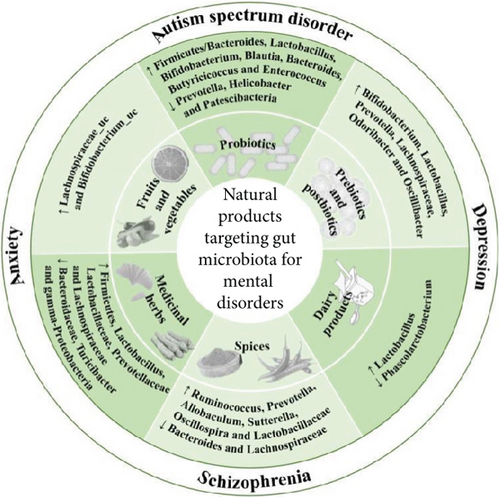
Therefore, dietary and microbial components such as probiotics (e.g., Lactobacillus and Bifidobacterium), prebiotics (dietary fiber), synbiotics, postbiotics (like SCFAs), dairy products, spices, fruits, vegetables, and medicinal herbs may have beneficial effects on mental disorders through GM modulation by improving its composition [56], as presented in Figure 3. This modulation can occur through prebiotic-like effects, where plant ingredients act as substrates for beneficial microbial growth, or through postbiotic-like effects, where active secondary metabolites produced by GM from nondigestible plant components exert physiological effects. Additionally, some medicinal herbs may exhibit antibiotic-like effects, reducing pathogenic bacteria levels and thus influencing mental health [27, 29].
Although not conclusive, significant differences in GM composition between healthy and depressed individuals have been observed. Despite limitations and inconsistencies, variations in several bacteria have been linked to psychiatric disorders. For instance, increased relative abundances of Enterobacteriaceae, Streptococcaceae, Eggerthella, Olsenella, Lactobacillus, Erysipelotrichaceae, Holdemania, Streptococcus, Desulfovibrio, and Paraprevotella, as well as decreased levels of Prevotellaceae, Sutterella, Clostridium, Escherichia/Shigella, and Coprococcus, are associated with depression. Similarly, enrichments of Enterobacteriales, Bacteroidaceae, Escherichia/Shigella, Bacteroides, and Tyzerella and reductions in Bacillota, Mollicutes, Prevotellaceae, Ruminococcaceae, Subdoligranulum, Coprococcus, and Dialister are correlated with anxiety [31].
A comparative study on the GM of 46 depressed patients and 30 individuals in a control group revealed that the levels of Bacteroidota, Proteobacteria, and Actinobacteria were significantly higher in depressed patients, whereas Bacillota levels decreased rapidly [21]. Another study discovered that individuals with MDD had significantly higher numbers of Prevotella and Klebsiella [57]. Moreover, the fecal microbiota composition of 165 MDD patients was compared with 217 healthy controls with the same age, gender, and body mass index. The microbiota of MDD patients showed more Enterobacteriaceae and Alistipes and less Faecalibacterium, Coprococcus, and Dialister as compared to the control group [58].
Although these findings point to potential microbial differences in psychiatric disorders, human studies in this field remain limited. Further research is required to clarify the intestinal microbiome’s role in mental health, how such microorganisms might influence brain function, and whether treatments targeting the microbiome could be effective for psychiatric conditions [55]. This field is still in its early stages, and while changes in GM have been associated with mood disorders, the causal relationship remains unclear.
Future studies employing multiple omics approaches (such as metagenomics, metaproteomics, and metabolomics) may allow the investigation of microbial functions, including the synthesis of proteins and metabolites and their roles in health and disease and are still needed to better understand these connections and their implications [59, 60]. Advancements in next-generation sequencing technologies have introduced various platforms and approaches to study the gut microbiome across different species and medical conditions [61]. According to Sarris et al. [35], an important area for future research involves studying how the microbiome effectively affects psychiatric disorders and medication use through advanced biomarker assays. Progress in multiomics approaches may also enable the customization of nutraceuticals for individuals based on factors such as existing deficiencies, genetic and microbiome profiles, or specific biochemical tests. Future research should use metagenomic approaches and their correlation with metabolomics to provide a more comprehensive understanding of microbial composition and function [62].
Moreover, current clinical research should be considered in light of several limitations. Many clinical studies are conducted with a small sample size, limiting its statistical power to detect individual or group differences, and a lack of information on participants’ use of medications, which could significantly influence the results. Also, there is a lack of dietary information, which could weaken observed associations. Dietary changes prompted by mental disorders symptoms may create a significantly different nutrient environment, contributing to GM alterations. There is also limited data on how antidepressants affect the human intestinal microbiome [62, 63].
5. Medicinal Plants and Herbal Medicines in the Management of Anxiety and Depression Through GM Modulation
Natural products and their bioactive compounds can alter the composition and diversity of GM and consequently the metabolites produced by gut microorganisms. These substances may also influence the protein structure of the intestinal tight junctions, gut mucosal immunology, and gut homeostasis, thus influencing the development and prognosis of certain diseases through a bidirectional interaction between natural products and GM [64]. Additionally, natural products can help regulate dysbiosis and reduce systemic inflammation caused by endotoxemia through various biological mechanisms. Furthermore, these bioactive compounds have been found to increase SCFA gut production, reduce intestinal barrier permeability, and thereby preserve its function [8, 53]. Some natural products’ consumption effects on GM are presented in Figure 4.

Phytochemicals produced by GM can acquire properties such as increased bioavailability, antioxidant activity, detoxification of xenobiotics, and prebiotic functions. These compounds can help reduce pathogenic microorganisms, affect the levels of some other microorganisms (like Bacillota, the Bacillota/Bacteroidota ratio, and Desulfovibrionaceae), decrease oxidative DNA damage and proinflammatory markers, promote healthy cell division, and regulate apoptosis [8]. Several studies have shown that, by affecting these microorganism levels in the intestine, phytochemicals can positively influence dysbiosis, alter microbial diversity, and maintain its stability [64, 65].
Only a small fraction (5%–10%) of dietary polyphenols are absorbed in the small intestine, while the majority (90%–95%) reaches the colon [20]. GM influences the bioavailability and biological activity of polyphenols through their biotransformation and metabolism. Due to their prebiotic-like characteristics, polyphenols and polyphenol-rich foods can alter the composition of the intestinal microbiota. Metabolites produced by the microbiota from polyphenols may be responsible for the anxiolytic and antidepressant effects attributed to their consumption [66]. However, further studies are necessary to determine if polyphenols themselves exhibit beneficial effects on psychiatric disorders or if these effects are primarily due to the metabolites produced by GM fermentation [6].
Interactions between intestinal microbiota and herbal medicines primarily occur through two main mechanisms: production of active small molecules and modifications in GM and its secretions. Such microorganisms possess extensive enzymatic capabilities that allow the breakdown of plant constituents. The primary metabolic interaction between GM and herbal medicines is deglycosylation. This enzymatic activity results in the production of metabolites with altered bioavailability and pharmacological effects [27, 67]. Reactions such as dehydroxylation, reduction, demethylation, and lactone ring scission can also be induced by the GM. These microbial transformations are influenced by the molecular structure, polymerization degree, and spatial configuration of the compounds [68].
Terpenoids and polyphenols from herbal medicines are deconjugated by GM glycosidases and esterases, making them more bioactive and easily absorbed than the original compounds. Gut bacteria are involved in the hydrolysis of sugar moieties from compounds like quercetin-3-O-glucoside, releasing the aglycone quercetin and producing lactate, acetate, formate, and ethanol. Moreover, some strains may metabolize quercetin, leading to the formation of taxifolin, SCFAs, and 3,4-dihydroxyphenylacetic acid [69]. Additionally, GM functions as a carrier, transporting compounds as berberine into the body and converting them into their active forms upon absorption [4].
Likewise, plant substances can influence the GM community composition. This interaction may promote the proliferation of beneficial bacteria and the production of beneficial health metabolites [27]. Thus, herbal medicines can impact the GM either directly or indirectly. They may alter the acidity, alkalinity, and transit time within the gastrointestinal tract, promote the growth of beneficial microorganisms, suppress the proliferation of harmful ones, and regulate the GM composition bidirectionally. Although research indicates that herbal medicines have therapeutic potential, the precise mechanisms underlying their interactions with GM during treatment remain incompletely understood [20, 31, 67].
Plant compounds like curcumin are transformed into metabolites by the microorganisms, which can also influence the composition and function of the intestinal microbiota [21]. Furthermore, the modulation of the GM composition by herbal medicines, resulting in increased diversity and beneficial microbial communities, often correlates with improved health effects. These include reduced inflammation, boosted immune responses, and better nutrient absorption [4, 70].
Therapeutic effects of herbal medicines in neurodegenerative diseases and mental well-being through GM modulation of GM have been documented as well [49]. Metabolites produced by the intestinal microbiome could offer new and effective therapeutic approaches for mood disorders. In addition, probiotic cultures may play a significant role in treating gastrointestinal dysbiosis and, therefore, as active supplements in improving adverse mood conditions by the GM modulation [2, 30].
It has been suggested that metabolites derived from GM can affect depressive behavior through either direct or indirect mechanisms, including (a) direct stimulation of central receptors; (b) peripheral stimulation of neural, endocrine, and immune mediators; and (c) epigenetic regulation of histone acetylation and DNA methylation [71, 72]. The elucidation of such mechanisms is essential to enhance our knowledge about the etiology of MDD and contributes to the development of new approaches related to the beneficial psychotropic effects of such metabolites.
Pathogen eradication from the intestinal environment allows beneficial strains to colonize, producing desired postbiotic metabolites. Herbal medicines used for mood disorders exert their effects through multiple mechanisms, including their impact on intestinal microbiota. Recent research indicates that herbal medicines and their compounds can significantly influence the GM structure [4, 49]. Moreover, certain metabolites produced by gut bacteria after herbal ingestion have been shown to improve depression-related behaviors in animal models. Modulation of the microbiota–gut–brain axis by these natural products may explain their therapeutic effects [31].
The knowledge on management of anxiety and depression through the modulation of GM by herbal medicines has been essentially derived from preclinical studies. The Chinese medicine formula Kai-Xin-San, comprising four herbal medicines (Acorus tatarinowii Schott., Panax ginseng C.A. Mey., Polygala tenuifolia Willd., and Poria cocos Schw. Wolf.), was able to alleviate depression-like behaviors in chronic unpredictable mild stress (CUMS) mice by modulating GM composition, inflammation, and stress system [14]. The antidepressant effect of Ginkgo biloba extract in mice could be attributed to the regulation of gut microbial metabolism. The potential role of Parasutterella excrementihominis and the bile acid metabolite ursodeoxycholic acid was revealed by bacterial profiling and metabolomics assay, suggesting that ginkgo reduces stress-induced depression through modulation of GM bile acid metabolism [15].
The antidepressant properties of total iridoids of Valeriana jatamansi and the influence of GM on their antidepressant effects were evaluated using a CUMS mouse model. While the control group showed significant depression-like behavior, the valerian treatment increased body weight, sucrose solution intake, and alleviated depression-like behavior. Moreover, the diversity and richness of GM were improved in comparison with the control group [73]. In another study, the in vitro PolyFermS model microbiota was used to investigate the interaction of herbal extracts and compounds, valerenic acid in valerian and hyperforin and hypericin in St. John’s wort, with artificial human microbiota. The exposure of compounds or herbal extracts had an overall minimal influence on SCFA production by the microbiota and minimal impact on bacterial viability at the tested concentrations of 30 μg/mL for compounds and 500 μg/mL for herbal extracts [74].
Moreover, the potential antidepressant and anxiolytic effects of several plants from traditional Chinese medicine have been reported. The same plants are also recognized for their anti-inflammatory action and positive modulation of GM in animal models. Some examples include ginseng (Panax quinquefolium L.) that increases the abundance of Bacillota and reduces the abundance of Bacteroidota and Verrucomicrobia, ameliorating gut dysbiosis [75], and Goji berry (Lycium barbarum) polysaccharides and anthocyanins that may increases the Bacillota-to-Bacteroidota ratio and the abundance of Lactobacillus spp., alleviating neuroinflammation in the gut–brain axis pathway [76, 77]. The extract of Ganoderma lucidum significantly preserved the intestinal barrier integrity, magnified the number of SCFA-producing bacteria, and reduced the relative abundance of Escherichia/Shigella group and Proteobacteria, an indicator of intestinal dysbiosis [78]. Rhizomes of Polygonatum species are used as tonic herbs, with positive effects on depression, inflammation, neurotoxicity, and modulation of GM. It can relieve DSS-induced colitis in mice by significantly reducing the production of proinflammatory cytokines, enhancing the expression of antioxidant genes, and selectively increasing the abundance of beneficial bacteria such as Bifidobacterium and Alistipes [79]. Particularly, both polysaccharides and methanol extract of Polygonatum odoratum can regulate GM, increasing the abundance of SCFA-producing bacteria. The methanol extract of P. odoratum, mostly comprising flavonoids and coumarins, decreases the number of H2S-producing bacteria, while the P. odoratum polysaccharides reduce the amount of Clostridium, Coprobacillus, and Sutterella [80, 81]. The stem of Dendrobium officinale Kimura et Migo can attenuate fatigue and depression by modulating the neuroendocrine-immune network. It modulates GM, decreasing the abundance of detrimental bacteria, such as Helicobacter, Sutterella, and Escherichia/Shigella, and increasing the abundance of beneficial SCFA-producing bacteria such as Allobaculum, Bifidobacterium, and Lactobacillus [82, 83].
As mentioned previously, clinical studies in natural product-based psychopharmacology have identified several promising herbal remedies for the treatment of mild mood disorders, especially St. John’s wort, kava, valerian, passionflower, hops, lavender, lemon balm, and roseroot. Effects of medicinal plants on depression and intestinal microbiota composition are summarized in Table 3. Unfortunately, these substances are understudied in the context of the gut–brain axis. Components in herbal medicines may act as prebiotics, promoting the growth of beneficial bacteria that produce SCFAs, known for their potential mood-enhancing effects. Although prebiotic supplementation alone may not significantly reduce depression scores, it can contribute to mood improvement [31].
| Herbal medicine | Beneficial effects on mental health and GM composition |
|---|---|
| Hypericum perforatum | St. John’s wort extract has been observed to change the proportions of several microbial genera, including increase in Lactobacillus, Akkermansia, and Bacillota, while decreasing Bacteroides. Higher amounts of Lactobacillus, which are linked to probiotic benefits, have been found in individuals with depression, hinting at positive impacts on mood |
| Crocus sativus | Research indicates that a carotenoid found in saffron called crocin alleviates depression symptoms and also affects GM composition in mice subjected to chronic restraint stress |
| Valeriana officinalis | A study involving different species of valerian has shown that their total iridoid content exhibited antidepressant activity in mouse models subjected to unpredictable mild stress, in addition to promoting GM richness and diversity |
- Note: Data compiled from Korczak et al. [31].
- Abbreviation:GM, gut microbiota.
Polyphenols can also regulate the GM and help maintain intestinal stability [21, 68]. These compounds, such as quercetin, have been shown to reduce depressive and anxious behaviors in rats. Dietary polyphenols influence neurotransmitter levels in the brain, development of the CNS, and immune function by modulating the SCFAs produced by gut bacteria. Additionally, these may help to treat depression by reducing stress-induced increases in brain cortisol via the vagus nerve. Strong evidence in preclinical studies indicates that dietary polyphenols can stimulate the abundance of health-promoting species in human GM [84, 85]. While it has been suggested that dietary polyphenols play a role in GM composition, the mechanisms by which this interaction occurs remain unknown, suggesting that more studies are necessary to elucidate the influence of dietary polyphenols on GM. The potential metabolism of polyphenols by the human intestinal microbiota is presented in Figure 5.
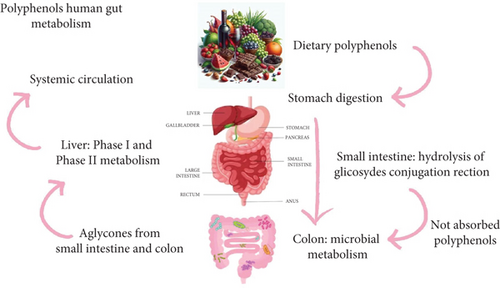
Rodríguez-Daza et al. [86], in order to understand the two primary mechanisms of action for polyphenols, have proposed the term “duplibiotics,” which expands the concept of prebiotics to include substrates that influence GM both by antimicrobial activity and by promoting the growth of beneficial bacteria. A duplibiotic refers to an unabsorbed substance that affects the GM through these dual actions. The duplibiotic effect of polyphenols may help mitigate metabolic imbalances and gut dysbiosis, suggesting their potential as dietary strategies with therapeutic benefits.
Despite the documented efficacy of phytotherapy in mild mood disorders, challenges persist in identifying biologically active substances and establishing consistency across epidemiological, clinical, and interventional studies, hindering their widespread acceptance as medicinal products. Future studies should investigate how phytoconstituents interact bidirectionally with intestinal communities to effectively treat mental disorders [31]. Clinical studies exploring the effects of herbs and spices on GM have shown variable results, possibly due to differences in microbiota community, dietary habits, small sample sizes, study durations, and designs. This variability underscores the need for larger scale studies to validate findings. Moreover, while culinary herbs and spices have been investigated for their potential health benefits, research specifically on their impact on GM remains limited and incomplete. Further studies are then necessary to fully understand these interactions and their implications in human health [20].
6. Conclusion
Medicinal plants and herbal medicines that are used in managing anxiety and depression through intestinal microbiota modulation constitute an emerging and promising research field in mental health therapy. Clinical studies have demonstrated anxiolytic and antidepressant effects by phytochemicals such as St. John’s wort, saffron, and valerian. These effects are mediated through complex interactions within the gut–brain axis, influencing neurochemical pathways, gut barrier integrity, and systemic inflammation. By modulating GM composition, plant-derived bioactive compounds facilitate the production of neuroactive metabolites that are critical in mood regulation. However, more studies are necessary in order to elucidate the mechanisms, optimal dosages, and long-term safety of these treatments. Advancing research in this field could lead to the development of novel, natural, and effective strategies for the treatment of anxiety and depression.
Nomenclature
-
- ANVISA
-
- Brazilian National Health Surveillance Agency
-
- ANS
-
- autonomic nervous system
-
- BBB
-
- blood–brain barrier
-
- BDNF
-
- brain-derived neurotrophic factor
-
- CAGR
-
- compound annual growth rate
-
- CNS
-
- central nervous system
-
- CUMS
-
- chronic unpredictable mild stress
-
- EMA
-
- European Medicines Agency
-
- GABA
-
- gamma-aminobutyric acid
-
- GAD
-
- generalized anxiety disorder
-
- GM
-
- gut microbiota
-
- HPA
-
- hypothalamic–pituitary–adrenal
-
- IL
-
- interleukin
-
- IFN
-
- interferon
-
- LPS
-
- lipopolysaccharide
-
- MCP
-
- monocyte chemoattractant protein
-
- MDD
-
- major depressive disorder
-
- RCT
-
- randomized controlled trial
-
- SCFAs
-
- short-chain fatty acids
-
- SSRIs
-
- selective serotonin reuptake inhibitors
-
- TNF
-
- tumor necrosis factor
-
- WHO
-
- World Health Organization
-
- ZO
-
- zonula occludens
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Priscilla Magro Reque: conceptualization, investigation, writing—original draft; Adriano Brandelli: visualization, investigation, writing—review and editing; Cristina Mayumi Sasaki Miyazaki: visualization, review, and editing; Ana Flávia Marçal Pessoa: conceptualization, visualization, review, and editing
Funding
No funding was received for this manuscript.
Acknowledgments
The authors express their gratitude to the Phytotherapy and Medicinal Plants Specialization Course at the University of São Paulo Medical School (FMUSP).
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




