The Impact of Niacin Administration on Plasma Lipids and Gene Expression in the Vervet Monkey Model (Chlorocebus aethiops)
Abstract
Investigations conducted in mice and humans have reported that the nature and the amount of lipids in plasma can predict the likelihood of cardiovascular disease (CVD) development. Although niacin has a history as treatment for dyslipidemia, only a handful of clinical trials have investigated its efficiency in the prevention of the morbidity and mortality associated with CVDs. Therefore, the purpose of this study was to assess the impact of a niacin formulation on gene expression and plasma lipids using 16 vervet monkeys (8 controls and 8 experimental). The control group was given a maintenance diet only, while the experimental group’s diet was supplemented with niacin (100 mg/kg) for a period of 3 months, followed by 4-week washout. The investigated plasma lipids were total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides. Gene expression of proprotein convertase subtilisin/kexin type 9 (PCSK9), cholesterol ester transfer protein (CETP), low-density lipoprotein receptor (LDLR), apolipoprotein B-100 (APOB-100) and sterol regulatory element–binding protein-2 (SREBP-2), which are involved in the reverse cholesterol transport (RCT) pathway, was also determined. Niacin administration resulted in statistically significant changes for total cholesterol and HDL-C, with the changes also significantly higher in females compared to males in the niacin-treated group. Furthermore, gene expression analysis revealed significant decrease in CETP during niacin treatment. The downregulation suggested that the vervet monkey model supports the HDL-C hypothesis. Future studies aimed at supporting these findings are directed towards exploring the epigenetic biomarkers influencing the RCT pathway to combat CVDs.
1. Introduction
There are several factors known to influence the development of cardiovascular diseases (CVDs); these include plasma lipid profile, blood pressure, obesity, and diabetes [1–4]. These risk factors are intermediate phenotypes, which are underpinned by inherent and environmental elements, such as nutrition, diet, hormones, smoking, alcohol intake, and physical inactivity [5–7]. Investigations conducted in mice and humans have revealed the nature and the amount of lipids in plasma to be a good predictor of the development of CVD [5–7]. High levels of total cholesterol and elevated low-density lipoprotein (LDL) levels have been found to have a positive and growing association with the development of ischemic heart disease [8]. Consequently, a significant number of clinical trials have validated the lipid theory of atherosclerosis, which consistently links reduced total cholesterol levels in plasma to a reduction in mortality from coronary heart disease (CHD), stroke, and CVDs [9–11].
The transport, maintenance, and elimination of plasma lipids including high- and low-density lipoprotein cholesterol (HDL-C and LDL-C) require a well-coordinated effort from a considerable number of protein molecules [8]. Hepatic uptake via the low-density lipoprotein receptor (LDLR) is one of the leading mechanisms employed for the removal of LDL particles from circulation. Another mechanism involves the gene that encodes proprotein convertase subtilisin/kexin type 9 (PCSK9), and this has become an exciting therapeutic target for the treatment of hypercholesterolemia [12]. It has been suggested that the core role of PCSK9 in humans is the posttranscriptional regulation of the number of cell surface LDLRs, thereby regulating the levels of plasma LDL-C [13, 14]. Current guidelines on CVD prevention and treatment recognize LDL-C lowering treatment as the primary therapy, with statins being the principal agents used and reported to significantly reduce residual cardiovascular risk [15]. Statins are also known to elevate PCSK9 levels thereby resulting in increased LDLR degradation and elevated LDL levels in plasma [16]. Although statins are currently the mainstay treatment to reduce LDL-C, many patients on statin therapy still present with atherogenic cholesterol levels higher than the recommended values [17]. Therefore, there is an increasing interest in developing more effective LDL-C-lowering drugs that might supplement statins. Niacin (nicotinic acid) is a lipid-altering drug that has been used to lower cholesterol and has been reported to favorably influence the plasma levels of HDL-C, LDL-C, and triglycerides [18].
Although niacin has a long history as treatment for dyslipidemia, only a handful of clinical trials have investigated its efficiency in the prevention of CVD-associated morbidity and mortality [15]. Despite being underused due to its unbearable side effects, niacin was for a long time second only to statins on the list of lipid-modulating agents [15] as the development of new formulations has reduced the severity of flushing symptoms by up to 20% [19, 20]. Niacin dosages between 1 and 3 g/day have been reported to lower the amounts of total plasma cholesterol, apolipoprotein B (APOB), triglycerides, very-low-density lipoprotein (VLDL), LDL, and lipoprotein (Lp) (a) while increasing HDL levels [21]. The impact of niacin on lipid metabolism was previously investigated by our research group using the vervet monkey as the model of choice [22]. The findings of this previous study were used as the anchor and reason to further study the genetic basis of niacin therapy in the same vervet colony. The major difference between these two studies is the determination of PCSK9 expression levels and the subsequent interactions with niacin. Since most of the clinical data that exists predates the statin era [15], there is limited literature on the association of niacin and PCSK9 studies. To date, this combination was reported to have a slight decrease in LDL-C levels by Khera et al. [23]. Therefore, the effect of niacin in reducing LDL-C levels through PCSK9 requires further investigation to bridge the gap that exists in literature.
The vervet monkey has long been viewed as an invaluable nonhuman primate (NHP) model for biomedical research, with some of the diseases that have been investigated relating to the brain, behavior, metabolism, and immunity [24]. This NHP species has been reported as an excellent model to study cholesterol metabolism, as it similarly responds to dietary cholesterol as humans [25]. Therefore, this study was aimed at investigating the effects of niacin administration on plasma lipids in the vervet monkey model and identifying any correlations between changes in plasma lipids and the expression of PCSK9, LDLR, SREBP-2, CETP, and APOB-100 genes implicated in the development of CVDs.
2. Materials and Methods
2.1. Animal Ethics
Studies conducted at the Primate Unit and Delft Animal Centre (PUDAC), the platform of the South African Medical Research Council (SAMRC), are designed according to the South African National Standard for the Care and Use of Animals for Scientific Purposes (South African Bureau of Standards, SANS 10386, 2008). The number of animals and the ethics clearance was obtained from the SAMRC Ethics Committee for Research on Animals (SAMRC/ECRA) (Ref: 11/18) as previously reported [26].
2.2. Group Selection and Compound Administration
Sixteen vervet monkeys (8 females and 8 males) were housed in similar housing conditions that have been reported by my research group [26, 27]. Animals were allowed a period of 2 weeks to acclimatize to the single cages, and baseline blood samples were taken thereafter. The monkeys were divided into two groups of eight animals consisting of a control and experimental (niacin) group matched according to their baseline lipid levels (Table 1). Following acclimatization, animals in the control group received a 30-g portion of the maintenance diet consisting of precooked maize meal, while animals in the experimental group received the same maintenance diet supplemented with sustained release (SR) of 100 mg/kg of niacin (Sigma, Missouri, United States) daily for the period of 3 months. The food bolus was voluntarily consumed by animals in the morning, and the rest of the food bolus (70 g) was offered only after the initial 30 g of food bolus was consumed in both groups. Food intake and wastage were monitored and recorded daily for both groups. Each animal was observed daily for general well-being and any signs of illness. Due to the known severe side effects of niacin such as skin flushing [28], special caution was given to the experimental group although the chosen dose (100 mg/kg) had been reported to produce no adverse effects [22, 29]. The clinical observation log sheet included behavior (alert, fearful, aggressive, confused, and depressed), motor function and activity (posture, coordination, locomotion, and active), and physical examination (coat, feces, urine, eyes, nose, ears, genitals, and rectal).
| Groups | Control | Experimental | p value |
|---|---|---|---|
| Sample size | 8 (4 M and 4 F) | 8 (4 M and 4 F) | — |
| Treatment | 30 g of maintenance diet | 30 g of maintenance diet and 100 mg/kg/day of niacin | — |
| Bodyweight | 4.70 ± 1.08 | 4.68 ± 0.98 | 0.97 |
| Cholesterol (mmol/L) | 3.26 ± 0.45 | 4.05 ± 0.80 | 0.07 |
| HDL-C (mmol/L) | 1.73 ± 0.22 | 1.90 ± 0.20 | 0.14 |
| LDL-C (mmol/L) | 1.18 ± 0.29 | 1.74 ± 0.59 | 0.05 |
| Triglycerides (mmol/L) | 0.76 ± 0.29 | 0.74 ± 0.28 | 0.88 |
| Non-HDL-C (mmol/L) | 1.75 ± 0.18 | 1.75 ± 0.90 | 0.20 |
| Cholesterol ratio | 1.96 ± 0.21 | 1.77 ± 0.41 | 0.82 |
- Abbreviations: F, female; M, male.
2.3. Blood Collection and Biochemistry Analysis
Blood sampling was conducted at baseline, end of the treatment, and after the 4-week washout period. Blood (2–4 mL) was collected into serum separator tubes (SSTs) and PAXgene Blood RNA Tubes (BRTs) via femoral venipuncture after putting the animals under sedation [26]. An analysis of the blood collected into SSTs for total cholesterol, LDL-C, HDL-C, and triglycerides was carried out by PathCare laboratories (Cape Town, South Africa). Body weight was also recorded at each blood sampling point, and all the animals were returned to the colony at the end of the washout period.
2.4. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
The PAXgene Blood RNA Kit (Qiagen, Venlo, Netherlands) was used to isolate and purify intracellular RNA from whole blood collected in the BRT according to our previously published procedure for RNA extraction, purification, and cDNA conversion [30, 31]. The RT2 qPCR primer assays (Qiagen, Venlo Netherlands), designed for SYBR Green-based RT-qPCR detection (Applied Biosystems, California, United States) were used for quantification of PCSK9 (PPQ16344A), CETP (PPH01386F), SREBP-2 (PPH00240F), APOB-100 (PPH02626E), and LDLR (PPQ00180A). The preparation of quantitative PCR (qPCR) standards and the analysis of the relative mRNA expression levels were performed according to the published protocol [26].
2.5. Statistical Analysis
In this study, the sample size was selected based on previous studies conducted in our unit and by other authors using the NHP model consisting of three to 16 animals [22, 32, 33]. The data for biochemistry (millimoles per liter) and relative mRNA expression (arbitrary units) for RT-qPCR were presented as means ± SD and analyzed using GraphPad Prism program version 6.01 (California, United States). Multiple comparison analysis was used to determine statistical significance from baseline to washout period and between the experimental and control groups. Statistical significance was calculated by using the Student t -test, and a value of p < 0.05 was considered statistically significant.
3. Results
3.1. General Observation and Biochemical Analysis
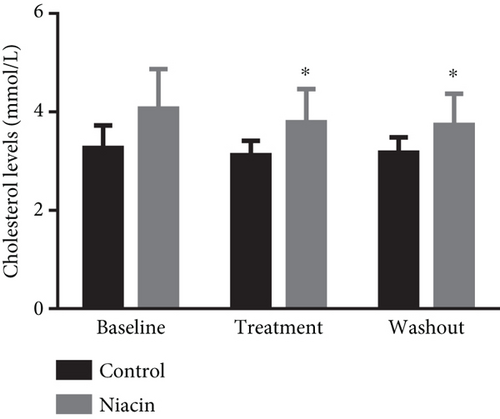
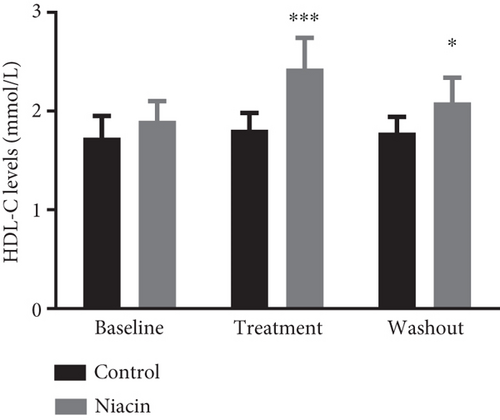
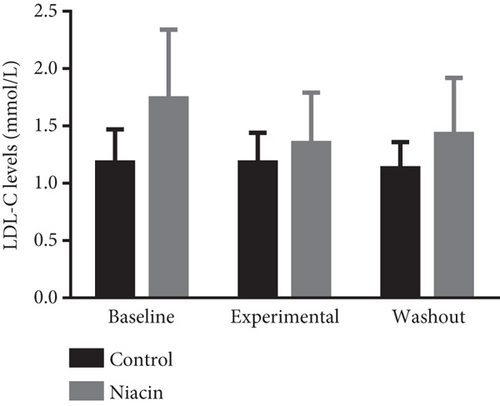
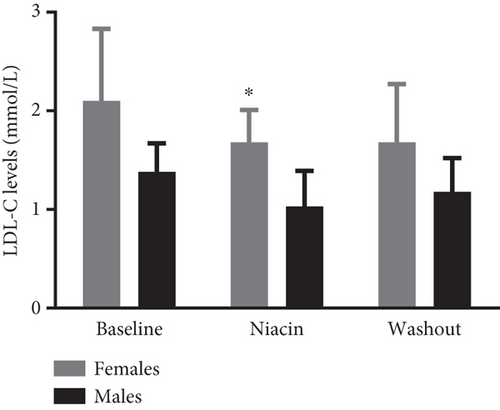
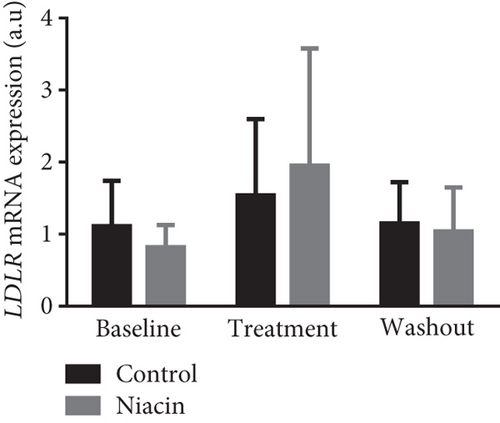
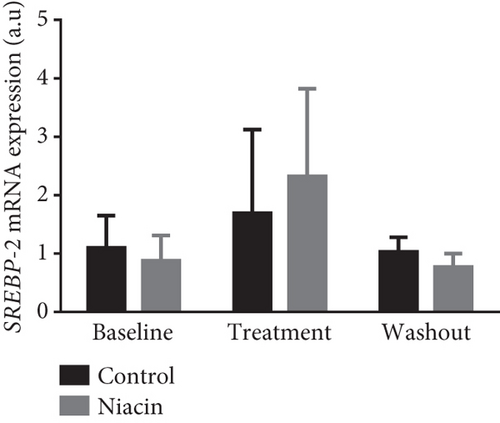
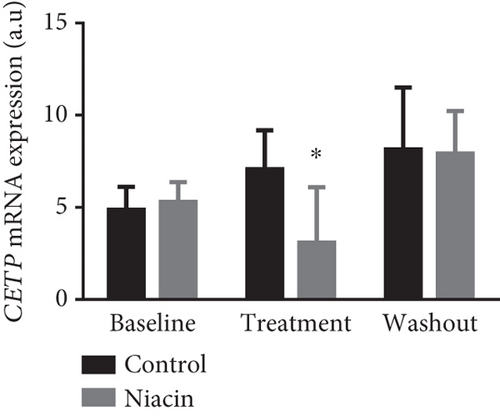
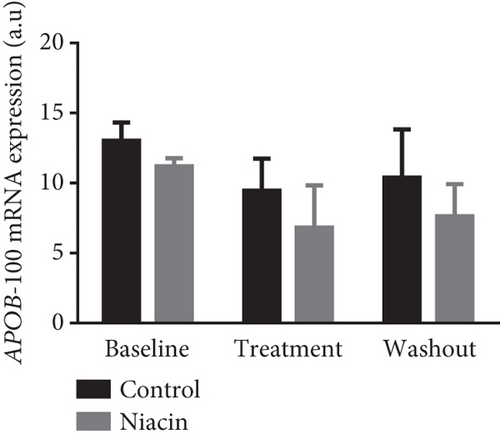
Food consumption in both groups was similar, with both groups consuming 100% of the food given. None of the treated animals showed any signs of illness during the treatment duration of 3 months. There was no statistical significant difference for body weight during the treatment (control: 4.83 ± 0.98 kg and niacin group: 4.78 ± 1.18 kg p value = 0.93, difference = −0.05). A decrease in total cholesterol (p value = 0.03, difference = −0.66) (Figure 1(a)) and an increase HDL-C levels (p value ≤ 0.0001, difference = 0.62) (Figure 1(b)) in the niacin group at the end of the treatment and washout periods were observed, which were statistically significant when compared to the control group. Total cholesterol and HDL-C levels remained relatively unchanged throughout the study period in the control animals. The increase in HDL-C levels in the experimental group however declined by 13.6% after withdrawal of niacin. Moreover, treatment with niacin resulted in 22.9% decrease in LDL-C (Figure 1(c)) and 33.9% triglyceride level decline, which were not statistically significant when compared to the control group. A significant decrease in LDL-C (p value = 0.04, difference = 0.65) was however observed between the males and females in the niacin group (Figure 2), with females having elevated levels. Furthermore, there were statistically significant changes in CETP gene expression between the control and experimental groups (p value = 0.01, difference = −3.99) (Figure 3(d)). The other two genes PCSK9 (p value = 0.44, difference = −0.46) and APOB-100 (p value = 0.15, difference = −2.64) had slight decreases from baseline to treatment period which were not statistically significant. Conversely, niacin slightly enhanced the expression of LDLR and SREBP-2, but the impact was reversed for all the genes after niacin withdrawal. Sex analysis for gene expression showed a difference between the vervet females and males; however, the change was not significant (Figure 2). The expression levels for PCSK9, LDLR, and APOB-100 was higher in males compared to females except for CETP which was high in females.

4. Discussion
In this study, the effect of niacin (100 mg/kg) on plasma lipids and gene expression was assessed in the vervet monkey model over a 3-month treatment period. There were no signs of ill health or discomfort observed in the monkeys for the duration of the study. The results indicated that niacin treatment induced biochemical changes in a manner similar to that reported in humans and other primate species [22, 34]. Vervet males weighed more than females, and this was expected as males are reported to have on average a bigger body size and more muscle fat mass ratio than females. Moreover, they are inclined to store additional amounts of intra-abdominal fat than females.
Niacin treatment resulted in a statistically significant reduction of total cholesterol which was reversed with the cessation of treatment (Figure 1(a)). Additionally, HDL-C levels were significantly increased at the end of the treatment period and discontinuing niacin treatment resulted in a significant decline in HDL-C levels at the end of the washout (Figure 1(b)). The significant increase and subsequent decline in HDL-C levels observed with the administration of niacin correlated with the expectations of CVDs being chronic and only managed by receiving continuous treatment. The increase in HDL-C levels also coincided with a statistically significant decline in CETP mRNA expression (p value = 0.01) (Figure 3(d)). This change was indicative of one of the mechanisms of niacin action in increasing HDLs via the RCT pathway utilizing the CETP gene [21]. This study suggests that niacin treatment had a role in the significant decrease of CETP while also increasing HDL-C in the treated animals compared to the control group. Therefore, the decline in CETP expression supported the notion that this gene is utilized in the HDL-C hypothesis. Furthermore, niacin administration is reported to result in a significant decrease in triglyceride levels by hindering the movement of fatty acids from adipose tissue as well as hepatic synthesis of fatty acids and triglycerides [35]. This might not be the case in the vervet monkeys, as they are known to be at the lower end of the triglyceride spectrum [36]. In monkeys, the triglyceride for cholesteryl ester exchange system occurs at a lesser rate as in humans, leading to subsequent triglyceride removal via Lp lipase being reduced [37].
Clinical studies have reported that niacin treatment elucidates an increase in HDL-C levels of 15%–35% and lowers triglyceride levels by 20%–50%, Lp levels by 24%–38%, and LDL-C levels by 5%–25% [38–40]. The results obtained in this study seem to correlate with those of the clinical trials. The slight decrease in LDL-C levels reported in this study can be attributed to the fact that niacin appears to have a modest LDL-C lowering efficiency [41]. Although niacin administration did not produce a significant decline in LDL-C, a slight reduction was observed in the treated animals (Figure 1(c)). Consequently, statins have been reported to be incapable of effectively clearing LDL-C in certain individuals, and one of the reasons for this is the ability to induce PCSK9 expression [42]. Niacin has been considered a mainstream treatment for elevated serum cholesterol levels due to its suppression of the expression of this gene. In this study, niacin administration led to a nonsignificant reduction in PCSK9 expression, with gene expression increasing at the end of the washout period (Figure 3(a)). The slight increase at the end of the washout period further supports the continuous treatment notion. Further studies are however required to understand the mechanisms underlying this observation.
Study limitations were noted which includes small sample size, noninvasive sampling techniques, and short treatment duration, and the study was possibly underpowered to detect differences. The current study used the minimum number/gender recommended by others [22, 32, 33], and this might have contributed to the reported nonsignificant changes. Due to the noninvasive nature of the study, liver biopsies were not performed to determine the level of gene expression at a tissue level. Therefore, it is speculated that the reported expression could be an underestimation of gene expression at tissue level. To overcome the above-mentioned limitations, this study recommends increasing the sample size by using one sex (males or females), incorporate tissue sampling for gene expression analysis, and extend the treatment duration by 3–4 months provided that the health and welfare of the animals will not be compromised. To be able to overcome some of the above-mentioned limitations, this study recommends that future investigations consider obtaining liver biopsy samples to evaluate tissue gene expression.
5. Conclusion
This current study was aimed at determining the impact of niacin in modulating blood lipids and mRNA expression of genes involved in the RCT pathway. The results revealed that niacin treatment was responsible for a statistically significant reduction in total cholesterol and the significant increase in HDL-C levels, while the decrease in LDL-C and triglyceride levels was not statistically significant. The results further confirmed that niacin is the most potent available drug to increase HDL-C levels. These findings were further supported by mRNA expression of associated genes which suggested that niacin induction slightly decreased the expression of PCSK9, even though it was not significant. The downregulation of the CETP gene suggested that the vervet monkey model can support the HDL-C hypothesis. These findings suggested that niacin has the potential of lowering cholesterol, LDL-C, and triglyceride levels in a reversible manner, while increasing HDL-C levels in the selected captive-bred vervet model. Future studies are aimed at supporting these findings by exploring the epigenetic biomarkers influencing the RCT pathway.
Ethics Statement
Ethical approval for the study was obtained from the South African Medical Research Council (SAMRC) Ethics Committee for Research on Animals (ECRA) (Ref: 11/18). This study was conducted in accordance with the South African National Standard for the Care and Use of Animals for Scientific Purposes (South African Bureau of Standards, SANS 10386, 2008).
Disclosure
This manuscript entitled “The Impact of Niacin Administration on Plasma Lipids and Gene Expression in the Vervet Monkey (Chlorocebus aethiops)” by Ngqaneka et al. was extracted from my original Master of Science (MSc) degree thesis titled “The Impact of Niacin on PCSK9 Levels in Vervet Monkeys (Chlorocebus aethiops)” which was submitted to the University of the Western Cape for graduation purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Mr. Thobile Ngqaneka and Dr. Zandisiwe Emilia Magwebu conceptualized and designed the study, carried out the experiments, and analyzed the data. Mr. Ngqaneka wrote the first draft of the manuscript under the supervision of Dr. Chesa Gift Chauke, Dr. Zandisiwe Emilia Magwebu, and Dr. Kenechukwu Obikeze. All authors contributed to the study since inception and approved the final draft of the manuscript.
Funding
This study was funded by the South African Medical Research Council (SAMRC/PUDAC).
Acknowledgments
The authors thank the animal technical team of the Primate Unit and Delft Animal Centre (PUDAC) of the South African Medical Research Council (SAMRC) for their excellent technical assistance and expertise in primate management. The authors also thank the SAMRC for financially supporting the study and the University of the Western Cape (UWC) for the opportunity to register this project.
Open Research
Data Availability Statement
The research data required to support the findings are incorporated in the manuscript.