Eco-Friendly Synthesis of Flower-Wrinkle Silica Nanoparticles From Rice Husk
Abstract
In this study, silica nanoparticles (SiNPs) with a flower-like wrinkled morphology were synthesized via a green method using rice husk (RH) as a sustainable silica precursor. The synthesis was performed without hazardous chemicals, highlighting the environmental compatibility and cost-effectiveness of the process. The structural and physicochemical properties of the nanoparticles were characterized using FTIR, XRD, scanning electron microscopy (SEM), dynamic light scattering (DLS), energy-dispersive X-ray spectroscopy (EDX), UV–vis, thermogravimetric analysis (TGA), and Differential scanning calorimetry (DSC) analyses. FTIR confirmed the presence of Si─O─Si and Si─OH groups, while XRD revealed that the synthesized particles exhibit a crystalline quartz structure rather than the amorphous form commonly obtained from RH. SEM images showed petal-shaped particles with hierarchical morphology. Thermal analysis indicated high stability up to 800°C. These findings suggest that the developed green synthesis method can yield structurally defined SiNPs suitable for further application in catalysis, adsorption, and nanomaterials development.
Summary
- •
Synthesizing SiO2 nanoparticles through eco-friendly and sustainable pathway.
- •
Preparing SiO2 nanoparticles with high surface area and low toxicity.
- •
Unique flower wrinkle-like structure of synthesized SiO2 from rice husk.
1. Introduction
Silicon dioxide (SiO2), commonly referred to as silica, is a fundamental material in nanotechnology, materials science, and biomedical fields due to its exceptional physicochemical stability, thermal resistance, high surface area, and biocompatibility [1]. Silica nanoparticles (SiNPs) have been widely utilized in applications, such as catalysis, drug delivery [2], sensors, adsorption systems [3], and composite materials. Their nanoscale size, controllable morphology, and ease of surface functionalization make them versatile platforms for both industrial and environmental technologies [3, 4]. Traditionally, SiNPs are synthesized via chemical routes involving silicon (Si) alkoxides like tetraethyl orthosilicate (TEOS) or sodium silicate under acidic or basic conditions [5, 6]. While these methods provide relatively good control over particle morphology and size distribution, they are often associated with significant drawbacks. The use of expensive precursors, high-temperature processing, toxic solvents, and complex purification steps contributes to environmental hazards, high energy consumption, and production costs [7].
In recent years, green synthesis has gained considerable attention as a safer and more sustainable alternative to conventional chemical and physical approaches for nanoparticle production [8–11]. Traditional chemical methods often involve toxic reducing agents and costly precursors, raising concerns about environmental safety and economic feasibility [12, 13]. In contrast, green synthesis utilizes biological resources, such as plant extracts, fungi, bacteria, and algae, offering a low-cost, one-pot strategy for nanoparticle fabrication [14, 15].
Nanoparticles synthesized through green routes not only demonstrate excellent biocompatibility and bioactivity, but also retain surface functionalities derived from natural reducing agents, making them highly effective across a range of applications [16]. For instance, green-synthesized nanoparticles have shown significant potential as nanocatalysts in chemical reactions due to their enhanced surface area and stability [17, 18]. In the biomedical field, they are widely investigated for antiparasitic activity, targeted drug delivery, and therapeutic applications, owing to their low cytotoxicity and functional versatility [19, 20]. Moreover, their use in solar cells contributes to improved energy conversion efficiency, while in photocatalysis, they assist in breaking down organic pollutants and enabling environmental remediation [21–23]. These diverse applications highlight the value of green-synthesized nanoparticles as multifunctional materials in both industrial and clinical settings [24, 25]. Among these, agricultural byproducts have emerged as promising alternatives to traditional chemical precursors. Rice husk (RH) is a low-cost and abundant agro-industrial byproduct that, despite its partial use in low-value applications, such as fuel or bedding, is still significantly underutilized in terms of its potential for producing high value nanomaterials like SiNPs [26]. In previous studies, researchers have demonstrated the feasibility of extracting silica from RH; however, these efforts have typically yielded amorphous, shapeless silica particles. Although the use of RH represents an important step toward sustainability, the lack of morphological control in these processes has restricted the application potential of the resulting material, especially in advanced fields where particle geometry critically influences performance [27–33]. Several studies have demonstrated the successful extraction of amorphous silica from RH through controlled thermal treatment. For instance, RH-derived SiNPs obtained at 700°C have been used as an environmentally friendly additive in mortar production [34]. Nguyen et al. extracted lignocellulose and silica from RH using a two-step alkaline-peroxide treatment [35]. Additionally, RH-derived SiNPs have been applied in dye adsorption processes, particularly for removing Safranin dye from aqueous solutions [36]. The structure and properties of the synthesized SiNPs, such as surface area, pore volume, and morphology, depend significantly on the calcination temperature, which typically ranges from 300 to 900°C [34, 37–41]. These factors are crucial in tailoring SiNPs for specific applications, including drug delivery, catalysis, and polymer reinforcement [42, 43]. Although these studies confirm the feasibility of silica extraction from RH, the resulting particles are predominantly amorphous and lack defined morphology. As far as our literature review suggests, no prior research has reported the synthesis of SiNPs with well-defined surface architectures, such as wrinkled or hierarchical structures, directly from RH [31, 44–50]. This absence of morphological control significantly limits the functional potential of the obtained materials, particularly in advanced applications where particle geometry plays a key role in surface interaction, catalytic activity, drug release behavior, and optical performance. Therefore, developing a green synthesis route capable of producing structurally tailored SiNPs from RH remains a critical challenge and a promising opportunity.
Given the critical influence of particle morphology on surface interactions, catalytic efficiency, and drug delivery performance, there is a growing demand for SiNPs with controlled and well-defined architectures. While most existing studies using RH as a silica source have yielded amorphous or irregularly shaped particles, limited attention has been paid to the development of structured morphologies through green synthesis. Therefore, this study aims to develop an eco-friendly and cost-effective method for synthesizing SiNPs with distinct hierarchical morphology using RH as a sustainable precursor. By optimizing synthesis conditions, such as pH, surfactant concentration, and reaction time, this work seeks to explore morphology-controlled production of silica suitable for advanced applications.
2. Experimental
2.1. Chemicals
RH was achieved from a local store. Sodium hydroxide, hydrochloric acid (HCl, 37%), nitric acid of analytical grade, cetyltrimethylammonium bromide (CTAB) and sulfuric acid were all obtained from Merck Company (Germany). Deionized water with electrical conductivity of 0.5 µS was used for the preparation of all the aqueous solutions.
2.2. Characterizations
Scanning electron microscopy (SEM) images and EDS analysis were recorded with a scanning electron microscope (Leo 1450VP, Germany). The average particle size, size distribution, and polydispersity index (PDI) of the synthesized SiNPs were all investigated via a particle size analyzer (Vasco, Cordouan Technologies, France). The FTIR spectrum of the synthesized SiNPs was obtained using an FTIR spectrometer (Nicolet AVATAR 370, USA) in the 4000–400 cm−1 region. UV−vis absorption spectrum was recorded using a UV–vis spectrophotometer (Nano Ar2015, Iran) with a 1 cm pathlength quartz cuvette. Also, by DSC thermograms at a rate of 10°C/min under inert flow gas (nitrogen) in the temperature range of 25–350°C (rate of 10°C/min) by Perkin Elmer DSC Q100 instrument. Thermogravimetric analysis (TGA) was performed with a TGA-Mettler device in the temperature range of 25–800°C with a scan rate of 10°C/min and under an inert gas of nitrogen.
2.3. Synthesis of Silica Nanoparticles With Flower-Wrinkle Structure
Raw RHs (10 g) were first washed thoroughly with deionized water to remove dust and surface impurities and then dried in a vacuum oven at room temperature (24 h) to eliminate residual moisture. The dried husk was then subjected to a two-step thermal treatment in a furnace: initially at 300°C for 2 h followed by 500°C for 8 h, see Figure 1. This controlled pyrolysis removed organic and carbonaceous matter while preserving the structural integrity of the silica. The resulting white ash was then calcined (700°C for 1 h) to obtain high purity amorphous silica powder, which served as the precursor. Subsequently, of the silica precursor (2 g) was dissolved in NaOH (100 mL of 2 M) and refluxed at 80°C (24 h) under continuous magnetic stirring, yielding a yellowish sodium silicate solution. The mixture was then filtered, and the pH was adjusted to 10 using acetic acid (2 M) to facilitate controlled gelation.

The resulting sodium silicate solution was slowly added dropwise to 10 mL of 10% (w/v) CTAB solution under continuous stirring. The reaction mixture was stirred (25°C for 24 h) in the dark condition to promote the self-assembly of silica around CTAB micelles, forming the desired radial wrinkled morphology. The final product was collected by filtration and air-dried at room temperature. Lyophilization was not performed. The precipitated product was then collected and dried in a hot vacuum oven (100°C for 5 h) to obtain the final SiNPs in powder form. The dried samples were stored in airtight containers for subsequent characterization (SEM, XRD, FTIR).
3. Results and Discussion
3.1. UV-vis Spectroscopy Analysis
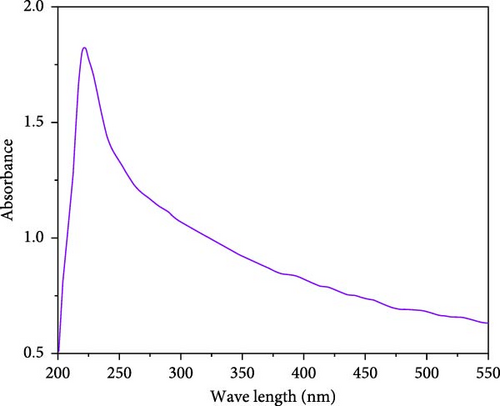
The optical properties of the synthesized SiNPs were investigated using UV–vis spectroscopy. As shown in Figure 2, a well-defined absorption peak appears at ~220 nm, which is consistent with previous studies reporting UV absorption bands for nanoscale silica structures [51–54]. The exact position and intensity of the UV absorption peak in SiO2 materials can vary depending on particle size, morphology, degree of aggregation, and synthesis conditions [54, 55]. In this study, the observed absorption band at 220 nm supports the successful formation of silica from RH under green synthesis conditions. Although UV–vis spectroscopy is not a direct method for confirming particle size, the sharpness and position of the absorption band align with characteristics typically observed in nanostructured silica materials.
3.2. FTIR Analysis
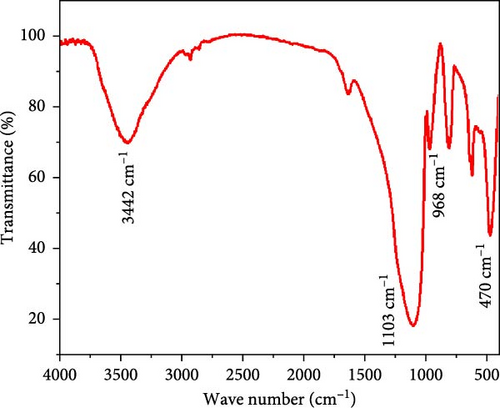
To identify the functional groups on the surface of the obtained silica nanoparticle, FTIR analysis in the range of 4000–400 cm−1 was carried out (see Figure 3). Two evident bands at 1103 and 470 cm−1 assigned to the Si─O─Si stretching bond [56–59]. Also, a characteristic band at 968 cm−1 corresponds to the Si─OH bond of silanol groups, which indicates a large amount of silicate exhibited in the synthesized nanoparticles. A broad absorption band centered at approximately 3440 cm⁻1 is attributed to the O─H stretching vibrations of surface silanol (Si─OH) groups and physiosorbed water molecules. These features are commonly observed in SiNPs and indicate the presence of hydrogen bonding on the particle surface [60, 61].
3.3. Particle Size Analysis
The particle size and PDI of the synthesized SiNPs were determined using dynamic light scattering (DLS) analysis (Figure 4). The results indicated that the nanoparticles exhibited a hydrodynamic diameter of approximately 163 nm with a PDI value of 0.31. This PDI reflects a relatively narrow size distribution, suggesting moderate uniformity among the particle population [62, 63].
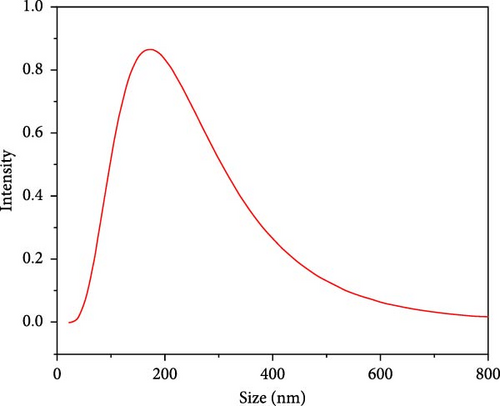
It is important to note that DLS measures the hydrodynamic diameter, which encompasses not only the solid particle core but also any surrounding solvation layer or surface-bound molecules. Therefore, the reported values may be slightly larger than the physical core size of the particles. Nonetheless, the DLS results confirm successful formation of nanoscale particles with consistent dispersion quality, which is essential for various applications, such as drug delivery, adsorption, and catalysis [64].
3.4. XRD Analysis
As reported in previous [41, 65, 66], increasing the annealing temperature beyond 800°C leads to a reduction in crystal size and results in the formation of amorphous silica particles [66]. In the present study, to achieve silica particles with a regular crystalline structure [67, 68], the calcination temperature was carefully controlled between 500 and 700°C [41]. It is well-established that SiNPs or microparticles can exhibit sharp XRD peaks under such conditions [68, 69]. The sharp peaks observed in Figure 5 suggest that the synthesized particles fall within the nano- or microscale range, which will be further confirmed in the SEM analysis.
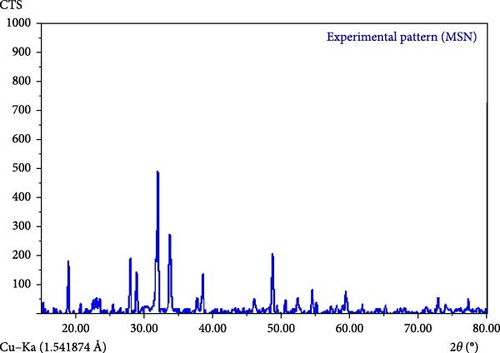
Moreover, according to qualitative XRD assessments (2θ range of 10°–80° at 20 mA) in the [67, 70], crystalline forms of silica, such as quartz, cristobalite, and tridymite, typically display three characteristic peaks: primary, secondary, and tertiary [70]. The peaks observed in Figure 5 closely match those of quartz, with slight shifts, and align with the standard JCPDS card number 46-1045 [68, 70]. Therefore, based on the XRD data, the lattice structure of the synthesized silica particles corresponds to that of crystalline quartz.
3.5. Morphological and Elemental Analysis
The surface morphology and elemental composition of the synthesized silica particles were characterized using SEM and energy-dispersive X-ray spectroscopy (EDX). As shown in the SEM images (Figure 6a,b), the particles display a three-dimensional flower-like morphology, consisting of radially arranged wrinkled structures that resemble petal-like formations. This hierarchical architecture is indicative of self-assembly during the synthesis process and contributes to the high specific surface area of the material.

The observed morphology suggests that the silica particles form dense and aggregated clusters rather than isolated spheres, making it difficult to define distinct boundaries for individual nanoparticles. Therefore, quantitative size analysis could not be accurately performed based on SEM images alone. Instead, the SEM evaluation highlights the textural complexity and surface roughness of the synthesized material, which are key attributes for applications, such as catalysis, adsorption, and drug delivery.
Additionally, the observed morphological order is in good agreement with the crystallinity confirmed by the XRD analysis. The diffraction pattern shows sharp peaks consistent with quartz-phase SiO2 (JCPDS card number 46-1045), further supporting the formation of a highly ordered silica structure.
The EDX spectrum (Figure 7) confirms that the particles are primarily composed of silicon (Si) and oxygen (O), consistent with the formation of SiO2. No significant signals from other elements were detected, indicating the purity of the synthesized material.

3.6. Differential Scanning Calorimetry (DSC)
DSC was performed to analyze the thermal behavior of the synthesized SiNPs in the temperature range of 25–400°C (Figure 8). The powder sample used for this analysis was dried in a vacuum oven (100°C for 5 h) following synthesis to remove residual moisture.
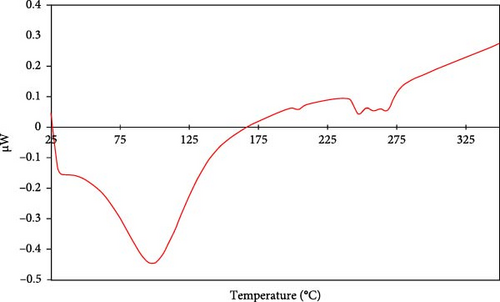
The DSC thermogram exhibits a broad endothermic peak centered around 99°C, which is attributed to the evaporation of physically adsorbed water molecules from the surface of the silica particles [71, 72]. This observation is consistent with the first stage of weight loss seen in the TGA analysis and is typical for silica materials that retain ambient moisture [15, 72].
No sharp or significant thermal events corresponding to crystallization or fusion were detected in the analyzed temperature range, which aligns with the expected thermal behavior of amorphous or partially crystalline silica. The small, broad fluctuation observed between 247 and 267°C may be related to the gradual desorption or condensation of surface hydroxyl (silanol) groups [15]. However, no conclusive thermal transitions indicative of phase changes was recorded, and no melting point was observed, as silica is known to have a melting point above 1600°C [67, 73].
These results confirm that the synthesized SiNPs exhibit typical thermal stability and moisture release characteristics of unmodified SiO2 particles, and that no structural transitions occur within the measured range.
3.7. TGA
TGA was conducted to investigate the thermal stability of the SiNPs over a temperature range of 25–800 °C under nitrogen atmosphere (Figure 9). The TGA curve reveals two major stages of weight loss. For a powder sample, the first stage occurs between 25 and 120°C and shows ~5% mass reduction, attributed to the evaporation of physiosorbed water molecules [15, 72].
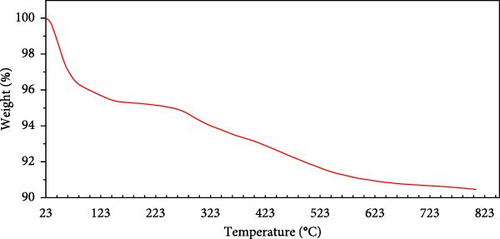
The second stage, between 120 and 400°C, presents an additional weight loss of approximately 5%, which is mainly due to the thermal decomposition of surface hydroxyl groups (Si─OH) [71]. Above 400°C, no significant mass loss was observed, indicating excellent thermal stability of the synthesized SiNPs up to 800°C. These findings are consistent with previous studies on unmodified SiNPs [72].
4. Conclusion
This study presents a comparatively eco-friendly approach for synthesizing SiNPs with a unique, flower-like, wrinkled structure from RH. The process adheres to several principles of green chemistry by using an agricultural waste-derived precursor, nontoxic aqueous conditions, and avoiding hazardous solvents or reagents commonly used in conventional silica synthesis. Unlike traditional studies that yield mostly amorphous or undefined silica particles, our method enabled the formation of highly crystalline, flower-like, wrinkled silica structures, confirmed by XRD, SEM, and other characterization techniques. This hierarchical architecture—rarely reported from RH-based synthesis—offers superior surface area and structural complexity, which are key for enhancing performance in catalysis, drug delivery, adsorption, and sensing. The process eliminates the need for toxic chemicals and harsh conditions, making it fully compatible with green chemistry principles. Thermal analysis demonstrated excellent thermal stability of the synthesized material, with more than 90% weight retention up to 800°C. Moreover, the particles exhibit a hydrodynamic diameter of around 163 nm with good dispersion characteristics, indicating suitability for nanotechnological applications. This study not only contributes a sustainable solution to silica nanoparticle production but also opens a new direction in the morphology-controlled green synthesis of nanomaterials using agricultural waste. The successful fabrication of a structured, crystalline silica from a low-value biomass source marks a significant advancement toward both environmental responsibility and functional material innovation. Future studies may focus on expanding this approach for large-scale production and exploring the morphology–property relationships in more depth. Ultimately, the synthesis strategy described herein represents a significant advancement in materials science. It demonstrates that by integrating the principles of green chemistry with innovative process design, it is possible to achieve a level of morphological control that was previously attainable only through expensive and hazardous chemical methods.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




