Thermal Ammonia Decomposition for Hydrogen-Rich Fuel Production and the Role of Waste Heat Recovery
Abstract
Hydrogen is an attractive future fuel with the potential to play a crucial role in reducing carbon dioxide emissions, but the major obstacles to implement hydrogen are associated with its storage, transportation, and safety. As a solution, ammonia has been recognized as a promising carbon-free hydrogen carrier; however, due to the poor combustion performance of ammonia fuel, this review first outlines the significance of on-site ammonia decomposition to generate COx-free H2-rich fuel. Furthermore, to demonstrate the potential of this approach in different fields, the main focus of this review is on the pivotal role of integrating waste heat recovery and thermal ammonia decomposition across various industries including power plants, transportation, and industrial furnaces, enabling researchers to gain insights and benefit from the work of each other. As a means to enhance fuel saving and reduce greenhouse gas emissions, two significant methods including thermochemical recuperation and autothermal reforming are investigated considering the impact of several factors like exhaust gas species, temperature, and the performance parameters of the main fuel consumers. Finally, the paper provides vital recommendations for research directions in waste heat recovery-based ammonia decomposition systems to promote sustainable and eco-friendly solutions applicable to various industries. These include but are not limited to conducting life cycle analyses considering green ammonia, optimization of the independency level for the decomposition system, and integrating it with various components such as dual-fuel engines, hydrogen purification, solar energy, and fuel cells.
1. Introduction
Due to the significant global population and associated industrial activities, the growing need for energy has resulted in substantial carbon and greenhouse gas (GHG) emissions which give rise to numerous pollution concerns and environmental challenges. The impact of CO2 on global warming constitutes approximately 30% of the overall effect, making it the most effective greenhouse gas in terms of elevating atmospheric temperatures [1]. Since the industrial revolution, there has been a notable rise in CO2 emissions so CO2 levels experienced a consistent annual increase of over two parts per million (ppm) for the 11th consecutive year in 2022 [2]. To reduce CO2 emissions, researchers are conducting studies on effective utilization of wasted thermal energy integrated with alternative carbon-free fuels, aiming to enhance the overall efficiency, conserve natural resources, and increase the feasibility of widespread adoption.
Hydrogen (H2) is one of the most important carbon-free fuels anticipated to play a significant role in mitigating CO2 emissions, aligning with the objectives of the Paris Agreement (keeping the temperature increase well below 2°C compared to preindustrial levels) [3]. Due to its zero-carbon content and high performance, it can be used for multiple purposes such as fuel for maritime, vehicles, power plants, oil refineries, and even in some advanced applications like aviation so that the global hydrogen demand from 2,000 to 2021 increased from 50 to 94 Mt [4, 5]. The present demand for hydrogen is met mainly by production from natural gas through methane steam reforming (MSR), while water electrolysis accounted for an estimated 30 ktH2 in 2020 which can be integrated with renewable technologies such as photovoltaic thermal systems (PVT) and wind turbines [6]. Some of the disadvantages of MSR process are the need for very high temperatures and pressures (800–1,000°C at 15–20 bar), the necessity of hydrogen purification which is highly energy intensive, and the release of CO2 emissions so that in 2020, the total global CO2 emissions related to hydrogen production was more than 900 Mt accounting for about 2.4% of the world emissions [7]. There are certain important obstacles to the general usage of hydrogen as fuel. One of such obstacles is that it has the lowest atomic weight of any substance and, therefore, has very low density both as a gas and a liquid so that its liquid density at 1 atm is about 10% and 17% of the liquid density of gasoline and methane, respectively [8]. Moreover, hydrogen gas molecules are smaller than those of any other gas, enabling it to permeate through numerous materials typically regarded as airtight or impermeable to other gases. Leaks of liquid hydrogen evaporate very quickly but due to very high buoyancy and diffusivity, leaked hydrogen rises and becomes quickly diluted. Although hydrogen has the highest heating value by mass among all fuels (LHV = 119.6 MJ/kg), it has a very low heating value by volume at 1 atm and 15°C which is one-third of methane gas [8]. Hydrogen is flammable over a very wide range of concentrations in air (4–75 vol%) and the flammability limits increase with temperature. As a result, even small leaks of hydrogen have the potential to burn. For this fuel, the autoignition temperature is relatively high at 585°C, which makes it difficult to ignite a hydrogen/air mixture based on heat alone without an additional ignition source [9].
The challenge of storing and transporting hydrogen represents one of the foremost obstacles to its practical use as a fuel. It is typically stored in two main ways, one of which involves storing as a highly compressed gas, reaching pressures of up to 700 bar at a temperature of 25°C. This is accomplished using materials like austenitic stainless steels, aluminum alloys, and carbon–fiber composites. Alternatively, hydrogen can be stored in its liquid form, which requires much lower temperatures, specifically −253°C at 1 bar. Theoretically, achieving liquefaction in this manner would require an energy input of approximately 11.6 MJ/kg [10]. The cryogenic liquefied form of fuels such as hydrogen with a very low boiling temperature continuously loses some portion of their mass due to temperature difference between the liquefied energy carriers and the ambient so these losses are called boil-off gas. Therefore, it is essential to consider the insulating qualities of the tank in order to efficiently manage this problem. Such storage systems are energy demanding and notably expensive imposing restrictions on tank materials and dimensions, which is a significant challenge to ensure the success of hydrogen-based applications [11]. So, the ways and means to carry it economically and safely are a challenge that needs to be solved.
To overcome the mentioned challenges, an alternative strategy involves storing hydrogen in the form of another compound containing hydrogen, which can be conveniently transported. Hydrogen is then produced on-site through a chemical reaction. To do so, the US Department of Energy (DOE) established certain criteria for technoeconomically feasible chemical storage of hydrogen so that storage materials should have at least 9 MJ/kg and 8.3 MJ/L gravimetric and volumetric energy as well as 9 wt.% and 80 g/L hydrogen content [11]. Figure 1 shows hydrogen storage capacity of different potential compounds in which the dashed lines are the criteria set by the DOE. In comparison with all compounds, ammonia (NH3) has been recognized as a promising carbon-free alternative hydrogen provider and a sustainable energy source since it has the highest gravimetric hydrogen density of 17.8 wt.% and proper LHV of 18.8 MJ/kg [14]. Moreover, Figure 1 illustrates the volumetric energy and gravimetric energy of different fuels as well as Li batteries so that ammonia has a volumetric energy of 11.3 MJ/L in its liquid form, which is far easier to achieve than cryogenically cooled liquid hydrogen.
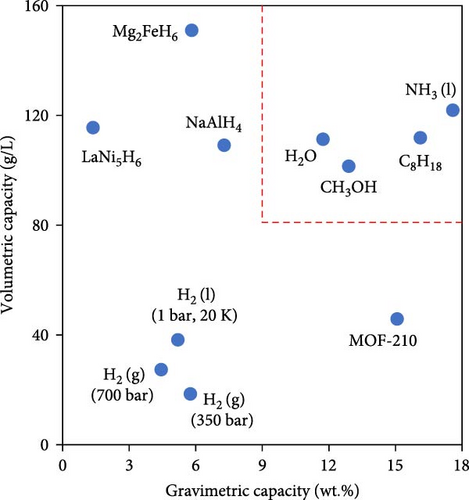
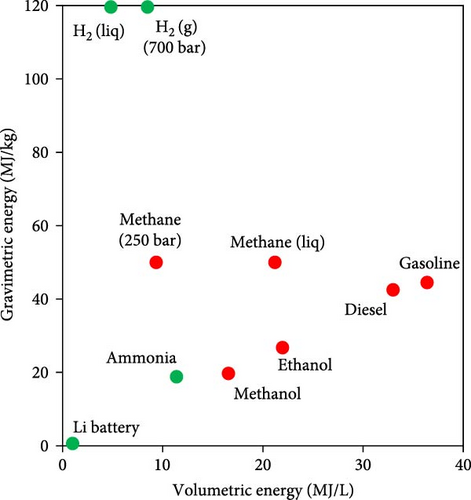
Given the abovementioned limitations with storage, transport, and safety of hydrogen, in this review, first in the following two sections, the advantages of ammonia as a carbon-free hydrogen carrier and the limitations of direct ammonia combustion are highlighted. Moreover, in two other specific sections, this paper underlines the significance of promoting ammonia combustion by blending with hydrogen and on-site thermal ammonia decomposition as a mean to extract H2-rich fuel since among the various techniques for producing hydrogen from ammonia, thermal catalytic decomposition (also known as thermal cracking) has emerged as the most mature, prevalent, and scalable method. In contrast, electrocatalysis, another possible technique, faces challenges such as slow hydrogen production rates and the inherent complexity of its systems [15, 16]. More importantly, considering the advancements in the waste heat recovery technologies (WHR) aimed at improving the efficiency in different industries as well as the use of carbon-free alternative fuels such as ammonia, the global energy demand on fossil fuels would surely decrease which would alleviate the impact of global warming. Therefore, given the significance of heat energy supply for the endothermic ammonia decomposition process, this paper aims to review and collect in a unique document the advances related to the significant role of WHR in thermal ammonia decomposition and explores different potential applications of such integration including power plants, transportation, alternative fuel production, and industrial furnaces through analysis of recent publications. Various types of decomposition techniques and sources of waste heat recovery are studied from theoretical and experimental perspectives, considering technical, environmental, and economical aspects.
2. Ammonia as a Hydrogen Carrier: Opportunities
Ammonia application as a means of energy storage or an alternative fuel presents various advantages. The extensive development of industrial ammonia production positions it as a well-established alternative fuel. It is ranked as the second most widely produced inorganic chemical commodity globally following sulfuric acid and its production has remained fairly stable in recent years. Its global production capacity is projected to increase from approximately 236 million tons in 2021 to nearly 290 million tons by 2030 [17]. Moreover, worldwide ammonia production today accounts for approximately 2% of total final energy usage. Around 72% of ammonia is produced through steam reforming of natural gas, while most of the remainder is via coal gasification (26%), about 1% from oil products and an even smaller fraction stems from electrolysis [4]. The global average energy intensity of ammonia production today is around 41 GJ/t [18]. Considering equivalent emission of carbon dioxide, the natural gas-based production has about 100 kgCO2/GJ which is higher than that of conventional fuel production, such as gasoline at around 16 kgCO2/GJ [19].
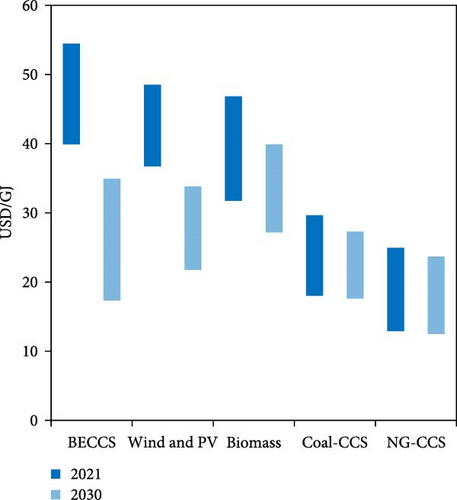
Different types of ammonia production include green ammonia (from carbon-free sources and near-zero carbon electricity), gray ammonia (typically from natural gas), brown ammonia (from coal or lignite), and blue ammonia (including carbon capture) [20, 21]. To mitigate the environmental effects associated with ammonia and hydrogen production, integrating carbon capture, utilization, and storage (CCUS) with biomass gasification has the potential to yield hydrogen and ammonia with a carbon-negative footprint. In 2021, the estimated production cost for electrolytic ammonia ranged from 37 to 48 USD/GJ compared to natural gas with CCUS at about 14–25 USD/GJ. It is anticipated that By 2030, the cost of electrolytic ammonia could decrease to as low as 22 USD/GJ (400 USD/tNH3) in the best locations (Figure 2) [4].
Unlike the challenges associated with storing and transporting hydrogen, ammonia can be liquefied under mild conditions and stored in a simple, inexpensive pressure vessel since the vapor pressure of ammonia at room temperature is 9.2 bar [22]. Moreover, the existing infrastructure for ammonia storage and transportation is well-established, primarily due to its use in the fertilizer industry. In the United States alone, there are more than 10,000 ammonia storage sites, most of which are linked to a pipeline network stretching more than 3,000 km [4]. Currently, no commercial ships can transport liquefied hydrogen and its liquefaction requires cooling work which is equivalent to 20%–30% of the energy content of the liquefied hydrogen itself [23].
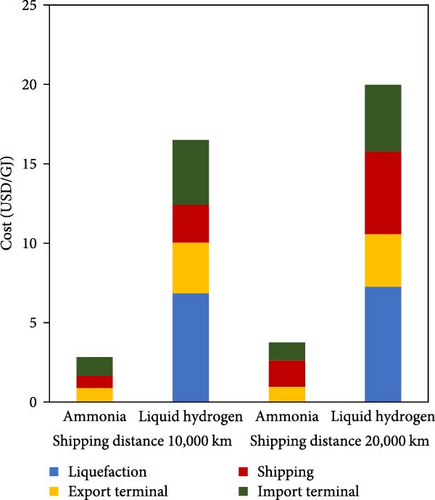
Ammonia also benefits from a lower boil-off rate, approximately 0.025 vol%/day, in comparison with hydrogen at about 0.52 vol%/day [24]. The combination of boil-off losses and the energy consumed in the liquefaction of hydrogen, leads to the short-term (7 days) storage efficiency of 53%, and a seasonal storage (182 days) efficiency of about 21%, while the overall efficiency for ammonia synthesis followed by liquefaction and storage is 85% [25]. According to Figure 3, the cost estimate for marine transport for a distance of 10,000 km is 14–19 USD/GJ for liquid hydrogen, while for ammonia, it is significantly lower at 2–3 USD/GJ [4]. Moreover, ammonia demonstrates a considerable advantage in terms of carrying hydrogen per unit volume compared to the hydrogen storage systems. Specifically, 1 mol of ammonia contains 1.5 mol of hydrogen so that 108 kg of hydrogen is embedded in 1 m3 liquid ammonia at 20°C. In contrast, cutting-edge hydrogen storage technologies such as metal hydrides can typically store hydrogen up to 25 kg/m³ [26].
Ammonia has a lower, but not negligible, explosion risk compared to other fuels such as hydrogen. The narrow flammability range of ammonia is from 15 to 28 vol% mixture in air, while this figure for methane and hydrogen is 5.3%–17% and 4%–75%, respectively [27, 28]. Moreover, ammonia requires a minimum ignition energy of 8 mJ, which is 30 times greater than methane and 470 times greater than hydrogen so it burns with difficulty in open air and will generally need a sustaining flame to keep burning [28]. In many transportation aspects, liquid ammonia is similar to liquid propane but with the advantage of being highly resistant to autoignition so that its autoignition temperature is high at 651°C compared to hydrogen and propane at 585 and 470°C, respectively [29, 30].
3. Ammonia as a Hydrogen Carrier and Fuel: Challenges
Despite the numerous advantages provided by ammonia usage, there are certain limitations especially related to net ammonia combustion as a fuel. Considering apparent toxicity as the ratio of vapor pressure to immediately dangerous to life or health (IDHL) concentration at room temperature, the apparent toxicity of liquid ammonia is 2.7 × 104, approximately three orders of magnitude higher than that of gasoline [31]. This underscores the significance of adhering to the National Institute for Occupational Safety and Health in the USA (NIOSH) recommended exposure limit (REL) of 25 ppm averaged over an 8-hr and a short-term exposure limit (STEL) of 35 ppm during any 15-min period in the day [32]. Moreover, implementing proper safety measures such as ventilation systems, gas detectors, and personal protective equipment for workers can help mitigate the risks associated with ammonia exposure.
Despite the potential negative effect of ammonia on aquatic environments, using it as a fuel appears to pose minimal risks. The significant difference between the temperature of water and the normal saturation temperature of ammonia causes that after spillage, a notable portion of the ammonia evaporates without polluting the water. Moreover, the ratio of the amount of ammonia used as fuel to the amount of surrounding water is so small that the concentration should not exceed the permissible values, which are 3–6 mg/L for short-term exposure and 0.3–2 mg/L for long-term exposure [33]. Implementing rigorous inspection and maintenance programs, along with g proper environmental management practices, such as spill prevention and response protocols, can help minimize the environmental impact of ammonia storage operations.
Combustion properties of alternative fuels are of vital importance since combustion serves as the primary process for power generation in different industries. So, because of its many technological advantages, it is expected to remain a major player for most of the twenty-first century [34]. Regarding ammonia combustion properties, one of the disadvantages could be its relatively low LHV which is 18.8 MJ/kg, but this is largely compensated by the low air/fuel ratio at stoichiometry (6.1 wt.%) compared to about 14.3 wt.% for diesel [35]. This implies that the energy content per unit mass of a stoichiometric mixture is just 7% lower than that of an equivalent diesel/air mixture [36]. Laminar burning velocity is one of the most important parameters for premixed flames [31]. Ammonia has a low laminar flame speed (SL = 15 cm/s at atmospheric conditions) compared to other fuels such as diesel at about 87 cm/s [35]. Low SL is associated with low burning efficiency in engines. To tackle such a drawback, flame enhancement by fuel addition is of interest with the aim of improving the SL, for example, methane, gasoline, or hydrogen addition [37, 38]. Considering the abovementioned properties of ammonia, Table 1 shows the comparison of ammonia properties with some other common fuels.
| Parameter | Ammonia (liquid) | Hydrogen (liquid) | Diesel | CNG |
|---|---|---|---|---|
| Molecular formula | NH3 | H2 | C10–22 hydrocarbons | CH4 |
| Density (kg/m3) | 602.8 | 71.1 | 825 | 187 |
| LHV (MJ/kg) | 18.8 | 119.6 | 42.5 | 38.1 |
| Volumetric energy density (MJ/L) | 11.33 | 8.54 | 36.05 | 7.13 |
| Molecular weight | 17.03 | 2.016 | 180–200 | 16.04 |
| Stoichiometric air/fuel ratio (kg/kg) | 6.14 | 34.3 | 14.4 | 17.2 |
| Flammability limit (vol%) | 16–25 | 4–75 | 0.6–5.5 | 5–15 |
| Laminar flame speed (cm/s) | 15 | 269 | 87 | 38 |
| Autoignition temperature (°C) | 650 | 585 | 230 | 540 |
Although ammonia combustion includes no SOx, CO2, or particulate emissions, its combustion leads to three other emissions that relate to safety, health, and climate: NH3 slip, NOx, and N2O [41]. While the emission of NOx is one of the drawbacks of ammonia use in internal combustion engines (ICE), selective catalytic reduction (SCR) can effectively mitigate this issue by converting NOx to nitrogen and water, with conversion efficiencies of up to 99% [38]. Moreover, this drawback can be addressed by adding oxygenated additives along with pilot fuels such as diesel [42]. N2O is a potential by-product of both ammonia combustion and after-treatment technologies, such as three-way catalysts (TWC) and SCR. It is also part of the combustion emissions of conventional engines such as diesel engines with emission levels around 0.03 g/kWhr [41]. The global warming potential of N2O over a 100-year period is 265 times higher than that of CO2, which is even higher than GWP of CH4 at 28 [43]. As expressed in the previous sections, the narrow combustion range of ammonia and the high autoignition make it resistant to ignite. So, when ammonia serves as a fuel in combustion, its chemical reaction rate is slower than conventional fuels. This causes ammonia to be emitted from the exhaust without burning (NH3 slip) [27]. Therefore, one of the most practical ways to enhance the chemical reaction rate of ammonia combustion, reduce NH3 slip and N2O is to use the promoters, especially hydrogen, in ammonia–air mixture.
4. The Importance of Hydrogen-Rich Gas
Considering the advantages of ammonia as a hydrogen carrier and due to the poor combustion performance of ammonia fuel, it is proposed to carry hydrogen in the ammonia-bound form and, immediately before use, decompose ammonia for producing H2-rich fuel which leads to a simpler fuel storage system and reducing the need for additional modifications in different applications, such as engines and turbines [44, 45]. Since hydrogen has an inherently higher adiabatic flame temperature (2,110°C) than that of ammonia (1,800°C), small amount of hydrogen could be used to promote ammonia combustion. Moreover, combusting together with ammonia, hydrogen can improve the laminar flame speed by a factor of 10 with a hydrogen percentage of as low as 30% [46]. The combustion of hydrogen leads to various radicals, including H, OH, and O, which facilitate the ammonia autoignition process. Thus, the combustion in dual fuel mode using ammonia–hydrogen are interactional due to the sharing of such radicals [47].
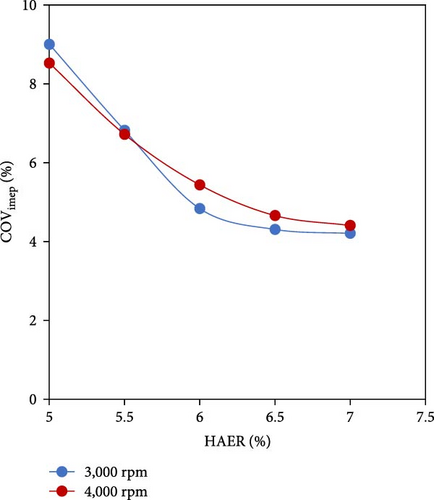
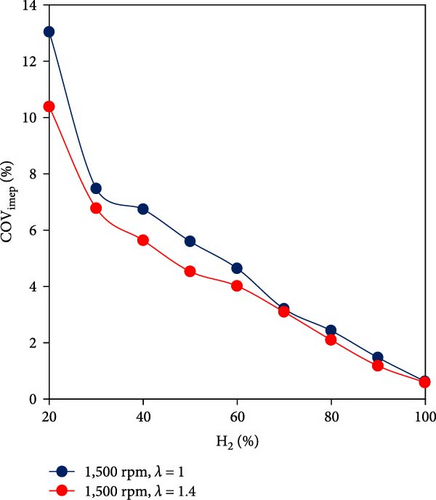
To investigate the benefits of using H2-rich gas, several research scholars explored the application of hydrogen as a fuel activator and combustion promoter when mixed with ammonia. Considering coefficient of variance (COV) of the indicated mean effective pressure (IMEP) as the criteria showing the consistency of the combustion process in different cycles, Frigo and Gentili [48] explored the combustion performance of ammonia–hydrogen fuel mixtures on a four-stroke spark ignition engine (SI) and concluded that it is necessary to add hydrogen to an air–ammonia mixture for improving ignition and increasing combustion velocity. The minimum hydrogen-to-ammonia energy ratio (HAER) to guarantee an acceptable engine behavior was about 7% at full load (Figure 4(a)). In a similar study, Xin et al. [49] showed that for a Miller-cycle SI engine, when the volumetric percentage of hydrogen decreased below 20%, the engine operation misfires (Figure 4(b)).
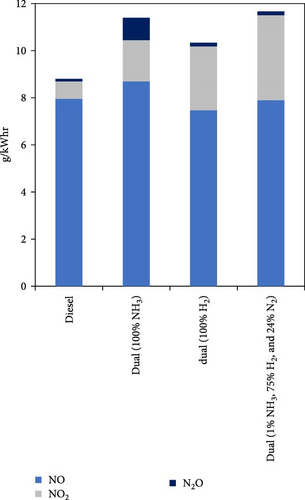
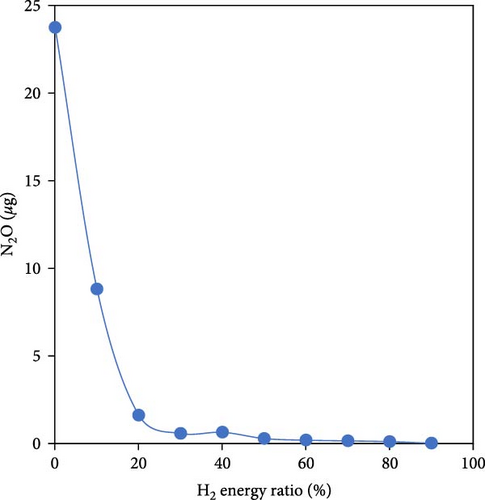
As well as the importance of reducing CO2 emissions by utilizing H2-rich gas, the emission triangle related to ammonia combustion (NOx, NH3, and N2O) needs to be addressed as part of developing ammonia pretreatment (such as using hydrogen–ammonia blend), dual-fuel engines, and after-treatment emission management technologies. Gill et al. [36] investigated cofueling a diesel engine in three different modes including only NH3, dissociated NH3 (H2-rich fuel) and pure H2 as the second fuel. They showed that substituting only 3% of the air intake with H2-rich gas led to a 15% reduction in diesel consumption and CO2 emissions. They also concluded that dissociated NH3 had similar emissions benefits to pure hydrogen so N2O decreased significantly (Figure 5). Yapicioglu and Dincer [50] also investigated the potential utilization of ammonia with combustion promoters in a SI engine and revealed that using H2-rich gas led to a reduction in harmful emissions, and the power output increased as the proportion of hydrogen was raised. Wang et al. [40] studied the effect of varying H2/NH3 ratio (10%–90% energy fraction) on different parameters of a single-cylinder diesel engine at constant speed. The results demonstrated that when the hydrogen blending ratio reached 30% compared to 0, the unburned NH3 was eliminated and N2O significantly decreased by 97% (Figure 5). To investigate the effect of using H2/NH3 blend on maximum temperature and NOx emissions, Dinesh et al. [51] conducted an experimental study on a single-cylinder four-stroke SI engine at two speeds of 1,400 and 1,800 rpm. They concluded that as the hydrogen content increased, the NOx formation due to ammonia decreased but the NOx formation related to peak temperature increased, especially at 1,800 rpm.
5. Thermal Ammonia Decomposition
Given the mentioned advantages of H2-rich gas as a fuel, the significance of ammonia decomposition becomes clear. Ammonia decomposition can generate a COx and sulfur-free stream of hydrogen, and unconverted ammonia can also be recovered in a one-step adsorption [52]. This is one of the most important advantages of ammonia decomposition in comparison with other hydrogen carriers such as light hydrocarbons (methane, ethane, or ethanol). Hydrocarbons decomposition systems must involve a series of purification steps, such as desulfurization and preferential oxidation to decrease COx levels in the outlet gas [53]. Even in the case of hydrogen purification, no COx byproducts are produced during the conversion of ammonia into hydrogen. In applications such as ships, separated nitrogen can be used as refrigerants to treat the reliquefication of boil-off gas [54]. Moreover, in this way, flexibility for using ammonia as hydrogen source after dissociation or directly as a fuel can be achieved.
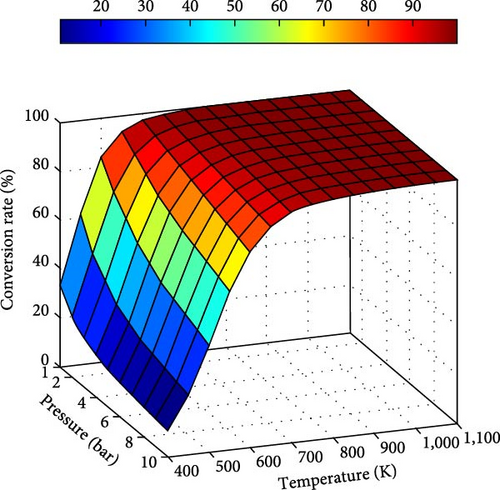
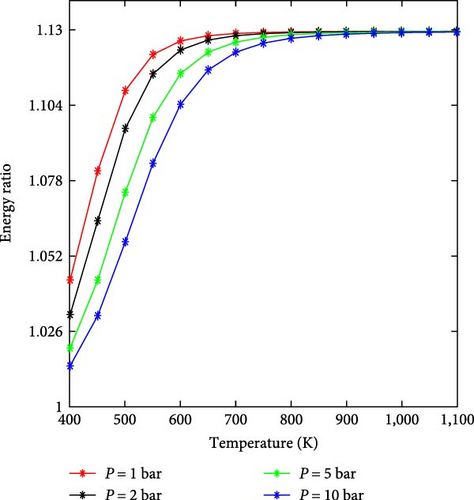
Practically, to produce hydrogen with very high purity and ensure the completion of the ammonia decomposition reaction, it is necessary to operate at high temperatures. This is especially necessary when the produced hydrogen is used for a fuel cell because it deteriorates irreversibly at low concentrations of ammonia [59]. To reduce the amount of energy required due to the high-temperature demands, research is being conducted to develop suitable catalysts that enable efficient operation at lower temperatures [55].
Figure 7(a) shows that the mechanism considered for the decomposition of ammonia involves a series of steps commencing with the adsorption of NH3 molecules onto the catalyst surface (NH3 ∗ generation), followed by dehydrogenation processes (N∗ and H∗ generation), and ultimately, the dissociation of hydrogen and nitrogen atoms into gaseous H2 and N2 [61]. Although the reaction is reversible, the ideal catalyst for the synthesis of ammonia differs from the ideal one needed for the ammonia decomposition [62]. For the ammonia decomposition, a catalyst capable of actively facilitating the recombinative desorption of nitrogen adatoms is necessary, while iron-based ammonia synthesis catalysts tend to strongly bind nitrogen adatoms, leading to surface saturation and subsequent poisoning at low temperatures [63]. Therefore, ammonia decomposition reaction is commonly catalyzed by noble metals (e.g., ruthenium, palladium, and rhodium), non-noble metals (e.g., nickel and iron), carbides and nitrides, bimetals and poly metals, metal amides and imide, etc. [61, 64].
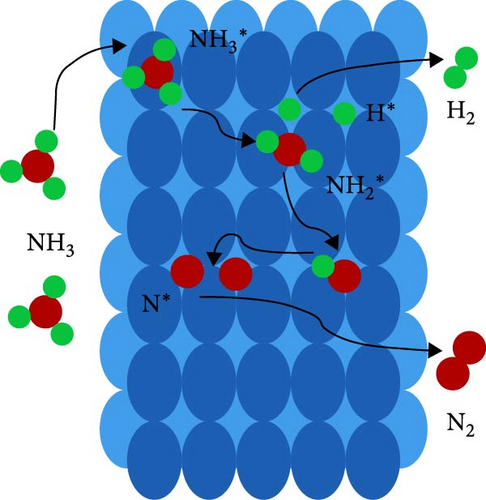
The recent review on ammonia decomposition catalysts by Ristig et al. [65] illustrates that the focus of 75% of the past research was on monometallic catalysts such as Ru and Ni so that Ru-based catalyst exhibits the highest activity at low temperatures, while Ni catalysts offer a more cost-effective option with lower conversion rates. The reactivity comparison for different transition metals is usually in the decreasing order of Ru > Ir > Rh > Ni > Pt > Pd > Fe, as reported by several researchers [11, 66, 67]. The effectiveness of ammonia decomposition relies not just on the catalytic phase but also on its supporting material. For instance, when using Ru catalysts, common supports include carbon nanotubes (CNTs), MgO, and Al2O3 [67]. Although a lot of studies have been reported in the field of different catalysts for ammonia decomposition, Table 2 summarizes the performance of some of the catalytic systems for ammonia decomposition based on sample studies on Ru, Ni, and Fe to emphasize the effect of the main parameters such as the type of catalyst, loading percentage, temperature, and flow rate on the conversion rate. In order to present a proper comparison, temperatures have been limited to 400–500°C.
| Catalysts | Supports | Loading (wt.%) | Reaction temperature (°C) | WHSV (mL/ghr) | Conversion rate (%) | References |
|---|---|---|---|---|---|---|
| Ru | Al2O3 | 2 | 400 | 13,800 | 37 | [55] |
| Ru | Al2O3 | 8.5 | 400 | 1,200 | 99 | [68] |
| Ru | CNTs | 7 | 450 | 5,200 | 50 | [69] |
| Ru | CNTs | 5 | 500 | 30,000 | 100 | [70] |
| Ru | MgO | 2.8 | 450 | 30,000 | 57 | [71] |
| Ru | MgO | 3.5 | 450 | 36,000 | 87 | [72] |
| Ni | Al2O3 | 20 | 500 | 7,500 | 28 | [73] |
| Ni | Al2O3 | 20 | 500 | 2,400 | 31 | [74] |
| Ni | MgO | 10 | 500 | 6,000 | 22 | [75] |
| Ni | MgO | 15 | 500 | 3,000 | 59 | [76] |
| Fe | CNTs | 5 | 500 | 6,000 | 15 | [77] |
| Fe | Al2O3 | 10 | 450 | 12,000 | 24 | [78] |
Regarding the decomposition reactors, mass and heat transfer are key criteria for the future industrial applications of ammonia decomposition. As well as fixed-bed reactors and microreactors, structured catalytic reactors coated with catalyst and membrane reactors are investigated for the decomposition of ammonia [79, 80]. Fixed-bed reactor as a well-established technology usually leads to the large pressure drop, temperature gradient and inconsistent flow, although can be optimized to provide proper volume, weight, and catalyst loading [81]. Microreactors are suitable for rapid catalytic reactions and widely used in laboratory scales, but the scale-up of such sub-millimeter-sized device to industrial scales is extremely difficult and leads to high-pressure drops [70]. Membrane reactors, which combine reaction, separation, and purification into a whole reactor unit, increase the conversion rate of ammonia by shifting the reaction equilibrium to the product side and can operate at lower temperatures than conventional systems. But, limited choices of membrane materials and catalyst compatibility as well as higher pressure drops are some of the challenges of such reactors [80, 82]. Structured reactors contain channels in a single block of inert or catalytic material. The main advantages of these reactors are low-pressure drop, proper scale-up, high surface area-to-volume ratio, enhanced mass transfer, and low weight [83]. Plana et al. [84] showed that by using a honeycomb cordierite reactor and a Ni/Al2O3 catalyst, higher ammonia conversion rates were obtained compared to the same catalyst but in a fixed bed reactor due to better heat distribution and mass transfer. Some of the challenges of structured reactors are difficult uniform loading of the catalyst onto the reactor structure and fouling over [83].
In some applications such as fuel cells and hydrogen production plants, the H2-rich gas at the outlet of the decomposition reactor should be separated and purified. For example, proton exchange membrane (PEM) fuel cells require a residual NH3 concentration less than 100 part per billion (ppb) [70]. Therefore, different methods of hydrogen separation and purification technologies can be utilized, one of which is the ammonia absorption by using some solid materials like MgCl2 and CaCl2, or acids such as H3PO4 which has been shown to be capable of 53 wt.% absorption capacity [70, 85]. Ammonia also can be dissolved in water to about 3.1 gNH3/g, which can be further increased by the addition of an acid such as H2SO4 [86]. Another technology is the cryogenic distillation which is costly due to working at high pressures and low temperatures with the maximum potential purity level of 99% of H2 [87]. Membrane separation based on various materials such as palladium, nickle alloys, and ceramics have been also developed for producing ultrapure H2 [86]. Pressure swing adsorption (PSA), based on the materials like zeolites, activated carbon, and silica compounds, is another important technique for generating ultrapure hydrogen larger than 99.999%, which can be used for both large industrial scales and small portable systems [88].
6. Ammonia Decomposition and Waste Heat Recovery
Approximately 20%–50% of the energy input in various industries, including furnaces, kilns, ovens, power plants, and ICEs, can be considered as the sources of waste heat recovery [89]. Various WHR technologies, especially heat exchangers, have been proposed to reduce energy losses and regenerate power by utilizing the available thermal energy from such sources. The recuperated waste energy can be used for fuel pretreatment, electrical power generation, preheating combustion air, or in a combined cooling, heating, and power system. The method and application of WHR technology largely depend on the temperature so the higher the gradient or absolute temperature of WHR, the better its quality, thereby enhancing the effectiveness of the process. WHR for high-temperature applications involves temperatures over 650°C. However, the intermediate temperatures range from 232 to 650°C and temperatures below 232°C are for the low-temperature range [90]. Table 3 shows temperature ranges of some of the industrial waste heat sources.
| Waste heat source | Temperature range (°C) |
|---|---|
| Jacket cooling water | 80–95 |
| Turbocharged ICE | 230–370 |
| ICE | 315–600 |
| Gas turbine exhausts | 370–540 |
| Catalytic crackers | 425–650 |
| Kiln system exhaust/preheater | 382–816 |
| Cooper refining furnace | 760–820 |
| Aluminum reverberatory furnace | 1,100–1,200 |
| Iron and steel making | 1,450–1,550 |
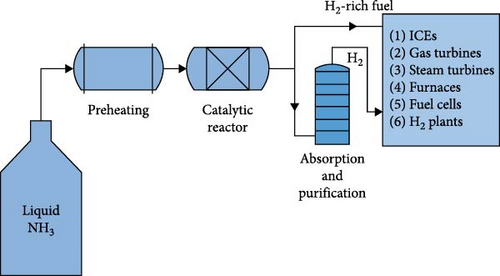
The use of WHR from flue gases or other sources is an important option for the on-board hydrogen or H2-rich gas production to carry out the catalytic decomposition or supply some of the heating energy needed for the process, especially preheating and gasification of ammonia. Figure 7(b) shows a schematic of some of the important applications which can be integrated by thermal ammonia decomposition systems. Therefore, different WHR-based ammonia decomposers for various applications have been investigated by researchers which can be categorized into two main groups including the systems based on thermochemical recuperation (TCR), in which the required energy for the reaction is supplied by waste heat only, and the systems based on autothermal reforming (ATR) in which transformation of wasted heat as well as partial oxidation are utilized for supplying the required energy.
7. WHR-Based NH3 Decomposition Systems Using TCR
TCR involves the integration of a recuperative heat exchanger within a thermochemical cycle to improve its overall energy efficiency. It is commonly used in power generation systems, such as gas turbines and internal combustion engines. The main concept of TCR is transformation of rejected heat from exhaust gases into chemical energy to produce a new synthetic fuel containing molecular hydrogen that has higher calorific properties. This new synthetic fuel produced in the reactor is used in the combustion chamber of fuel-consuming equipment to reduce specific consumption of the primary fuel [93, 94]. Different fuels can be used for TCR as reported in many publications on fossil-fuel-based TCR including natural gas pretreatment for gas turbine power plants and industrial furnaces [95, 96], reforming of liquid hydrocarbons such as alcohols and gasoline in internal combustion engines [97, 98], as well as TCR by coal gasification [99]. For example, Poran and Tartakovsky [100] showed that TCR based on methane can lead to a substantial rise in energy efficiency (up to 25%) for the industrial furnaces and demonstrated that for a spark-ignition engine, using TCR based on methanol led to at least 18% increase in the indicated efficiency and 73% reduction in NOx compared to gasoline.
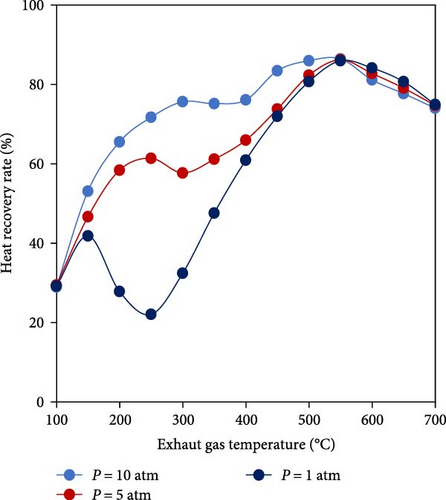
Since the decomposition of ammonia is an endothermic reaction and the produced H2-rich gas can be utilized in different applications such as furnaces, gas turbines, steam turbines, and internal combustion engines as well as the fact that in all of the mentioned systems, waste heat with different temperatures is available, using TCR for ammonia decomposition emerges as a highly effective solution to enhance energy efficiency and reduce environmental pollutants. Considering the application of TCR for industrial furnaces, Pashchenko and Mustafin [101] carried out the thermodynamic analysis of an ideal TCR system for ammonia decomposition using Aspen HYSYS, with and without the condensation of steam in the combustion products. It was found that a TCR system, by using exhaust gas of a typical fuel consumer had a maximum efficiency of about 80% in a temperature range above 500°C so that at temperatures higher than 550°C, pressure did not significantly affect the efficiency of the system and the maximum heat recovery rate was in the temperature range of 500–550°C (Figure 8). At low pressures, a heat deficit appeared in the TCR system and to compensate for the highest heat deficit at 300°C and 1 bar, approximately 13% of water from exhaust gas needed to be condensed.
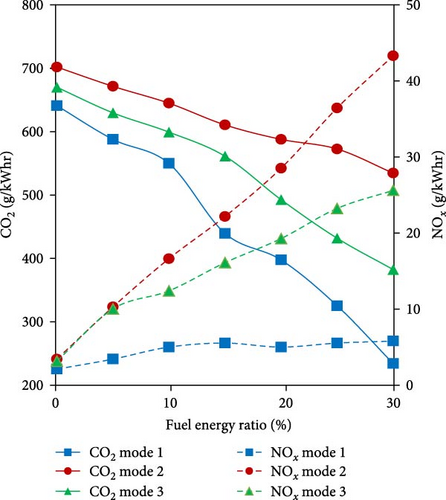
There are some studies available in the literature using WHR and ammonia decomposition in vehicular dual-fuel engines. Kane et al. [102] developed a TCR with the objective of improving a dual-fuel truck diesel engine including the diesel oxidation catalyst. The TCR utilized both exhaust waste heat and unburned diesel to supply the heat for the ammonia decomposition catalytic reactor which included ruthenium on an alumina support. The engine was operated in dual fuel mode with up to 40% replacement of diesel energy considering different loads and speeds. They concluded that the performance of the WHR-based reactor was significantly dependent on the engine operation conditions as well as ammonia flow rate. Increasing diesel substitution by H2-rich gas increased exhaust temperature across all operation modes due to the recovered sensible energy from the fumigant. Higher ammonia flow rates increased heat exchange coefficients within the reactor, but also decreased residence times and increased the rate at which sensible energy was removed from the reactor. This made the reactor more efficient with lower ammonia-to-diesel ratios and higher temperatures. Top of FormBrake thermal efficiency was also improved by up to 5.0% using TCR for the optimum operation mode (low speed, high load) since the exhaust gas temperature was not sufficient for low loads and high speeds. In another study using the same TCR system and different modes of operation, Kane and Northrop [103] conducted a technical and environmental analysis. They showed that overall engine thermal efficiency increased for modes with a high exhaust temperature where ammonia decomposition conversion in the TCR reactor was high, but decreased for all other modes due to poor combustion efficiency. Although CO2 decreased up to 63% due to the combination of WHR and diesel replacement, NOx and N2O were found to increase due to recovering heat by unburned ammonia oxidation over the diesel oxidation catalyst (Figure 9).
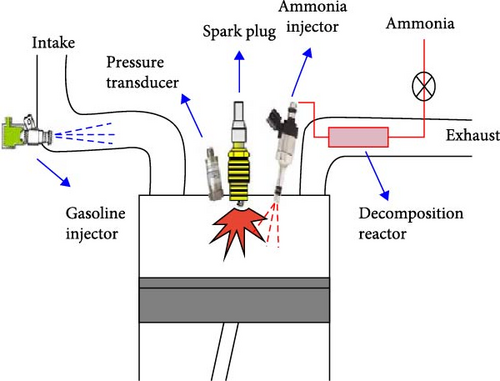
In order to investigate the effect of load and speed of the engine on the performance of a WHR-based ammonia decomposition system, Comotti and Frigo [37] developed an onboard hydrogen generation system based on the fixed-bed reactor and ruthenium catalyst to be coupled to a small vehicular SI engine (21 kW). The findings indicated that the minimum required hydrogen and exhaust gas temperature was more affected by engine speed rather than the load. Efficiency of the cofueled engine was about 28% and comparable with that obtained with the original gasoline version, especially in the leaner mixtures. Moreover, due to the changes of the exhaust gas temperature in different loads, rpm, and fuel/air ratios of the engine, they showed that the required temperature for proper catalytic reaction could not be supplied by exhaust gas in all modes, which was more important during the cold start of the engine.
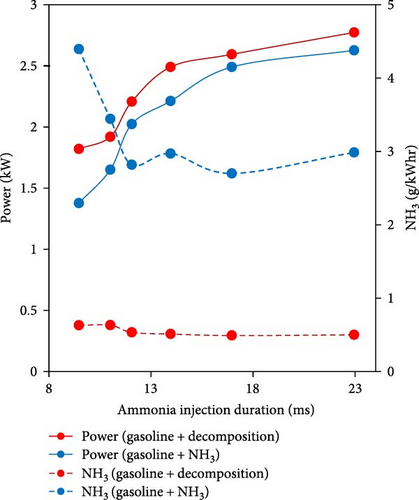
In another study and to compare with ammonia operation mode, the effect of cofueling a gasoline vehicular engine by H2-rich gas (using WHR and ammonia decomposition) investigated by Ryu et al. [104]. They assessed the performance of a single-cylinder dual-fuel SI engine running at constant speed (1,800 rpm) and the WHR-based ammonia dissociation catalytic reactor (Figure 10(a)). The catalyst coated with 2% ruthenium on 3.2 mm alumina pellets operated at higher conversion rates at low load conditions due to the lower ammonia flow rates (higher residence time). The operation with dissociated ammonia using direct heat energy from exhaust gases resulted in about 19% higher power output and decreased fuel consumption due to the existence of hydrogen, which elevated the in-cylinder combustion. Also, NH3 emissions were reduced by about 80% considerably for the operation with H2-rich gas (Figure 10(b)). However, despite the reduction in fuel-NOx emissions, thermochemical decomposition led to elevated thermal NOx levels due to the higher concentration of nitrogen from ammonia decomposition converting to NOx at the prevailing higher burning temperature with more hydrogen.
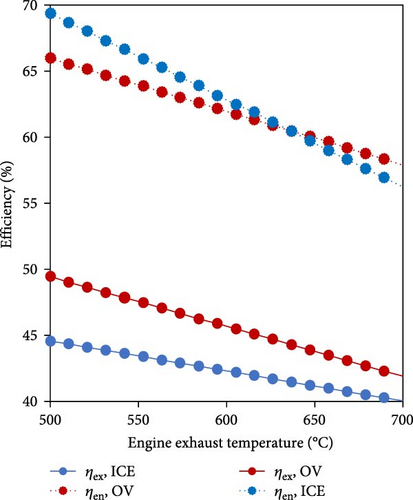
To achieve the maximum utilization and higher overall energy and exergy efficiencies, Siddiqui and Dincer [105] developed and thermodynamically investigated WHR in a novel ammonia-based integrated system including ICE, fuel cell, and ammonia decomposition to produce power and cooling. The proposed system provided an effective means to improve the current ammonia-based power generation systems so that the overall energy and exergy efficiencies of the system were determined to be 60% and 52%, respectively. Moreover, increasing the exhaust gas temperature of the ICE resulted in a decrease in both the overall system energy and exergy efficiencies, primarily due to the diminished work output of the ICE (Figure 11).
The usage of H2-rich gas is promising not only in ICEs but also in gas turbines with the possibility of using existing power plants after minor modifications [106]. Kumar and Meyer [107] numerically showed that for the blends of ammonia–hydrogen with the ammonia energy fraction below 20%, the flame speed could be the same as for methane combustion. Moreover, Medina et al. [108] demonstrated that the combustion of an ammonia–hydrogen mixture with 30 vol% of hydrogen was stable and NOx emissions for an air/fuel equivalence ratio above 1.3 were at an appropriate level below 10 ppm.
In order to further reduce CO2 emissions and increase overall efficiency, such gas turbine-based power plants can be equipped with precombustion decarbonization of fuel based on the concept of using ammonia decomposition and waste heat recovery [109]. Therefore, as well as TCR systems, other additional recuperation strategies are proposed to fully exploit the waste heat, especially in the field of gas turbines. Su et al. [110] theoretically developed an innovative power cycle including partial ammonia decomposition, ammonia vaporizer, and a 200 kW microturbine so that waste thermal energy from the gas turbine was used to preheat the air entering the combustor and drive the ammonia decomposition process (Figure 12). The results indicated that in the same electric power output condition and considering high-temperature flue gas (500°C), 34.5 kW heat was recovered for the ammonia decomposition process so that hydrogen molar ratio reached 40% for the novel design. Due to the waste heat recovery, the combustion efficiency and thermal efficiency of the novel cycle reached 94.8% and 36%, respectively, which were higher than that of the reference system (based on pure ammonia) at 81% and 31.8%.
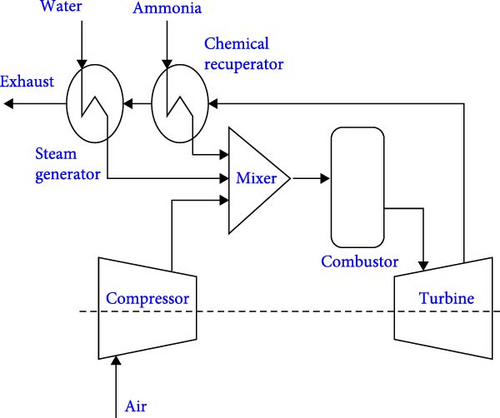
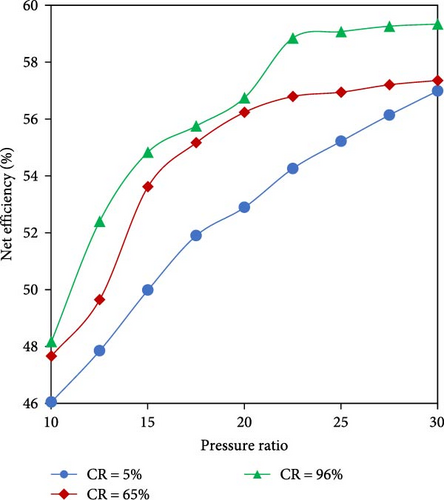
Among the potential heat-recovery systems, humidified gas turbine (HGT) cycle also is recognized as one of the practical processes due to its superior effectiveness in improving the efficiency and reducing the emissions based on the injection of water or steam into the combustion process of the gas turbine leading to an increase in mass flow rate [111]. A challenge associated with this cycle is that the steam injection leads to a reduction of flame speed and reactivity, which makes the combustion with an alternative fuel such as ammonia even harder to sustain. Therefore, a promising solution is using ammonia decomposition to obtain H2-rich fuel, leading to a higher reactivity [112]. Shen et al. [113] proposed a new chemical recuperated and humidified gas turbine cycle (CHGT) concept to optimize the WHR in electricity generation so that the heat from the turbine exhaust gas was recovered in the chemical equilibrium reactor and the steam generator successively (Figure 13(a)). All process components were simulated using Aspen HYSYS and the results revealed that the maximum efficiency of the CHGT cycle increased compared to the simple ammonia-fueled Brayton cycle by 20.6%, corresponding to a fuel saving of 17% which highlighted the significant benefits of the WHR and H2-rich fuel integration. Moreover, the ammonia decomposition from 5% to 96% led to the increase of the net electrical efficiency up to 4.8% which was affected by pressure ratio as well (Figure 13(b)).
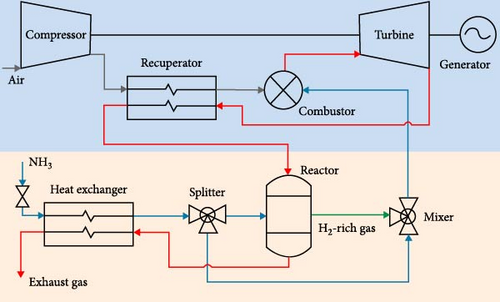
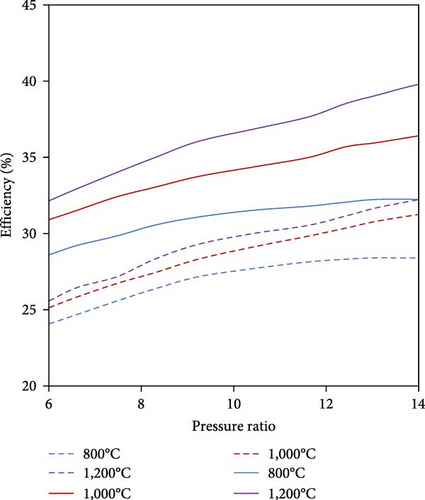
Assuming an ideal Gibbs reactor and neglecting chemical kinetics, the effect of catalyst, and residence time, Pashchenko et al. [114] thermodynamically analyzed WHR from the gas turbine combined with a thermochemical recuperation (CRGT) for ammonia decomposition using Aspen HYSYS. The exhaust gases from the gas turbine (GT) were passed through the ammonia reactor and the regasifier. They concluded that to produce H2-rich gas and to use it in the combustion chamber, up to 43% of the wasted energy was recovered by the thermochemical exhaust heat recuperation system, leading to 3.5%–7.5% increase in gas turbine efficiency, especially in the turbine inlet temperature range above 800°C (Figure 14). Moreover, the temperature of the exhaust gases after the TCR system still had a potential for the addition of other heat recovery systems such as a steam injection.

In another study in the field of power generation integrated with ammonia, Malenkov et al. [115] conducted simulation of a large-scale ammonia decomposition unit with a heat recovery steam-turbine cycle using Aspen Plus and considering Ni/Al2O3 as the catalyst to be exposed to an ammonia flow rate of 16 kg/s. The maximum efficiency of the ammonia decomposition system was attained when the least amount of heat was provided to the heat recovery steam turbine cycle. This aligned with the highest temperature difference of the heated gas within the ammonia decomposition zone. With an inlet temperature of 1,100 °C to the decomposition zone, it was feasible to reach an efficiency of the hydrogen separation from ammonia of about 70%. According to the abovementioned studies, it is worth mentioning that most of the investigations in the field of ammonia decomposition integrated with gas turbines are numerical modeling and simulation since ammonia-fired gas turbines are still relatively new technology and there is limited experimental data on their performance compared to conventional gas turbines, especially for real-scale power plants.
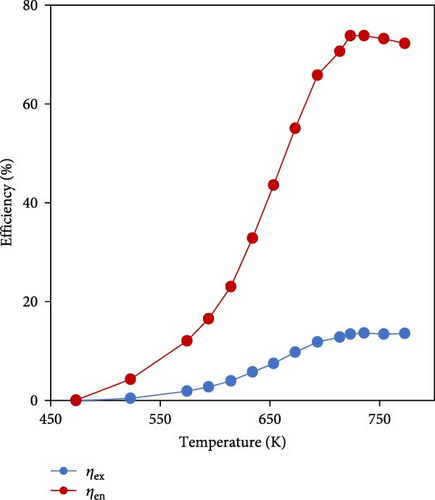
Many large-scale industries, such as the iron, steel, or glass, release waste heat during manufacturing processes, which can be used to meet energy and climate goals for a low-carbon economy. Although there are very limited studies in the field of WHR-based ammonia decomposition integrated with furnaces, one of the most important applications of ammonia fuel could be the integration with the recovery of the heat from exhaust gases in the furnace stacks. El-Shafie et al. [116] conducted energy and exergy analyses on a glass furnace regenerator and hydrogen production through thermal catalytic ammonia decomposition. Considering a constant flow rate of ammonia and using a ruthenium catalyst in a cylindrical-type reactor, it was found that the energy and exergy efficiencies increased as the heating temperature raised at lower pressures so that the maximum energy and exergy efficiencies of the system were 73.5% and 13.3%, respectively, at a feeding pressure of 100 kPa for the catalytic thermal decomposition (Figure 15).
Due to the presence of unreacted NH3 and N2 at the outlet of the thermal ammonia reactors, the combination of the WHR, ammonia decomposition, and hydrogen purification is another important aspect in the field of alternative fuels production and utilization. Using Aspen Plus, Devkota et al. [117] performed process development of a large-scale reactor with a single or multicatalytic packed beds for ammonia decomposition including intermediate heating, temperature swing adsorption (TSA) beds and pressure swing adsorbers (PSA) for purification of hydrogen. The waste heat from both the product and flue gas streams was recovered for the burned air–fuel mixture and the required regeneration energy for TSA. They concluded that the single-bed reactor had a maximum conversion rate of nearly 35%, while the combination of multibed reactor in series, with intermediate heating and WHR reached an almost equilibrium conversion of 97%. Moreover, preheating of the process fed with ammonia and an air–fuel mixture using WHR from exhaust flue gas increased the fuel saving by about 22% and improved the product yield by about 7%.
8. WHR-Based NH3 Decomposition Systems Using ATR
Wang et al. [45] studied ammonia ATR to produce H2-rich gas for a single cylinder, naturally aspirated dual-fuel diesel engine operating at a constant load and speed indicating that ammonia conversion rate was significantly dependent on the O2/NH3 ratio, and the temperature decreased in the reactor along the catalyst bed due to the presence of CO2 and H2 in the exhaust gases. The conversion rate of the studies reactor was about 40% and 56% at 500 and 600°C, respectively. Moreover, they showed that when the produced H2-rich gas by WHR supplied 5% with the primary diesel fuel, carbon emissions were reduced effectively, while NO2/NO ratio increased.
To compare WHR-based ammonia decomposition either by only utilizing the exhaust heat of a gasoline direct-injection engine or by reaction with part of the exhaust, Sittichompoo et al. [57] investigated on-board H2-rich gas production by using a rhodium–platinum catalyst. Rhodium exhibits superior selectivity in promoting ammonia decomposition, while platinum demonstrates greater effectiveness in catalyzing ammonia oxidation. They found that H2-rich gas from the exhaust heat-driven recovery contains up to 15% more energy than the reactant ammonia, and almost complete decomposition of ammonia was achieved at 550°C so that only about 25% of available heat energy was required for ammonia decomposition. Moreover, ammonia reforming with exhaust gas species led to an enhancement of up to 32% in fuel saving and reduction in CO2 emissions, which was only 2% more than that of heat-driven recovery.
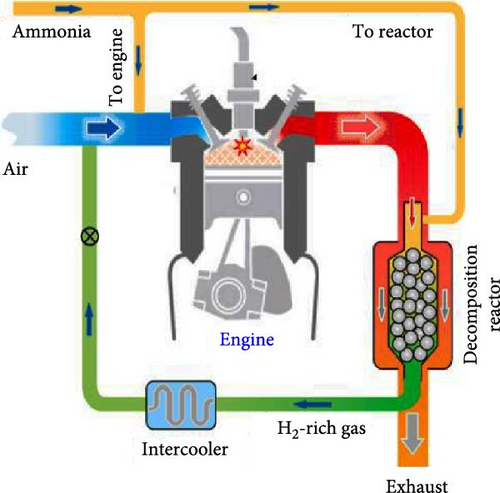
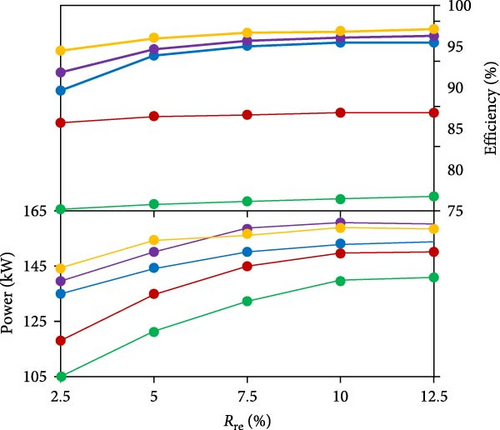
In order to investigate the effect of using ATR on the technical and environmental performance of a SI engine, Zhang et al. [44] proposed a reformed exhaust gas-fuel recirculation system (REGR) and numerically investigated H2-rich gas addition, under the assumption of complete ammonia consumption in the reactor and removal of steam via an intercooler (Figure 16(a)). By defining the reformate blending ratio (Rre) as the ratio of the volume of reformate added per cycle to the total volume of intake mixture, they revealed that combustion efficiency and engine power were improved by utilizing waste heat for ammonia decomposition at a given air–fuel equivalence ratio (λ) so that the maximum combustion efficiency of about 96% could be attained when Rre reached 12.5% under stoichiometric conditions. Since the flame temperature increased with the increase of Rre, the lowest N2O emissions were observed at richer conditions with high Rre (Figure 16(b)).
Dynamic performance and cold-start emissions have been considered as another aspect associated with the use of ammonia as a fuel in dual-fuel applications integrated with WHR. Koike et al. [120] evaluated the cold start performance of an ammonia fueled four-cylinder spark ignition engine modified to run on H2-rich gas including an ATR unit for ammonia decomposition as well as a three-way catalyst and an ammonia adsorber unit for minimizing NOx and unburned NH3 emissions. It was found that NH3-free emissions were achieved during cold start with less than 10% for the hydrogen fuel ratio. The hydrogen concentration drastically increased with time, reaching 47% at 8 s. Approximately 3 s was required to initiate combustion in the engine and the hydrogen fuel fraction at that point was about 15%, while each segment of the reactor required about 20 s to achieve temperature stability so that the engine could be started due to the presence of hydrogen.
Kim and Kwon [121] experimentally investigated the effects of various operating parameters and catalyst types on the performance of an ammonia reforming system to determine a cost-effective candidate. They considered an annulus-type microreforming system to produce hydrogen, integrated with a heat-recirculating microcombustor, and with heat extracted from exhaust gas for preheating fresh propane–air mixtures. Under optimal conditions, the microreforming system employing a ruthenium–iridium catalyst generated 5.4 W of hydrogen with a conversion rate of 98% and an overall system efficiency of 13.7%. For all the test conditions, the performance for Ru and Ir was almost the same as individually and better than that for Ni. In another similar study by considering the same configuration but based on the H2-rich gas as the fuel for microcombustor and just the catalyst Ru for the reformer, Kim et al. [122] showed that the production rate of hydrogen, the conversion rate of ammonia, the overall efficiency of the microreforming system and the NOx concentration in the exhaust gas from the microcombustor for optimized operating conditions were 5.4 W, 97.0%, 10.4%, and 158 ppm, respectively. The overall system efficiency increased with increasing the fuel inlet velocity until the critical condition since flame was already stabilized in the microcombustor and thus further enhanced velocity resulted in wasting fuel input without increasing hydrogen output.
9. WHR-Based NH3 Decomposition for Hydrogen Production Plants
In addition to the studies focusing on technical and environmental analysis of the WHR-based ammonia decomposition systems, the technoeconomic performance of a hydrogen production system using 300 Nm3/hr ammonia was evaluated by Lin et al. [123], with a focus on the effects of different separation configurations including PSA, membrane separators (polymeric and Pd) and the combination of the two methods. Several heat exchangers were considered to recover the heat from the reactor outlet and the flue gas. By defining specific total cost (STC) as the sum of the specific investment costs and the specific operation costs, it was concluded that ammonia decomposition by WHR at an elevated pressure was preferable to atmospheric pressure considering the reduction of about 4.1% in STC due to the possibility of avoiding an extra compressor for PSA, and the hybrid purification configuration had the lowest STC. Moreover, they demonstrated that the efficiency of a combined system based on the PSA and membrane could reach to about 0.85 with a H2 recovery greater than 95% compared to 0.59% and 66% for PSA-based system.
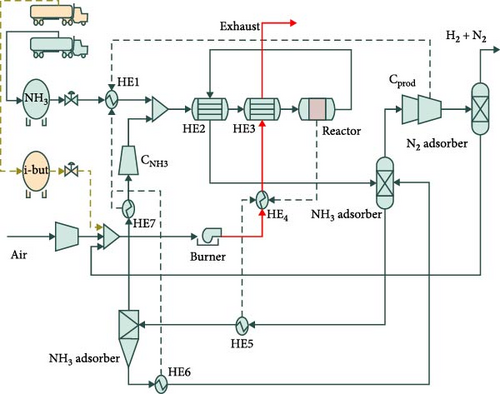
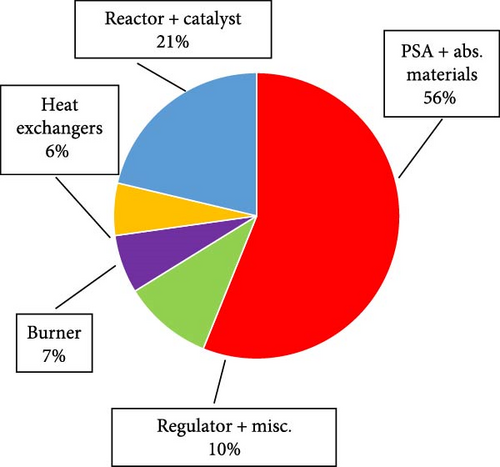
Cha et al. [124] also conducted a technoeconomic analysis of hydrogen production from green ammonia reforming by using a 12-faceted reactor and Ru/La–Al2O3 pellet catalyst equipped with the N2 PSA. Two heat exchangers in series used to preheat the mixture of the feed ammonia and recovered ammonia by utilization of the heat from the burner flue gas and outlet gas of the reactor up to 525°C. The results revealed that the dehydrogenation catalytic activity remained stable over an extended period of approximately 6,700 hr with the ammonia conversion rate of over 99.7% at 550°C. Moreover, to produce pure hydrogen, the most important factor affecting the system capital cost was the PSA, accounting for 56% of total cost. This figure for the reactor with the initial catalyst material and heat exchangers was just 21% and 6%, respectively (Figure 17). In another technoeconomic study in the field of hydrogen production from ammonia decomposition incorporated by combined cycle gas turbines, Richard et. al [125] showed that the Pd-based membrane reactor system outperforms the conventional tubular reactor, with a 30% increase in thermal efficiency, while its capital expenditure reached to about 26 €/MWhr compared to 20.6 €/MWhr for the basis reactor.
10. Conclusions
This paper outlines the significance of on-site ammonia decomposition as a proper mean to extract H2-rich fuel to address the limitations associated with pure ammonia combustion. Furthermore, the main novel emphasis of this review is on the pivotal role of waste heat recovery in thermal ammonia decomposition and the applications of such integration in different industries. Two key decomposition methods including TCR and ATR are investigated so that researchers in different fields can draw insight and benefit from the work of each other. The main findings of this study can be summarized into the following points.
Using appropriate catalyst and reactor, thermal ammonia decomposition has the potential to integrate various energy systems, particularly gas turbines, vehicular engines, maritime engines, stationary engines, and furnaces. These systems reject hot exhaust gases so that the combination of waste heat recovery and using H2-rich gas leads to an enhanced fuel saving and to reduced GHG emissions. Based on the specification of the source of waste heat, adding preheater and regasification units to the ammonia decomposition techniques, play an important role in rising the advantages of such systems. Moreover, technoeconomic analyses showed that the costs associated with the thermal catalytic reactor, even when utilizing precious catalysts like ruthenium, are much lower than the costs related to other equipment such as hydrogen purification.
Several factors affect the performance of WHR-based thermal ammonia decomposition systems, especially exhaust gas temperature and species, ammonia flow rate, the engine operating condition, as well as H2/NH3 ratio. Therefore, various criteria should be considered to achieve an optimum application scenario for the WHR-based ammonia decomposition, ensuring maximum effectiveness and practicality. These criteria include the limitations of the application like volume and weight, scale of the application, the existence of a consistent demand for H2-rich fuel, potential environmental benefits, quality and quantity of the available heat, economic criteria, and the chance of being integrated by other technologies.
Given the points mentioned above, it is imperative to conduct further research encompassing various aspects of integrating waste heat recovery and ammonia decomposition. One such aspect involves performing multicriteria analyses to evaluate the long-term performance of the integrated system, considering factors such as catalyst durability. Additionally, life cycle analyses can provide valuable insights into the overall system performance, considering the usage of green ammonia in different industries. Further optimization studies are also needed to increase the level of independency of the decomposition system for all modes of operation. To do so, integrated design with renewable energies such as solar concentrators could be useful to enhance the performance and safety of the system. Moreover, the usage of ammonia decomposition and waste heat recovery could be more investigated in two significant modes of transportation including ships and trains by considering the integration of various components such as dual-fuel engines, thermal ammonia decomposition, hydrogen purification, and fuel cells.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank Serge Michaud, from Chantier Davie Canada Inc., for his supportive role and collaboration during the research project. The project was financed by Chantier Davie Canada Inc., as Canada’s premier shipbuilder, based on the project in the field of using alternative fuels for marine industry.




