Eculizumab Versus Rituximab for Refractory Antiacetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: A Single-Center Experience
Abstract
Background: Rituximab (RTX) and eculizumab (ECU) are treatment options for refractory myasthenia gravis (MG), but comparative clinical data derived from real-world experience are limited. Here, we describe the baseline characteristics, treatment, and safety outcomes of patients with antiacetylcholine receptor antibody-positive (AChR+) generalized myasthenia gravis (gMG) treated with ECU and/or RTX in our clinic.
Methods: Patients with refractory AChR+ gMG who received ECU or/and RTX treatment for more than 1 year at the Department of Neurology, Istanbul Faculty of Medicine were included in this observational study. After obtaining written patient consent, data were collected retrospectively from medical records.
Results: Twelve patients treated with ECU and 25 patients treated with RTX were included in the analysis. Groups were comparable with regard to demographic and clinical characteristics, including age at onset of MG, disease duration, and history of thymoma. ECU was associated with significantly better outcomes compared with RTX, as measured by decreases in the mean MG activities of daily living score at 1 (p = 0.024), 3 (p < 0.001), 6 (p < 0.001), and 12 (p < 0.001) months of treatment; steroid-sparing effect after 1 year of treatment (decrease in mean [standard deviation] daily prednisolone dose of −21.8 mg [13.5] vs. −6.6 mg [9.4] with RTX; p < 0.001); and need for rescue treatment and number of myasthenic crisis episodes during treatment (p < 0.001). No new safety signals were observed with either treatment.
Conclusion: Our data provide real-world evidence supporting ECU over RTX to treat patients with refractory AChR+ gMG.
1. Introduction
Myasthenia gravis (MG) is a rare autoimmune disorder affecting the neuromuscular junction that is caused by antibodies against postsynaptic membrane proteins [1]. Most patients (~85%) carry antibodies against the muscle acetylcholine receptor (AChR) [2]. Standard treatment options for MG include acetylcholinesterase inhibitors, immunosuppressive drugs, and/or thymectomy which are tailored to the patient’s characteristics [3]. At least 10% of patients develop refractory MG, which is characterized as inadequate response to standard treatment, ongoing symptoms, frequent exacerbations, and adverse reactions to corticosteroid and immunosuppressive therapies, and fewer than one-quarter of patients with MG achieve complete stable remission with contemporary treatment [4].
Rituximab (RTX) is a monoclonal antibody against CD20 antigen on B-cells [5]. RTX has several approved indications and is used off-label to treat certain autoimmune conditions including refractory MG [6]. RTX has been shown to be effective for refractory muscle-specific tyrosine kinase (MuSK) + generalized myasthenia gravis (gMG) [7–12], whereas its role in refractory antiacetylcholine receptor antibody-positive (AChR+) gMG is less clear [13–19]. Nevertheless, due to the absence of more effective and safe treatment options, until relatively recently, a significant proportion of patients with refractory AChR+ gMG received RTX.
In AChR+ gMG, terminal complement activation leads to AChR antibody-mediated destruction of the motor endplate, disrupting neuromuscular transmission [20]. Eculizumab (ECU) is a humanized monoclonal antibody that binds to complement protein C5 and inhibits the activation of terminal complement, thus protecting the neuromuscular junction from these destructive effects [21]. A Phase 3 study (REGAIN) in refractory AChR+ gMG failed to show a statistically significant benefit for ECU over placebo in the primary efficacy endpoint [22]. However, the open-label extension phase of REGAIN, as well as observational studies and case series/reports, have suggested favorable outcomes and safety with ECU [23–27]. Preplanned and post hoc sensitivity analyses of primary and secondary outcomes of the REGAIN study also supported the efficacy of ECU over placebo [21]. ECU is approved for use in AChR+ gMG in the United States, Europe, and Japan and, more recently, for refractory AChR + gMG in Türkiye. To date, a single study from Germany has compared the effectiveness of ECU and RTX in patients with refractory AChR+ gMG [28].
In this study, we compared baseline characteristics, treatment, and safety outcomes of patients with refractory AChR+ gMG treated with ECU and/or RTX at our clinic.
2. Methods
This retrospective observational study involved patients with refractory AChR+ gMG who received ECU and/or RTX for at least 1 year, between 2016 and 2022, at the Department of Neurology, Istanbul Faculty of Medicine. In Türkiye, RTX has been used off-label for refractory AChR+ gMG since 2016, and ECU was approved for use in this indication in 2020. After obtaining written informed consent from patients, relevant data were abstracted from written and electronic medical records.
Patients were included if they had (1) a diagnosis of refractory AChR+ gMG, (2) a Myasthenia Gravis Foundation of America (MGFA) clinical classification of III–V, (3) a myasthenia gravis activities of daily living (MG-ADL) total score of ≥ 6 (scores on this eight-item survey of MG symptoms range from 0 [normal] to 24 [most severe]), and (4) previous treatment of adequate duration and dosage with at least two immunosuppressants and/or intravenous immunoglobulin (IVIG) or plasma exchange at least four times in 12 months, without symptom control. Excluded from the study were patients with (1) antiacetylcholine receptor antibody-negative gMG, (2) ocular MG (MGFA Class I), (3) thymectomy within 12 months of treatment initiation, and (4) a follow-up period of less than 1 year.
2.1. Treatment Regimens
RTX was administered as two 1000 mg induction doses given 14 days apart. A single 1000 mg maintenance infusion was administered at 6 months.
ECU was administered as a 900 mg induction dose on Day 1 and at Weeks 1, 2, and 3, followed by a maintenance dose of 1200 mg at Week 4 and every 2 weeks thereafter. Patients were vaccinated against Neisseria meningitides at least 2 weeks before starting ECU. Patients who were not vaccinated at the appropriate time received prophylactic antibiotics until 2 weeks after vaccination.
2.2. Outcome Measures
During treatment with RTX or ECU, outcome measures were changed in the mean MG-ADL total score, change in the mean daily prednisolone dose, and need for IVIG rescue therapy/disease exacerbation/myasthenic crisis. A myasthenic crisis is defined as a life-threatening worsening of myasthenic weakness requiring intubation or noninvasive ventilation [29]. Upon completion of RTX or ECU treatment, the outcome measure was MG Foundation of America Postintervention Status (MGFA-PIS). A favorable MGFA-PIS outcome is defined as achieving minimal manifestations (MMs), meaning that “the patient has no symptoms of functional limitations from MG but has some weakness on examination of some muscles” or better, namely, complete stable remission or pharmacologic remission. MM grades range from MM-0 (the patient has received no MG treatment for at least 1 year) to MM-3 (the patient has received cholinesterase inhibitors or other symptomatic therapy and some form of immunosuppression during the past year). A change in MGFA-PIS is described as improved, unchanged, worsened, exacerbation, or deceased [30].
Safety was assessed by the occurrence of adverse events, serious adverse events (SAEs), and hospital admissions. SAEs are defined as any untoward medical occurrence that results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, may have caused a congenital anomaly/birth defect, or requires intervention to prevent permanent impairment or damage.
2.3. Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Istanbul University Institutional Review Board for Research with Human Participants (2023/2017) and was conducted in accordance with the Declaration of Helsinki. Data were anonymized and collected retrospectively. All patients provided written informed consent for the use of their data.
2.4. Statistical Analysis
Results are reported descriptively as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables and as counts (n) and percentages for categorical variables. The normality of continuous variables was investigated by the Shapiro–Wilk test. The Friedman test was used to compare continuous variables for more than two dependent groups. Categorical variables were compared using the chi-square test (or Yates’s continuity correction/Fisher’s exact test where available). For post hoc comparisons, the Bonferroni adjusted Wilcoxon’s signed-rank test was used. Statistical significance was accepted when the two-sided p value was less than 0.05. Statistical analyses were performed using MedCalc Statistical Software Version 19.7.2 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021).
2.5. Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
3. Results
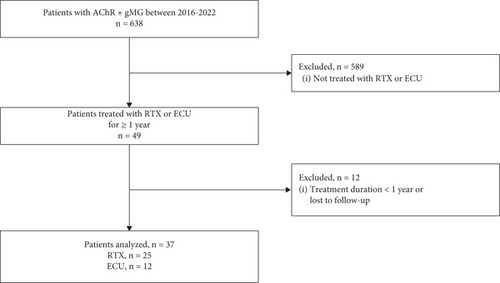
Of 638 total patients with AChR+ gMG identified in our clinic over the observation period, 49 with refractory AChR+ gMG had received ECU or RTX. Of these, 12 were excluded from the analysis due to insufficient treatment duration or loss of follow-up. The 37 remaining patients, 25 treated with RTX and 12 treated with ECU, fulfilled the inclusion/exclusion criteria and were included in the analysis (Figure 1).
There were no significant differences in baseline demographic and clinical characteristics between the ECU and RTX groups (Table 1). The female-to-male ratio was approximately equal in both treatment groups. The mean (SD) age at diagnosis was 30.1 (15.3) years in the ECU group and 27.9 (13.2) years in the RTX group. Most patients (89%) had early-onset (< 50 years) MG. Mean AChR-Ab titers were similar between groups. The majority of patients in both treatment groups (83% vs. 92%) had previous thymectomy, of which six ECU-treated patients (50%) and nine RTX-treated patients (39%) had a history of thymoma. Thymectomy was performed at least 1 year prior to treatment in all patients except for two patients with late-onset gMG. A MGFA classification of ≥ 3 (moderate or severe MG) was recorded in all patients at baseline, and most patients (83.7%) had predominantly bulbar involvement (MGFA 3B or 4B). The mean MG-ADL score at baseline was > 8 points in both groups. Mean (SD) disease duration prior to treatment with ECU or RTX was 10.3 (7.9) years in the ECU group and 11.3 (8.7) years in the RTX group. All patients were receiving immunosuppressive therapies at the time of treatment initiation.
| Characteristic | Eculizumab (n = 12) | Rituximab (n = 25) | p value |
|---|---|---|---|
| Female sex, n (%) | 6 (50) | 14 (56) | 1.00a |
| Age at MG diagnosis, years | 0.833b | ||
| Mean (SD) | 30.1 (15.3) | 27.9 (13.2) | |
| Median (IQR) | 25.5 (19–43.5) | 26 (17.5–33.5) | |
| MG subgroups, n (%) | 0.582c | ||
| < 50 years (early onset) | 10 (83.3) | 23 (92.0) | |
| ≥ 50 years (late onset) | 2 (16.7) | 2 (8.0) | |
| AChR-Ab titers (nmol/L), mean (SD) | 13.5 (7.0)d | 14.2 (7.6)e | |
| Previous thymectomy, n (%) | 10 (83.3) | 23 (92.0) | |
| Thymic pathology, n (%) | NA | ||
| Thymoma | 6 (60.0) | 9 (39.1) | |
| Thymic hyperplasia | 4 (40.0) | 11 (47.8) | |
| Atrophic thymus | — | 3 (13.0) | |
| Time from thymectomy (years), mean (SD) | 4.0 (5.8) | 8.0 (5.7) | |
| Previous ventilator support, n (%) | 7 (58.3) | 14 (56.0) | 1.00a |
| MGFA, n (%) | NA | ||
| 3A | 1 (8.3) | 3 (12.0) | |
| 3B | 5 (41.7) | 12 (48.0) | |
| 4A | 1 (8.3) | — | |
| 4B | 5 (41.7) | 9 (36.0) | |
| 5 | — | 1 (4.0) | |
| MG-ADL | 0.974b | ||
| Mean (SD) | 8.8 (2.6) | 9.1 (4.1) | |
| Median (IQR) | 8.5 (6.3–11.5) | 7.5 (6–12) | |
| Duration of disease before ECU/RTX (years) | 0.987b | ||
| Mean (SD) | 10.3 (7.9) | 11.3 (8.7) | |
| Median (IQR) | 9.5 (3–16.8) | 9 (2–19) | |
| Previous treatments, n (%) | NA | ||
| PRD and AZA | — | 2 (8.0) | |
| PRD, AZA, and IVIG | 2 (16.7) | 7 (28.0) | |
| PRD, AZA, MMF, and IVIG | 1 (8.3) | 6 (24.0) | |
| PRD, AZA, PE, and IVIG | 2 (16.7) | 3 (12.0) | |
| PRD, AZA, and MMF | — | 2 (8.0) | |
| PRD, AZA, MMF, PE, and IVIG | 1 (8.3) | — | |
| PRD, AZA, MMF, CP, and IVIG | — | 4 (16.0) | |
| PRD, AZA, RTX, and IVIG | 3 (25.0) | — | |
| PRD, AZA, CP, PE, and IVIG | — | 1 (4.0) | |
| PRD, AZA, RTX, PE, and IVIG | 3 (25.0) | — | |
| Age at first dose of ECU or RTX (years) | 0.758b | ||
| Mean (SD) | 40.4 (16.5) | 39.2 (14.6) | |
| Median (IQR) | 35 (30.5–48.8) | 34 (29–51.5) | |
| Treatment at the time of ECU or RTX initiation, n (%) | NA | ||
| PRD, AZA, and IVIG | 4 (33.0) | 14 (56.0) | |
| PRD, MMF, and IVIG | 8 (67.0) | 11 (44.0) |
- Abbreviations: AChR-Ab, acetylcholine receptor antibody; AZA, azathioprine; CP, cyclophosphamide; ECU, eculizumab; IQR, interquartile range; IVIG, intravenous immunoglobulin; MG, myasthenia gravis; MG-ADL, MG activities of daily living; MGFA, MG Foundation of America; MMF, mycophenolate mofetil; PE, plasma exchange; PRD, prednisolone; RTX, rituximab; SD, standard deviation.
- aYates’s continuity correction.
- bMann–Whitney U test.
- cFisher’s exact test.
- dValues available for eight patients.
- eValues available for 14 patients.
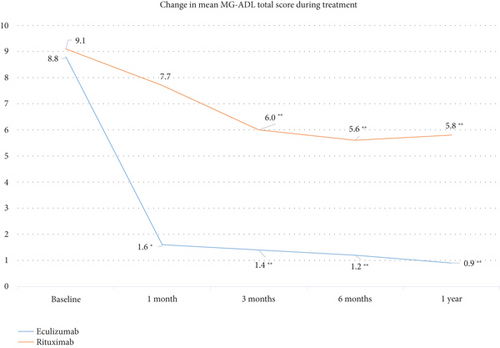
3.1. Change in MG-ADL Total Score
The change in the mean MG-ADL total score from baseline to 1 year was statistically significant in both treatment groups (p < 0.001; Friedman’s test) but numerically greater with ECU (Figure 2). In the ECU group, changes from baseline in mean MG-ADL scores were statistically significant at 1 month (p = 0.024), 3 months (p < 0.001), 6 months (p < 0.001), and 1 year (p < 0.001). In the RTX group, changes from baseline in mean MG-ADL scores were statistically significant at 3 months, 6 months, and 1 year (all p < 0.001). The mean (SD) reduction in MG-ADL scores from baseline to 1 year was −7.9 (3.1) in the ECU group and −3.3 (2.8) in the RTX group, and the between-group difference was statistically significant (p < 0.001; the Mann–Whitney U test).
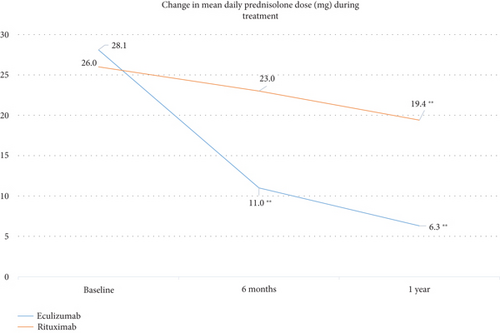
3.2. Change in Daily Prednisolone Dose
Change in the mean daily dose of prednisolone from baseline to 1 year was statistically significant in both treatment groups (p < 0.001; Friedman’s test) but numerically greater with ECU (Figure 3). The mean (SD) daily dose of prednisolone in the ECU group was 28.1 mg (14.7) at baseline and 6.3 mg (3.6) at 1 year, for a reduction of 77.6%. Corresponding values in the RTX group were 26.0 mg (13.4) at baseline and 19.4 (12.6) at 1 year, for a reduction of 25%. The between-group difference of −21.8 mg (13.5) for ECU and −6.6 mg (9.4) for RTX was statistically significant (p < 0.001; the Mann–Whitney U test). Changes in the mean daily dose of prednisolone were statistically significant from baseline to 1 year (p < 0.001), from 6 months to 1 year (p = 0.018) in the ECU group, and from baseline to 1 year (p < 0.001) in the RTX group.
3.3. Need for IVIG Rescue Treatment/Disease Exacerbation/Myasthenic Crisis
No patient treated with ECU required IVIG rescue treatment or experienced a myasthenic crisis during observation. One ECU-treated patient had disease exacerbation at 6 months. In the RTX group, seven patients (29.2%) required IVIG rescue treatment, three patients (12.0%) experienced myasthenic crisis, and 15 patients (60.0%) had disease exacerbation during observation. The between-group difference in the number of disease exacerbation episodes after 1 year of treatment with ECU or RTX was statistically significant (p < 0.001; Yates’s continuity correction).
3.4. MGFA-PIS Evaluations
The rate of favorable MGFA-PIS evaluation was statistically significantly greater with ECU than RTX at each evaluation timepoint (Table 2). Seven patients in the ECU group achieved MM-3 by 1 month of treatment and maintained their status throughout the year. Two additional ECU-treated patients achieved MM-3 status during treatment for a total of nine patients (75%) at 1 year. The change in status after treatment in the remaining three ECU-treated patients was described as “improved.” In the RTX group, one patient achieved MM-3 status by 3 months postinduction. Six additional patients achieved MM-3 status after the second cycle of RTX, bringing the total to seven patients (28%) at 1 year. The change in MGFA-PIS after treatment with RTX was described as “improved” in seven patients, “unchanged” in eight patients, and “exacerbation” in three patients.
| Timepoint (status), n (%) | Eculizumab (n = 12) | Rituximab (n = 25) | p value |
|---|---|---|---|
| 1 month (MM-3) | 7 (58.3) | 0 (0) | < 0.001a |
| 3 months (MM-3) | 8 (66.7) | 1 (4.0) | < 0.001a |
| 6 months (MM-3) | 8 (66.7) | 4 (16.0) | 0.006a |
| 1 year (MM-3) | 9 (75.0) | 7 (28.0) | 0.019b |
- Abbreviation: MM-3, patient who has received cholinesterase inhibitors or other symptomatic therapy and some form of immunosuppression during the past year.
- aFisher’s exact test.
- bYates’s continuity correction.
3.5. Outcomes in Patients Who Received RTX Prior to ECU
Six patients in the ECU group had previously been treated with RTX without improvement. The evolution in mean MG-ADL scores is shown in Table 3. The mean (SD) MG-ADL score in these six patients was 11.7 (9.8) at the start of RTX treatment and 10.5 (2.2) at 6 months (after two cycles). At the time of switching to ECU, the mean (SD) MG-ADL score was 10.0 (2.4), reducing to 1.7 (1.0) at 1 month and to 0.7 (1.2) at 1 year. The between-group difference in mean (SD) MG-ADL scores was statistically significant at 1, 3, 6, and 12 months of treatment (all p = 0.027; Wilcoxon’s signed-rank test). Five of the six patients who switched from RTX to ECU achieved MM-3 by 3 months and maintained their status through 1 year of treatment.
| Timepoint, mean (SD) | Rituximaba (n = 6) | Eculizumab (n = 6) | p valueb |
|---|---|---|---|
| Baseline | 11.7 (9.8) | 10.0 (2.4) | 0.180 |
| 1 month | 11.8 (3.2) | 1.7 (1.0) | 0.027 |
| 3 months | 10.8 (2.8) | 1.3 (1.0) | 0.027 |
| 6 months | 10.5 (2.2) | 1.2 (1.5) | 0.027 |
| 1 year | 10.0 (2.4) | 0.7 (1.2) | 0.027 |
- Abbreviation: SD, standard deviation.
- aPatients received rituximab for at least 6 months (two cycles) before being assessed as nonresponders.
- bWilcoxon’s signed-rank test.
3.6. Safety
Adverse events reported during treatment with ECU or RTX, irrespective of causality, are summarized in Table 4. Two patients in the RTX group died from COVID-19 during the pandemic. Information about their vaccination status was not available. Four patients in the ECU group (all vaccinated) experienced COVID-19 infection without any serious complications. No pattern of adverse events was observed with either RTX or ECU.
| Event, n (%) | Eculizumab (n = 12) | Rituximab (n = 25) |
|---|---|---|
| Serious adverse events | — | 2 (8.0)a |
| Death due to COVID-19 | — | 2 (8.0)a |
| Adverse events | 6 (50.0) | 11 (44.0) |
| Headache | — | 2 (8.0) |
| Tachycardia | 1 (4.0) | |
| Joint pain | 1 (8.3) | 1 (4.0) |
| Numbness in hand | 1 (8.3) | — |
| Muscle pain | — | 1 (4.0) |
| Nasopharyngitis | 2 (16.7) | — |
| Pneumonia | 1 (8.3) | 1 (4.0) |
| Upper respiratory tract infection | — | 2 (8.0) |
| Nasal furuncles | 1 (8.3) | — |
| Thrombocytopenia | — | 1 (4.0) |
| Atrial fibrillation | — | 1 (4.0) |
- aBoth patients died from COVID-19.
4. Discussion
As emerging options to manage refractory AChR+ gMG in clinical practice may ultimately lead to alterations in therapeutic algorithms, evidence should ideally derive from randomized controlled studies. However, real-world evidence in less-selected populations also has a role in guiding treatment selection. This observational study compared RTX and ECU for the treatment of refractory AChR+ gMG in routine clinical practice. ECU was associated with statistically significant better outcomes than RTX at study timepoints up to 1 year of treatment, including improved functional ability, a greater steroid-sparing effect, and superior disease control. No new safety issues arose with either RTX or ECU.
The thymoma rate was high in our patient cohort, consistent with a report suggesting that thymomatous patients are strongly overrepresented in the refractory MG population [31]. As patients with a history of thymoma or thymic neoplasms were excluded from the REGAIN study, information specific to this group was lacking. A postmarketing study of ECU in Japanese patients with AChR+ gMG showed that effectiveness outcomes were broadly similar in patients with/without a history of thymoma [24]. Other case series/reports have described the effectiveness of ECU in thymomatous MG [27, 32, 33]. Of the six refractory thymomatous patients we treated with ECU, four failed to respond to RTX. All six patients achieved MM-3 status by the first year of ECU treatment, further supporting its effectiveness in this patient group.
The 3.1-point reduction in the mean MG-ADL score from baseline to 3 months in the RTX group was clinically meaningful, as a reduction of ≥ 2 points is considered predictive of clinical improvement [34]. The 3.5-point reduction observed at 6 months was maintained through 1 year. In ECU-treated patients, the 7.2-point reduction in the mean MG-ADL score observed at 1 month (p = 0.024) persisted at 3 months, 6 months, and 1 year of treatment (p < 0.001). Numerically, the extent of improvement in the mean MG-ADL score was more than twofold greater with ECU than RTX at 3 months, and the rate of improvement was significantly in favor of ECU (p < 0.001; the Mann–Whitney U test). These findings align with previously published real-world experience of ECU in (mainly) refractory AChR+ gMG [25, 32]. The only previous comparison of ECU and RTX in the routine practice setting concluded that outcomes were better with ECU [28]. However, because this study used the change in the quantitative myasthenia gravis (QMG) score at 12 and 24 months of treatment as the outcome measure, the results cannot be compared directly with ours.
In our cohort, patients treated with ECU versus RTX were more likely to achieve permanent MM-3 status within the first year of treatment (75% vs. 28%). The response was rapid, as seven (58%) ECU-treated patients achieved MM-3 status at 1 month compared with none in the RTX group. Although RTX is known to cause rapid B-cell depletion [5], seven nonresponders after the first course of RTX achieved MM-3 status after the second cycle, likely due to more complete B-cell depletion as described in RA [35]. These data suggest that slow responders to RTX may exist in the refractory AChR+ gMG population. Response to RTX may have been greater had the observation period been longer than 1 year, as seen during longer term follow-up [36, 37]. Alternatively, other therapeutic options can be considered for nonresponders or insufficient responders to RTX. Given that five of six patients who failed to improve with RTX responded after switching to ECU, ECU may be a reasonable alternative for RTX nonresponders. Subgroup analyses of REGAIN and its extension study showed that ECU is effective in patients with refractory AChR+ gMG irrespective of previous exposure to [38].
The mean disease duration of > 10 years before treatment with either RTX or ECU may also have contributed to the lower response with RTX, as retrospective studies [37, 39] and a randomized clinical trial (RINOMAX) [40] have shown benefit with RTX in new-onset gMG. As autoantibodies are produced mainly by long-lived plasma cells, intervening early to prevent the buildup of a disease-associated, antibody-producing plasma cell pool may provide a more effective way to target the disease through B-cell depletion in new-onset patients [41, 42].
Despite only limited evidence from randomized controlled trials to support the use of corticosteroids in MG [43], experts largely agree on their effectiveness while recognizing the potential harmful effects of long-term use (e.g., increased cardiovascular risk, diabetes mellitus, weight gain, osteoporotic fractures, and cataracts) [44]. To achieve the goal of using the lowest possible dose of corticosteroid to maintain the therapeutic effect, the steroid-sparing effect of an intervention should be evaluated as a primary outcome measure in studies of MG. Since clinical trial protocols typically mandate that immunosuppressive doses remain stable throughout the treatment period, real-world data can have a role in providing the necessary insight. In our cohort, the mean corticosteroid dose decreased by 25% from baseline to 12 months with RTX. By comparison, in BeatMG, 60% of patients with AChR+ gMG treated with RTX (and 56% treated with placebo) achieved a ≥ 75% reduction from baseline in their mean daily prednisone dose [18]. The lower steroid-sparing effect of RTX in our study relative to BeatMG can be explained by differences in disease characteristics of the patient populations: refractory disease in our study versus predominantly mild-moderate disease in BeatMG. Importantly, the mean corticosteroid dose was reduced by a clinically significant 77% from baseline to 12 months among ECU-treated patients in our study.
ECU and RTX were both well tolerated. No SAEs were reported with ECU. Two RTX-treated patients died from COVID-19 infection, but a causal relationship cannot be certain. In both treatment groups, adverse events (i.e., headache, nausea, fever, tachycardia, joint pain, and infections) were similar to those reported in previous studies of RTX [18] and ECU [22] in AChR+ gMG. No meningococcal infections were recorded with ECU during the observation period.
The main limitations of this study are its retrospective observational design and small sample size. As outcomes were compared only during the first 1 year of treatment with ECU or RTX, any benefit beyond this period (e.g., in slow responders to RTX) could not be detected. The QMG score, which is frequently used as an outcome measure in clinical trials, was not routinely evaluated in our daily practice when RTX treatment was started. Lastly, the study scope is limited to a comparison of RTX and ECU, as newer FDA-approved treatments for refractory AChR+ gMG such as efgartigimod [45], ravulizumab [46], rozanolixizumab [47], and zilucoplan [48] are not yet approved in Türkiye.
Recently, it has been reported that, beyond complement inhibition, ECU may have off-target effects (e.g., inhibiting the generation of downstream proinflammatory leukotrienes) that account for its effectiveness in MG [49].
5. Conclusion
Our study provides real-world evidence that complement inhibition is more effective and has a faster onset of action than B-cell depletion in the treatment of longstanding, refractory AChR+ gMG. ECU led to a rapid improvement in functional ability, as evidenced by a twofold greater decrease than RTX in the mean MG-ADL total score at 3 months. This level of improvement in functional ability with ECU was maintained over the 12-month observation period. The time to achieve MM-3 status was significantly shorter with ECU than with RTX, and ECU had a significantly greater steroid-sparing effect than RTX. ECU was effective even in patients who had failed to respond to RTX. ECU appears to be a valuable alternative to RTX for patients with refractory AChR+ gMG.
Disclosure
This study was presented as a poster at the 76th Annual Meeting of the American Academy of Neurology, Denver, CO, United States, 13–18 April 2024 (https://www.neurology.org/doi/10.1212/WNL.0000000000204958#:%7E:text=Eculizumab%2520was%2520associated%2520with%2520a,9.4)%252C%2520the%2520need%2520of%2520rescue).
Conflicts of Interest
H.D. and Y.G.P. have received travel grants and honoraria as speakers from Alexion. The other author declares no conflicts of interest.
Author Contributions
Hacer Durmus: study conception and design, data acquisition, analysis and interpretation of results, draft/revision of the manuscript for content, including medical writing for content. Arman Çakar: study conception and design, data acquisition, analysis and interpretation of results. Yesim Gülşen Parman: study conception and design, data acquisition, analysis and interpretation of results, draft/revision of the manuscript for content, including medical writing for content. All authors read and approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Medical writing assistance was provided by Kerry Dechant, ISMPP CMPP, on behalf of Content Ed Net (Istanbul, Türkiye), which was funded by Alexion Pharmaceuticals (Istanbul, Türkiye). Statistical analyses were performed by Arzu Baygül on behalf of Content Ed Net (Istanbul, Türkiye), which was funded by Alexion Pharmaceuticals (Istanbul, Türkiye).
Acknowledgments
Medical writing assistance was provided by Kerry Dechant, ISMPP CMPP, on behalf of Content Ed Net (Istanbul, Türkiye), which was funded by Alexion Pharmaceuticals (Istanbul, Türkiye).
Statistical analyses were performed by Arzu Baygül on behalf of Content Ed Net (Istanbul, Türkiye), which was funded by Alexion Pharmaceuticals (Istanbul, Türkiye).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




