Diarrheagenic and Uropathogenic Escherichia coli: Biofilm Production and Virulence Factors in Clinical Isolates From Argentina
Abstract
Biofilm formation by Escherichia coli contributes to the persistence of infections. In order to deepen the knowledge about the pathogenesis of E. coli infections, the objective was to assess the biofilm production and the expression of virulence factors of human clinical isolates of E. coli from enteric and urinary infections from Argentina. We analyzed 105 isolates of diarrheagenic E. coli (DEC) and uropathogenic E. coli (UPEC). All isolates (except one) were biofilm producers. The moderate biofilm former (MBF) category was prevalent (38.1%). Curli fimbriae was the most highly expressed virulence factor (94.3%), whereas cellulose, hemolysins, and hemagglutinins were detected at lower frequencies (43.8%, 35.2%, and 33.3%), respectively. The number of DEC isolates that express cellulose was significantly higher than the number of UPEC isolates (p < 0.01). The multifactorial nature of biofilm of E. coli indicates the need for new approaches to understand the pathogenesis and persistence of biofilm-related infections.
1. Introduction
Escherichia coli is a highly versatile and worldwide distributed bacterium that can exist as a harmless commensal in the mammalian colon or as a pathogen. E. coli is broadly categorized into two pathogroups based on the presence of specific virulence factors: diarrheagenic E. coli (DEC) and extraintestinal pathogenic E. coli (ExPEC) [1, 2]. Many virulence factors such as adhesins, fimbriae, hemolysins, and aerobactin production are encoded in specific regions of the chromosome, called pathogenicity islands, and are associated with survival and fitness [3]. The DEC pathotypes include enteropathogenic E. coli (EPEC), Shiga toxin–producing E. coli (STEC), enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), diffusely adherent E. coli (DAEC), and adherent invasive E. coli (AIEC) [1, 2]. ExPEC has virulence factors that allow it to invade, colonize, and induce several diseases, including urinary tract infections, bacteremia, septic shock, and neonatal meningitis. Although no specifically pathogenic virulence factors are associated with ExPEC, several pathotypes have been described: uropathogenic E. coli (UPEC), neonatal meningitis E. coli (NMEC), avian pathogenic E. coli (APEC), and sepsis-associated E. coli (SEPEC) [4].
Biofilms play a significant role in human health during infections. As reported by the National Institutes of Health, approximately 80% of human infections are associated with biofilms and are involved in most chronic infections [5–7]. Biofilm-related diseases increase the morbidity and mortality rates of patients and cause significant economic burdens such as substantial healthcare costs and extended patient hospitalization [6]. Microorganisms can colonize a wide range of medical devices, including intravenous and urinary catheters, orthopedic prostheses, and endotracheal tubes. Furthermore, biofilms present on contaminated surfaces in hospital environments can serve as a source of infection via the periodic release of planktonic bacterial cells into the environment [5, 7].
Growth in organized bacterial communities constitutes a paradigm shift in the pathogenesis of infectious diseases and reinforces the importance of new approaches for detecting and combating biofilm-associated infections. Limited information is available on biofilm formation by E. coli pathotypes and the role of biofilm-associated components in this process. Moreover, previous studies have emphasized that the outcomes derived from nonpathogenic strains may not accurately represent the biological processes that occur in all isolates [8, 9]. In order to deepen the knowledge about the pathogenesis of E. coli infections, the objective of the study was to assess the biofilm production capacity and the expression of four virulence factors of clinical isolates of E. coli that produce enteric and urinary infections of patients treated in hospitals in the province of Buenos Aires, Argentina.
2. Materials and Methods
2.1. Bacterial Isolation and Identification
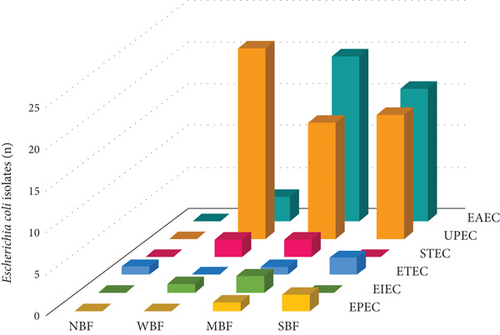
This was an observational, descriptive, retrospective, and cross-sectional study. The study group consisted of 105 E. coli isolates, 53 were DEC and 52 were UPEC. The DEC isolates were recovered from feces of patients with diarrhea. Pathotypes were identified by molecular amplification of eight characteristic virulence gene fragments using two multiplex PCRs (mPCR1 and mPCR2). The genes investigated were those encoding intimin (eae), heat-labile toxin LT (lt), heat-stable toxins STP (stp) and STH (sth), invasion plasmid antigen H (ipaH), transcriptional activator R (aggR), and Shiga 1 (stx1) and Shiga 2 (stx2) toxins. Molecular detection was performed by using a two-stage diagnostic method as previously described [10, 11]. Briefly, the first stage consisted of performing two multiplex PCRs with DNA from confluent cultures. If amplification of any gene was observed, the second stage consisted of identifying positive colonies (with virulence genes). For this purpose, DNA from 10 to 20 isolated colonies was used as a template for individual PCRs. The colonies that presented amplification were identified using phenotypic methods (VITEK2, bioMerieux) to confirm the bacterial species [10, 11]. DEC pathotypes included EAEC (n = 39), ETEC (n = 4), STEC (n = 4), EPEC (n = 3), and EIEC (n = 3). UPEC isolates were obtained from urine samples of patients with urinary tract infections. The isolates were identified by automatic phenotypic methods (VITEK2, bioMerieux). All isolates were recovered from patients treated in public hospitals from Buenos Aires, Argentina, and stored in the cryobank using an alphanumeric code and permanently dissociated from patient information.
2.2. Biofilm Formation Capacity
To estimate the amount of biofilm produced by each isolate, the experiments were carried out in sterile flat-bottomed 96-well polystyrene microtiter plates incubated at 37°C using Luria–Bertani (LB) broth. Each well was inoculated with a 12-h LB liquid cultures previously adjusted to an OD600 of 0.2 (Beckman DU-640 Spectrophotometer, United States). Cultures were incubated 4 h statically to allow bacterial adhesion and further incubated for 18 h for biofilm production. After washing the plates with sterile phosphate-buffered saline (PBS) to remove the planktonic cells, sessile cells were dried 60°C for 20 min and stained for 10 min with 200 μL of 0.1% crystal violet solution (Certistain, Merck Química Argentina S.A.I.C). The stain absorbed by the biofilms was dissolved adding 250 μL of 96° ethanol for 15 min. The supernatants were transferred to another plate, and the optical density (OD) was measured at 590 nm using a microtiter-plate reader (Benchmark Plus, Bio-Rad Laboratories Inc.). Each assay was performed in triplicate in two independent replicates. E. coli ATCC 25922 strain was used as a positive control of the assay. For the negative control, 12 wells per plate were inoculated with 200 μL of LB broth. The biofilm formation capacity for each isolate was estimated based on Stepanović et al.’s [12]. For this purpose, we determined the cut-off optical density (ODc) to be three SDs above the mean OD of the negative control. The tested isolates were classified into each of the following four groups: nonadherent strain or nonbiofilm former (NBP) (OD ≤ ODc); weak biofilm former (WBF) (ODc < OD ≤ 2 ODc); moderate biofilm former (MBF) (2 ODc < OD ≤ 4 ODc); and strong biofilm former (SBF) (OD > 4 ODc) [13].
2.3. Phenotypic Assessment of Virulence Factors
2.3.1. Curli Fimbriae and Cellulose
The expression of curli fimbriae and cellulose production was evidenced by culture on Congo Red Agar according to Serra, Richter, and Hengge [14]. Macroscopic appearance and pigmentation of the colonies were used to classify the isolates into morphotypes according to the expression of curli fimbriae and/or cellulose: Red, dry, and rough (RDAR) morphotype corresponds to isolates that express both curli and cellulose; brown, dry, and rough (BDAR) morphotype expresses only curli; and smooth and white (SAW) morphotype demonstrates that neither curli nor cellulose is detected [15].
2.3.2. Hemagglutinins and Hemolysins
The expression of hemagglutinins was revealed by agglutination of erythrocytes. Briefly, the bacterial isolates were incubated in LB broth at 37°C for 48 h. Equal volumes of each bacterial suspension were mixed with human erythrocytes on slides. Red blood cells were the zero-negative blood group, collected with EDTAK3, washed with physiological solution, and suspended (3%). Hemagglutination was detected by observing agglomerated erythrocytes in the presence of bacteria [16].
The expression of hemolysins was detected in blood agar. For each bacterial isolate, 10-μL aliquots of a 12-h LB liquid culture previously adjusted to an OD600 of 0.1 were inoculated onto agar supplemented with sheep blood (Britania, Argentina) and incubated at 37°C for 24 h. Hemolysis was identified by the presence of a clear zone around the colony [17].
2.4. Statistical Analysis of Data
The statistical analyses were descriptive techniques using summary measures, such as means and percentages (GraphPad Prism v8). Biofilm data were analyzed using Kruskal–Wallis ANOVA. The proportional distributions of the independent variables were compared using the Wald test. Statistical significance was set at p ≤ 0.05.
3. Results
3.1. Biofilm Formation Capacity in E. coli Pathotypes
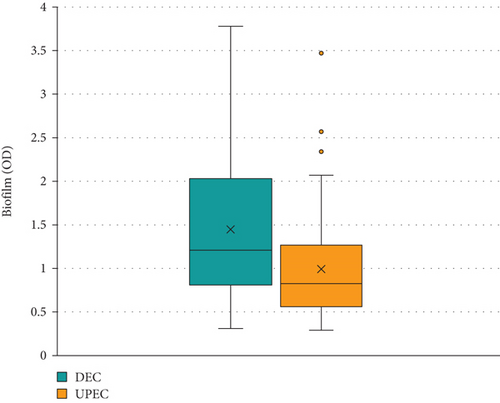
All the isolates, except one, were able to produce biofilm under our experimental conditions. The average of the amount of biofilm produced by DEC and UPEC isolates estimated by OD was 1.45 ± 0.81 and 0.99 ± 0.64, respectively. Significant differences were observed between both groups (p = 0.002) (Figure 1). The average amount of biofilm (OD) produced by different pathotypes of E. coli varied between 0.725 and 1.93, while E. coli ATCC 25922 presented the lowest value (0.485). The amount of biofilm produced by DEC and UPEC isolates was significant in SBF category (0.031) (Table 1).
| Categories | IC (95%) | p | |||
|---|---|---|---|---|---|
| DEC | UPEC | DEC | UPEC | ||
| Nonbiofilm former (NBF) | 0.31 | — | — | — | — |
| Weak biofilm former (WBF) | 0.55 ± 0.16 | 0.52 ± 0.13 | [0.38; 0.71] | [0.46; 0.58] | 0.666 |
| Moderate biofilm former (MBF) | 1.09 ± 0.43 | 0.94 ± 0.20 | [0.91; 1.27] | [0.82; 1.07] | 0.263 |
| Strong biofilm former (SBF) | 2.24 ± 0.59 | 1.74 ± 0.67 | [1.96; 2.52] | [1.38; 2.13] | 0.031 |
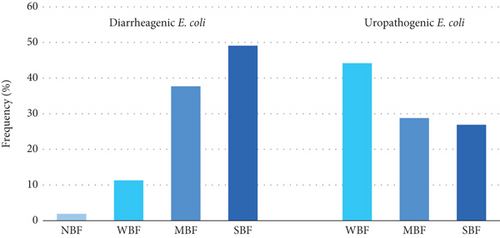
The MBF category was prevalent, representing 38.11% (n = 40) of all isolates. The SBF category was second in frequency (33.33%, n = 35), and the WBF ranked third (27.61%, n = 29). A single isolate was NBF (0.95%, n = 1) (Figures 2 and 3).
3.2. Expression of Virulence Factors in E. coli Pathotypes
Only 46 (43.8%) isolates showed the RDAR morphotype (expression of both curli and cellulose), whereas the BDAR morphotype (expression of curli) was found in 53 (50.4%) isolates, and the SAW morphotype (absence of both components) in six (5.7%) isolates (Figure 4). The most highly expressed virulence factor was curli fimbriae (94.3%; n = 99). Other factors, such as cellulose (43.8%; n = 46), hemolysins (35.2%; n = 37), and hemagglutinins (33.3%; n = 35), were expressed at lower frequencies. The number of cellulose-producing DEC isolates is significantly higher than the number of cellulose-producing UPEC isolates (p < 0.01) (Table 2).

| Virulence factor | Diarrheagenic E. coli | Uropathogenic E. coli | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Curli fimbriae | 51 | 96.2 | 48 | 92.3 | 0.290 |
| Cellulose | 33 | 62.3 | 13 | 25.0 | < 0.01 |
| Hemagglutinins | 19 | 35.8 | 16 | 30.8 | 0.194 |
| Hemolysins | 16 | 30.2 | 21 | 40.4 | 0.137 |
4. Discussion
Pathogenic E. coli infections remain a major global public health issue worldwide [1–4]. To ascertain the correlations of genotypic and phenotypic characteristics and pathotype with biofilm formation, we investigated 105 clinical E. coli isolates (EAEC, EPEC, ETEC, EIEC, STEC, and UPEC) from feces and urine of patients treated in public hospitals from Buenos Aires, Argentina. In addition to the evaluation of biofilm-forming capability, isolates were examined for the presence of virulence factors such as curli fimbriae, cellulose, hemagglutinins, and hemolysins.
Biofilm formation capacity was detected in 99% of the E. coli clinical isolates analyzed. In line with our results, several studies have reported biofilm production of 92%–100% of human clinical isolates [13, 18–20]. Similarly, Rodrigues da Silva et al. [21] reported 100% biofilm production by E. coli recovered from environmental water samples. Other studies have reported lower percentages of biofilm formation capacity. For example, Hashemizadeh et al. [22] found values lower than 84% for UPEC and 6% for commensal E. coli. Other work realized by Maheswari et al. [16] analyzed UPEC recovered from hospitalized patients and observed that E. coli from urinary catheter-associated urinary tract infections (CAUTI) produced less biofilm than those recovered from patients with kidney stones. A lower frequency of biofilm production in UPEC isolated from hospitalized patients has also been reported [23, 24]. Although most isolates of E. coli formed biofilms, there was high variability between them. These differences could be due to the origin of isolation (feces and urine), virulence (commensal and pathogenic), underlying pathology (diarrhea, UTI, and CAUTI), and patient status (ambulatory and hospitalized), among other factors.
Biofilms significantly enhance the virulence of E. coli by providing a protective environment that promotes its survival and persistence under hostile conditions. The extracellular polymeric substance (EPS) matrix of biofilms acts as a barrier against environmental stressors, including antibiotics, and host immune responses, thereby increasing resistance and promoting chronic infections. To date, few studies have compared biofilm formation among different E. coli pathotypes. In this study, all the pathotypes tested produced biofilms. However, E. coli isolates retrieved from feces (DEC) showed a significantly higher capacity for biofilm formation than those recovered from urine (UPEC). Bacterial biofilms play an important role in the development of intestinal diseases by contributing to dysbiosis and inflammation and lead to diseases such as inflammatory bowel disease (IBD) and colorectal cancer. The increased production of biofilms by gut bacteria like DEC could be due to intrinsic or acquired bacterial virulence factors that allow it to survive in anatomical sites with different pH, availability of nutrients, osmolarity, composition of the microbiota, presence of antimicrobial substances, and humoral and cellular factors of the immune system [13, 25].
EIEC is a pathotype implicated in bacterial cell invasion. However, all EIEC isolates examined were biofilm formers (one WBF and two MBFs). This fact has been described previously by other authors. For example, Iqbal et al. [26] obtained an isolate of EIEC from a Colombian child with severe gastroenteritis with the ability to invade and produce biofilms (BF-EIEC). Moreover, this emerging pathogen was previously detected in outbreaks of Europe and Latin America [27].
In our study, bacterial isolates that produced moderate amounts of biofilms (MBF) were prevalent. These results are consistent with other previous studies. For example, David et al. [28] examined DEC isolates from children with diarrhea and reported that moderate biofilm-forming isolates were prevalent. Similar results were reported for UPEC isolates. For 1 year, Ponnusamy, Natarajan, and Sevanan [29] isolated 100 nonrepetitive strains of E. coli from the urine samples of UTI patients from tertiary care hospitals in India. The authors pointed out that moderate biofilm production is prevalent. This category was also predominant in three studies of UPEC isolated from patients with urinary tract infections and kidney disease [30–32]. However, several authors have reported differences. For example, two recent studies on UPEC have reported that the main biofilm category was strong [19, 33]. Unlike previous authors, Naziri et al. [20] studied 100 UPEC isolates from outpatients and inpatients and found that weak producers were the prevalent category.
The EAEC pathotype produced significantly more biofilm than UPEC in the SBF category. EAEC are well known for their ability to adhere to the intestinal mucosa and form biofilms. This difference could be related to virulence factors, as EAEC is associated with persistent diarrhea, the pathogenesis of which is based on aggregative adherence and biofilm formation [3]. Wakimoto et al. [34] studied approximately 1000 E. coli isolates from children with diarrhea and reported that EAEC isolates exhibited significantly stronger biofilm formation than non-EAEC isolates. Mohamed et al. [35] screened E. coli isolates from travelers in developing countries and confirmed that biofilm formation is a widespread phenomenon in the EAEC pathotype.
In addition to the evaluation of biofilm-forming capability, E. coli isolates were phenotypically analyzed for the presence of curli fimbriae, cellulose, hemagglutinins, and hemolysins. Curli fimbriae is a surface protein relevant for biofilm development by E. coli. This structure interacts specifically with host matrix proteins, such as fibronectin, laminin, and plasminogen, to initiate adherence and invasion of host cells, activation of the immune system, bacterial aggregation, and biofilm formation [1–3, 8]. In our study, the most frequently detected virulence factor was curli fimbriae (92%–96%). This result is similar to that reported by Milanov et al. [36]. These authors documented that curli fimbriae were found in all the E. coli isolates. In contrast, Schiebel et al. [13] reported curli expression lower than 60%. Similarly, Özdemir and Arslan [37] detected this adhesin in 50% of E. coli recovered from vegetables.
Cellulose is an architectural element in spatially structured E. coli biofilms [14]. In this study, the difference in cellulose expression between DEC (62.3%) and UPEC (25%) was significant. Similar results were reported by Sivaranjani et al. [38] in E. coli recovered from birds. The intestine is an anatomical site with different availabilities of nutrients, presence of microbiota, and effectors of the immune system. Higher cellulose production by DEC strains could improve intestinal colonization and allow bacterial survival in this environment. DEC can be transmitted to human through consumption of contaminated food, including vegetables. Özdemir and Arslan [37] examined DEC survival in fresh vegetables and noted that 70% of isolates were capable of producing cellulose. In other study, the authors [39] screened cellulose production in Salmonella enterica serotype Enteritidis, an intestinal pathogen, and concluded that cellulose plays a critical role in biofilm development and resistance to chlorinated disinfectants in abiotic surfaces.
The relationship between toxins and biofilm formation is multifaceted and plays a crucial role in the pathogenicity of E. coli. Toxins can cause damage to host tissues and disrupt normal physiological processes, while biofilms help bacteria adhere and resist clearance mechanisms. Moreover, toxins can further modulate this immune response, allowing bacteria within biofilms to persist in the gastrointestinal and urinary tracts. Approximately one-third of the isolates produced hemolysin (35.2%) and hemagglutinins (33.3%). These results are like those reported in several studies [24, 40]. In contrast, other researchers have reported different values [19, 23, 41, 42]. This difference between expressions of virulence factors could be due to multiple aspects, such as the virulence of the isolate or the clinical characteristics of the patient (age, site of infection, comorbidities, consumption of antimicrobials, and recurrence of the infectious disease). According to this, a recent study reported different frequencies of hemolysis between pathogenic and commensal E. coli isolates [24]. Similarly, Maheswari et al. [16] examined UPEC isolates from two populations, patients with CAUTI and lithiasis, and reported significant differences in the frequency of hemolysin detection between both groups of patients.
Several studies have explored the influence of cell surface components on biofilm formation in nonpathogenic E. coli K-12 strain [43–45]. Pratt and Kolter [46] demonstrated that motility mediated by flagella and Type 1 fimbriae contributed to the formation of biofilms in the reference E. coli K-12 strain. This finding was subsequently corroborated by Wood et al. [47], who established a direct correlation between E. coli K-12 motility and its capacity to form biofilms. However, another study revealed that the presence of mannoside and absence of motility did not hinder the ability of an EAEC strain to form biofilms. This observation highlights that the results obtained from nonpathogenic strains may not accurately reflect the biological processes that occur in all E. coli isolates. So far, most of the studies on biofilm and virulence factors expression have been focused on nonpathogenic and reference E. coli strains. Thus, there is quite limited information available on the capacity of biofilm formation by clinical isolates belonging to different E. coli pathotypes and information regarding the role of biofilm-associated factors.
The results of this study constitute a relevant contribution to medicine as they provide new relevant information on the production of biofilms in pathogenic E. coli. In this investigation, only biofilms on abiotic polystyrene surfaces and static cultures were examined, which may not accurately represent the properties of biofilms in the human body.
Biofilm formation and regulation exhibit remarkable variability, although distinct regulatory patterns of biofilm formation emerge in certain pathovars and clones. Environmental conditions, host-derived signals, and commensal microbiota within the gastrointestinal tract contribute to shaping E. coli biofilm formation within multicellular communities in a complex and integrated manner. Although several major regulatory networks, adhesion factors, and extracellular matrix components constituting E. coli biofilms have been identified, these processes have been primarily characterised in vitro. However, the vast number of genes encoding surface appendages in the E. coli genome suggests that the complexity of the biofilm process is far from being fully elucidated.
5. Conclusions
Growth in organized bacterial communities constitutes a paradigm shift in the pathogenesis of infectious diseases and reinforces the importance of new approaches for detecting and combating biofilm-associated infections. Limited information is available on biofilm formation by E. coli pathotypes and the roles of virulence factors in this process. Moreover, previous research has emphasized that the outcomes derived from nonpathogenic strains may not accurately represent the biological processes that occur in all isolates. This study showed that the capacity of biofilm formation was detected in 99.05% of isolates. All E. coli pathotypes were capable of producing biofilms, and among 105 clinical isolates tested, the MBF category was prevalent. Interestingly, a difference was evident between the DEC and UPEC isolates in the SBF category (p = 0.031). The virulence factor with higher frequency was curli fimbriae (94.3%), whereas cellulose (43.8%), hemolysins (35.2%), and hemagglutinins (33.3%) were detected at lower frequencies. The difference in cellulose expression between DEC (62.3%) and UPEC (25%) was significant (p < 0.01). Biofilm formation is a key virulence factor for several microorganisms that cause chronic infections. The multifactorial nature of biofilm development and tolerance to antimicrobials and disinfectants poses major challenges for the application of conventional treatments and indicates the need for new approaches to understanding the pathogenesis and persistence of biofilm-related infections.
Ethics Statement
This article did not contain any studies with human participants or animals performed by any of the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by grants from Universidad Nacional de La Plata (PPID/M011).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




