Effectiveness Evaluation of a Graded Pharmaceutical Care Model in Women with Intrahepatic Cholestasis of Pregnancy: A Before-After Study
Abstract
Objective. Intrahepatic cholestasis of pregnancy (ICP) significantly impacts the maternal and fetal safety. Research on the role of clinical pharmacists in guiding drug therapy for this condition remains limited. This study aimed to evaluate the effectiveness of graded pharmaceutical care for women with intrahepatic cholestasis of pregnancy and to provide a theoretical foundation for clinical pharmacist services. Study Design. This study comprises a pre-and-post analysis of women with intrahepatic cholestasis of pregnancy (ICP) treated between December 2019 and June 2023 at a tertiary hospital in Jiangsu province. Each group consisted of 102 participants. The control group received standard treatment, while the guardianship group received graded pharmacological care provided by a clinical pharmacist. The effectiveness of pharmacological monitoring by clinical pharmacists was assessed by comparing and analyzing clinical outcome indicators, quality management indicators, safety indicators, and economic factors. Results. The guardianship group exhibited a noteworthy 12.8% reduction in combined adverse pregnancy outcome and more effective management of total prenatal bile acids compared to the control group (16.05 µmol/L vs. 22.85 µmol/L, P < 0.05). The guardianship group displayed superior rationalization of therapeutic drugs and medication duration (P < 0.05). The cost-benefit analysis revealed a favorable economic impact concerning medication costs but did not indicate economic significance regarding total inpatient costs. Conclusion. The implementation of a graded pharmaceutical care model by a clinical pharmacist holds the potential to enhance outcomes for women experiencing intrahepatic cholestasis during pregnancy, mitigate adverse pregnancy results, optimize the rational utilization of therapeutic medications, and yield positive economic results.
1. Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a prevalent and distinct complication of pregnancy, presenting with clinical signs of pruritus and elevated bile acid levels, often accompanied by abnormal liver function parameters [1]. Pregnant women with ICP are at risk of preterm labor, meconium-stained amniotic fluid, fetal distress, and the possibility of fetal mortality [2]. The pathophysiological mechanisms underlying ICP primarily involve genetics, reproductive hormones, and disease susceptibility [3]. Elevated levels of reproductive hormones could heighten the susceptibility. Risk factors for ICP include advanced maternal age, oral contraceptive use, personal or family history of ICP, multiple pregnancies, high estrogen fertility treatments, hepatitis C, cholelithiasis, and nonalcoholic fatty liver disease (NAFLD) [4, 5]. Currently, there are no established or efficacious interventions [6]. Medications used in drug treatment for ICP include ursodeoxycholic acid (UDCA), polyene phosphatidylcholine, S-adenosyl-L-methionine (SAMe), glutathione, antihistamines, and various herbal remedies [7]. UDCA reduces cholestasis by promoting bile acid excretion and protecting cholangiocytes [8–10], while SAMe has a role in cholestasis reversal although its mechanism of action remains unclear [11].
The concept of pharmaceutical care (PC) was introduced by American academics Hepler and Strand in 1987, aiming to identify and resolve real or potential drug difficulties, prevent future medication problems, and enhance patients’ quality of life [12]. Clinical pharmacists are currently prioritizing research and implementation of medication therapy management and pharmaceutical care in various areas including diabetes, cardiovascular disease, cancer, hemato-oncology, organ transplantation, respiratory disease, and obstetric-related conditions. These studies have demonstrated varying degrees of improvement in patient medication adherence, reduction in adverse event rates, and potential cost savings in healthcare [13, 14].
Currently, research on ICP drug therapy focuses on comparing and optimizing treatment regimens, as well as evaluating their effects on the mother and fetal [15, 16]. Pharmacists do not yet play a supervisory role in ICP pharmacotherapy. Globally, comprehensive pharmacological services for patients with ICP are not yet fully developed. Maternal and fetal safety issues need prompt attention. UDCA is mainly used in the treatment of ICP, but it may lead to side effects [17]. Polyenyl phosphatidylcholine injections may pose a threat to fetal development, and ICP severity is associated with the risk of preterm birth, amniotic fluid abnormalities, and stillbirth [18]. According to the guidelines, ICP of varying severity requires specific treatment and monitoring [19].
Therefore, to ensure the safety of both mothers and fetuses, providing graded pharmaceutical care for ICP women based on the severity of the disease is essential. In this study, we aimed to categorize pharmaceutical care for ICP women into three levels based on their varying needs for pharmacological treatment, pathophysiological status, and medication characteristics. We also developed corresponding care models and investigated the effects of this graded pharmaceutical care approach on ICP women.
2. Methods
2.1. Study Design and Setting
The study was a before-and-after controlled study conducted at a tertiary hospital in Jiangsu Province. The historical control group comprised women diagnosed with ICP at this hospital from December 2019 to December 2021, who received the standard diagnostic and treatment approaches. The guardianship group included women diagnosed with ICP at this hospital from March 2022 to June 2023, who additionally received a graded pharmaceutical care model.
2.2. Study Participants
To ensure comparability of the statistical data, inclusion and exclusion criteria were established. The inclusion criteria were (1) pregnant women with a definite diagnosis of ICP in accordance with the diagnostic standards of the World Health Organization [20] and (2) ICP women receiving pertinent therapeutic medicine while being treated in a hospital. The exclusion criteria were (1) ICP women without relevant medication while hospitalized; (2) ICP women without relevant indices for review after medication; and (3) ICP women who were unable to actively cooperate.
2.3. Intervention Methods
2.3.1. Control Group
The control group received standard treatment, wherein the attending physician selected therapeutic drugs based on the patient’s condition. Meanwhile, the clinical pharmacist conducted routine follow-ups on the effectiveness and safety of drug therapy. However, the patient was not monitored in accordance with the criteria of the graded pharmaceutical care model.
2.3.2. Guardianship Group
Drawing upon guidelines from the American Society of Health-System Pharmacists, the American Society of Maternal-Fetal Medicine (2021) [21], the RCOG guidelines on Intrahepatic Cholestasis of Pregnancy (No.43) (2022) [19], recent high-quality evidence-based literature, and in consideration of previously identified challenges, obstetric clinical pharmacists formulated a standardized pharmaceutical care protocol for grading ICP. This protocol received approval from the Hospital Pharmacy Administration and the Pharmacotherapy Committee. ICP women in the guardian group underwent graded pharmaceutical care upon admission, ensuring comprehensive management and continued follow-up for six months. This care approach was implemented following the diagnosis of ICP, therapeutic protocols, clinical recommendations, and medication guidelines (refer to Table 1). Detailed criteria and contents, as well as the pharmacist team supervision process and supervision key points, are shown in Tables 2 and 3 and Figure S1.
| Reasons for grading | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| TBA level |
|
|
|
| Liver enzyme levels | AST > 120 U/L, ALT > 120 U/L | 40 < ALT < 120 U/L | AST, ALT within normal range |
| 40 < AST < 120 U/L | |||
| Pregnancy situation | TBA ≥ 100 µmol/L or ICP pregnant women with risk factors or comorbidities (e.g., vaginal bleeding, gestational diabetes, preeclampsia, and multiple pregnancies) | TBA < 100 µmol/L | TBA < 40 µmol/L |
| TBA control situation | Poor TBA control | TBA within the normal range | TBA value within the target range several times in a row |
| Medication characteristics |
|
|
UDCA-based bile acid reduction |
| Adverse events | (i) Severe abdominal pain and diarrhea in oral formulations or severe allergic reactions in injectable formulations, etc | Mild gastrointestinal reactions | No adverse events |
| Compliance | (i) Poor compliance; | (i) Fair compliance; | (i) Good compliance, |
| (ii) Unauthorized dose changes, frequent missed doses | (ii) Occasional missed doses | (ii) Take medication on time | |
- TBA, total bile acids; ALT, alanine aminotransferase; AST, alanine aminotransferase; ICP, intrahepatic cholestasis of pregnancy; UDCA, ursodeoxycholic acid; SAMe, S-adenosyl-L-methionine.
| Grade 1 | Grade 2 | Grade 3 | ||
|---|---|---|---|---|
| Pharmaceutical inquiry | Within 24 hours of admission | |||
| Pharmaceutical ward round | Joint medical check-up | The attending doctor is responsible | ||
| Pharmacy independent ward round | Once a day | Twice a week | Once a week | |
| Prescription review | Once a day | Twice a week | Once a week | |
| Medication education | Once a day | All medicines when guidance is needed | ||
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Pharmacy rounds |
|
|
|
| Physician order review |
|
|
(i) Adjust dosage of dosage forms according to therapeutic effects |
| Medication education |
|
(i) Educate on how to take therapeutic drugs, the course of treatment, and the safety of use during pregnancy and lactation to improve medication adherence | (i) Educate on how to take therapeutic drugs, the course of treatment, and the safety of use during pregnancy and lactation to improve medication adherence |
2.4. Clinical Outcome Indicators
2.4.1. Laboratory Indicators
Total bile acid (TBA), total bilirubin (TBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured in ICP women at three points following admission to the hospital for treatment, i.e., prenatal (have been admitted to the hospital for treatment until delivery), postnatal, and discharge. These measurements were utilized to evaluate the treatment’s effectiveness.
2.4.2. Adverse Pregnancy Outcomes
The primary outcome measure was the incidence of adverse pregnancy outcomes. Combined adverse pregnancy outcome (defined as a composite of intrauterine fetal death or known neonatal death for up to 7 days or preterm delivery (less than 37 weeks gestation) or neonatal ward admission f complication or at least 4 hours (from delivery to discharge of the infant)). Each infant was counted once within this composite. In addition, the incidence of each adverse pregnancy outcome was calculated, encompassing perinatal death, preterm delivery (less than 37 weeks’ gestation), meconium-stained amniotic fluid, neonatal asphyxia, neonatal respiratory distress syndrome, neonatal pneumonia, neonatal heart disease, and low birth weight infants.
2.5. Quality Management Indicators
2.5.1. Duration of Therapeutic Drug Administration and Proportion of Intravenous Drug Use
ICP women are presented with a diverse selection of therapeutic medications. Due to the considerable compliance with oral medication regimens and the potential developmental risks to the fetus associated with injectable medications, clinical pharmacists implement targeted interventions and adjustments tailored to improve medication management quality, contingent upon the individual patient’s circumstances. Assessment indicators comprise the duration of therapeutic drug utilization and the ratio of intravenous administration, denoting the percentage of intravenous drug formulations among the total variety of prescribed medications.
2.6. Safety Indicators
2.6.1. Adverse Effects
Adverse reactions related to therapeutic drugs include diarrhea or loose stools, constipation, allergy, headache, dizziness, dry mouth, and vomiting.
2.7. Cost-Benefit Analysis
In this study, cost-benefit analysis (CBA) was utilized for economic evaluation, whereby costs and benefits were compared in monetary terms yielding clear and intuitive outcomes [22]. Costs primarily encompass expenses associated with the implementation of pharmacy services. The determination of pharmacy service costs per capita in the intervention group involved multiplying the total intervention hours by the clinical pharmacist’s hourly rate and then dividing by the number of patients who received the intervention. As pharmacy services were not utilized in the control group, their cost was deemed zero. The benefit-cost ratio (B/C) was delineated as the ratio of benefits to costs throughout the intervention phase. A B/C ratio exceeding 1 signified the economic feasibility of the program [23].
2.8. Sample Size
Previous studies indicated that the incidence of the combined outcome (perinatal death or preterm delivery at less than 37 weeks of gestation, or combined neonatal asphyxia or neonatal respiratory distress syndrome) after pharmacological treatment was 46.7% [24]. The sample size was calculated using a two-sided test with 90% power, a 5% error level, and a 20% dropout rate. Therefore, 194 cases (97 in each group) were identified to test the incidence of the combined outcome in the two patient groups for a 20% relative difference between the groups. Risk ratios were utilized to assess the risk in the guardianship group compared to the control group, following the methodology outlined in the literature on adverse pregnancy outcomes in ICP women (a risk ratio less than 1 indicates a more favorable outcome for the guardianship group).
2.9. Statistical Analysis
IBM R © SPSSTM Statistics v21.0 software was used for statistical analysis. Measured data were expressed as the mean ± standard deviation () using the t-test and rank sum test if the data did not conform to normal distribution. Count data were expressed as frequencies and percentages. Comparisons between two groups were made using the chi-square test. Data that did not obey normal distribution were described by median and quartiles (M (Q1, Q3)), and differences were considered statistically significant if P < 0.05. In addition, in cases where the test indicators (TBA, TBIL, ALT, and AST) were missing, the median was substituted using univariate interpolation.
3. Results
3.1. Demographics of Patients in the Two Groups
A total of 204 ICP women were included, 102 each in the guardianship and control groups (Figure 1). There were no statistical differences in age, body mass index (BMI), gestation week, admission TBA, admission TBIL, admission ALT, admission AST, multiple pregnancy, preeclampsia, hypertension in pregnancy, gestational diabetes, primary production, and ICP history between the two groups (P > 0.05, Table S1).
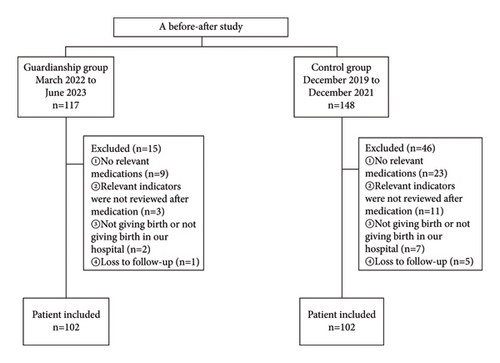
3.2. Comparison of Clinical Results between Two Groups of ICP Women
The prenatal TBA values in the guardianship group were significantly lower compared to the control group (16.05 (9.13, 28.68) vs. 22.85 (12.53, 44.13), P < 0.05; refer to Figure 1(a)). There were no statistically significant differences in TBIL, ALT, and AST between the two groups of women. Comparative analysis within each group showed a significant decrease in the indicators before and after treatment in both groups (P < 0.01; refer to Figure 2).
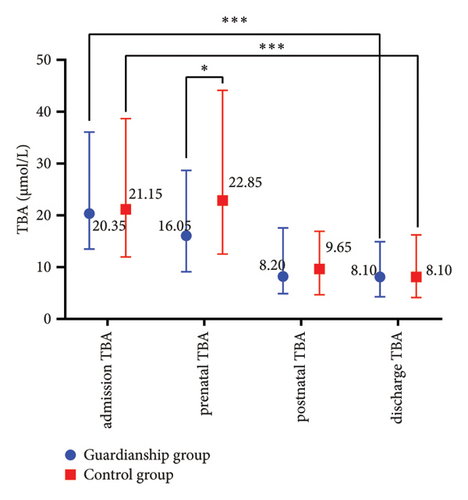
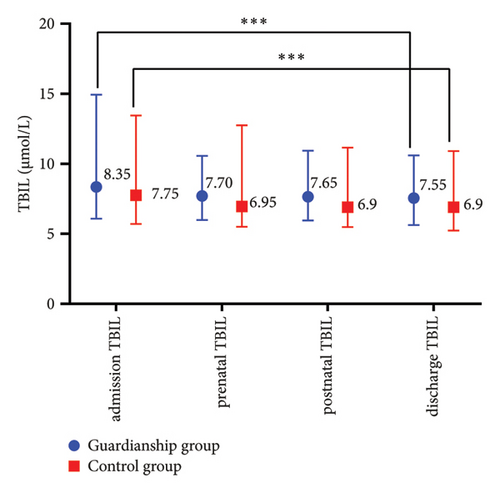
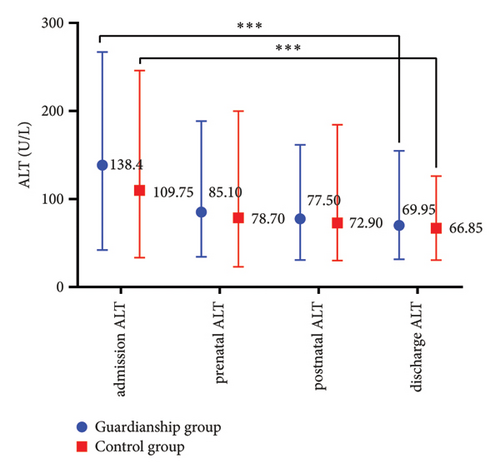
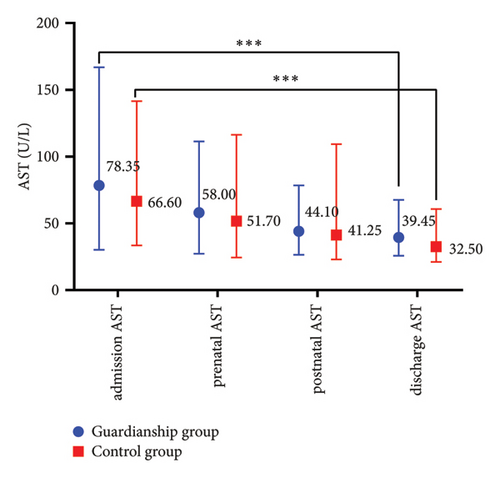
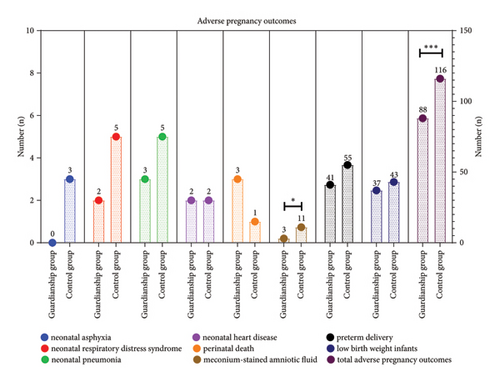
3.3. Comparison of Pregnancy Outcomes between the Two Groups of ICP Women
The results demonstrated a noteworthy reduction in combined adverse pregnancy outcome within the guardianship group, where the incidence rate was 37.2%, in contrast to the control group’s incidence rate of 50.0%. This translates to an absolute risk reduction of 12.8% (RR = 0.74, 95% CI (0.56–0.99), P < 0.05). Furthermore, the guardianship group experienced a lower incidence of meconium-stained amniotic fluid compared to the control group (2.9% vs. 10.9) (Figure 3).
3.4. Comparison of Therapeutic Drug Regimen and Proportion of Intravenous Drug Use between the Two Groups of ICP Women
Comparing the medication regimens of the two groups, it was found that the duration of UDCA treatment in the guardianship group significantly exceeded that in the control group. In addition, the guardianship group exhibited a significantly lower rate of intravenous drug usage compared to the control group (45.8% vs. 62.9%, P < 0.05) (Table 4). However, there was no significant difference in the total therapeutic drugs dosage between the two groups (P < 0.05, Table S1).
| Group | Days of medication (d) | Proportion of intravenous drug use (%) | |||
|---|---|---|---|---|---|
| UDCA | SAMe | Polyene phosphatidylcholine capsules | Polyene phosphatidylcholine injection | ||
| Guardianship group | 8.0 (4.0, 15.0) | 6.0 (4.0, 13.0) | 5.0 (3.0, 9.0) | 9.0 (7.0, 13.3) | 45.8 |
| Control group | 5.5 (3.0, 13.8) | 6.0 (3.5, 10.0) | 5.0 (3.0, 7.0) | 8.5 (7.0, 13.0) | 62.9 |
| P value | 0.047 | 0.813 | 0.719 | 0.597 | 0.030 |
- UDCA, ursodeoxycholic acid; SAMe, S-adenosyl-L-methionine.
3.5. Comparison of Medication Safety between the Two Groups of ICP Women
As indicated in Table 5, a total of 204 women participated in this study, with 31 cases of adverse drug reactions (ADRs) reported, constituting 15.2% of the total cases. Among these cases, 13 ADRs occurred in the guardianship group, while 18 were observed in the control group, representing 12.7% and 17.6% of the respective patient populations. There was no significant difference in the rate of ADRs reported between the two groups (P > 0.05). All reported adverse reactions were mild. Ten ICP women who developed constipation experienced symptom improvement after taking lactulose oral solution, while three ICP women with diarrhea were treated with montmorillonite powder.
| Group | ADR types (n) | Incidence rate (%) | P value | ||||
|---|---|---|---|---|---|---|---|
| Diarrhea or loose stools | Constipation | Dizziness | Dry mouth | Vomiting | |||
| Guardianship group | 1 | 10 | 1 | 1 | 0 | 12.7 | 0.45 |
| Control group | 2 | 13 | 1 | 1 | 1 | 17.6 | |
- ADR, adverse drug reaction.
3.6. Comparison of Cost-Benefit Analysis between the Two Groups of ICP Women
In this study, clinical pharmacists in the guardianship group were compensated at a rate of $12.2 per hour. On average, they dedicated 0.5 hours per day to interventions. Over the 15-month trial period, with interventions occurring on 20 days each month, the total intervention time added up to 150 hours. The overall time cost amounted to $1830. Consequently, the average time cost per patient for pharmacy services was $17.9. In contrast, the cost of clinical pharmacist services in the control group was zero. When compared to the control group, patients in the guardianship group experienced reductions of $19.5 in average total drug costs and $11.6 in average total inpatient costs. The benefit-to-cost ratios (B/C) were 1.09 and 0.65, respectively. This indicates that the pharmacy interventions by clinical pharmacists yielded a positive economic impact concerning medication costs but did not show economic significance regarding total inpatient costs (Table 6).
| Group | n | Intervention costs | Relevant costs | Benefits | ||
|---|---|---|---|---|---|---|
| Average time cost per patient ($) | Total drugs cost ($) | Total hospitalization cost ($) | Reduced total drugs cost ($) | Reduced total hospitalization costs ($) | ||
| Guardianship group | 102 | 17.9 | 250.45 | 1987.38 | 19.52 | 11.6 |
| Control group | 102 | 0 | 269.97 | 1998.98 | ||
4. Discussion
Studies have shown that clinical pharmacists can optimize antibiotic usage, reduce hospitalization and readmission rates, improve patient medication adherence, decrease adverse drug reactions, and potentially lower treatment costs [25–27]. Most recent research involving clinical pharmacists in obstetrics has focused on prescription reviews and drug safety issues [28, 29]. However, there exists a dearth of research pertaining to medication management and pharmacological intervention in the treatment of obstetric conditions. The incidence of ICP exhibits significant global variation, with particularly high prevalence observed in regions like China [30]. The enforcement of China’s “three-child policy” has resulted in a rise in the proportion of women experiencing advanced maternal age, consequently contributing to a surge in the prevalence of comorbidities. To enhance obstetric pharmaceutical care, this study employed a cohort of Chinese ICP women to devise a graded pharmaceutical care strategy, guided by the pathophysiological and medication profiles of ICP women, from the vantage point of a clinical pharmacist, aiming to deliver refined therapeutic interventions for ICP sufferers. By conducting an exhaustive examination of both domestic and international literature and leveraging the available infrastructure and clinical pharmacists’ expertise, we advocate for the implementation of a graded pharmaceutical care regimen tailored for Chinese ICP women.
TBA levels are strongly correlated with the severity of the condition. With each 1 µmol/L rise in TBA levels, the incidence of adverse perinatal outcomes, including spontaneous preterm labor, asphyxiation events, and meconium staining of amniotic fluid, placenta, and membranes, increases by 1-2% [31]. Early research indicates minimal risk to the fetus at low bile acid levels, particularly when serum bile acid levels exceed 40 μmol/L. The mechanism of preterm birth in ICP remains unclear, but in vitro studies suggest increased responsiveness to oxytocin stimulation in uterine muscle cells of ICP women [8]. Elevated maternal bile acid levels can stimulate fetal colonic motility, resulting in the expulsion of meconium [10]. The likelihood of green staining of the placenta and membranes increases progressively and nearly linearly with elevated bile acid levels [31]. In addition, elevated bile acid levels are believed to impact alveolar enzyme function, resulting in the inactivation of surface-active substances and triggering an inflammatory response in the lungs, ultimately leading to respiratory distress syndrome [32]. Previous studies have demonstrated that abnormally elevated bile acid levels induce adverse pregnancy outcomes, so lowering bile acid to within the normal range may reduce the risk of adverse pregnancy outcomes by upstream blocking of the signalling pathways that cause adverse pregnancy outcomes with high bile acids. The study results revealed significantly lower prenatal TBA levels in the guardian group compared to the control group. This outcome was attributed to individualized graded dosing and treatment tailored to different TBA levels in pregnant women. In addition, graded pharmaceutical care resulted in a 12.8% reduction in adverse pregnancy outcomes in pregnant women with ICP compared to the control group. These findings suggest that adjusting prenatal TBA levels through graded medication is likely to significantly enhance pregnancy outcomes. Recent studies have shown that the aspartate aminotransferase to platelet ratio index (APRI) is a key predictor of ICP and its consequences. APRI seems to be more relevant than traditional methods of TBA prediction [33, 34]. Consequently, future investigations could center on the crucial role of APRI in ICP women.
The graded pharmaceutical care model developed in this study instructs clinical pharmacists to focus on the treatment regimens of ICP women at different levels and promptly adjust treatment plans based on clinical performance and TBA levels (e.g., dose adjustments, changes in dosage forms, and monitoring of patient medication adherence). The study results indicated a reduced use of polyene phosphatidylcholine injection in the guardianship group in comparison to the control group. This reduction was a result of clinical pharmacists’ thoughtful consideration of the overall risk-benefit profile, leading them to decrease the administration of polyene phosphatidylcholine injection that contains benzyl alcohol, a substance capable of crossing the placental barrier, in the guardianship group. Furthermore, the study’s results revealed that the guardianship group had a longer duration of ursodeoxycholic acid treatment and days on medication compared to the control group. This outcome is attributed to the active role of clinical pharmacists in the pharmacological care of ICP women and the emphasis on the significance of bile-acid-lowering therapy, which contributed to enhanced patient compliance. Thus, in our opinion, might be one of the factors contributing to the guardianship group’s better TBA management and lower incidence of unfavorable pregnancy outcomes.
Numerous studies over the years have shown that clinical pharmacy interventions provide significant economic benefits to the economy, society, and cost benefit [35, 36]. The study employed cost-benefit analysis to evaluate the costs and benefits linked with delivering graded pharmaceutical care for ICP women. Findings indicated that clinical pharmacist interventions led to considerable economic advantages, notably by lowering medication expenses and minimizing wastage. However, a systematic evaluation study [37] unveiled disparities in the cost-effectiveness of pharmacy service implementation across various nations. Specifically, the median cost-effectiveness ratio of pharmacy services across 17 countries stood at 5.05 (3.08, 11.28), contrasting with the median ratio reported by Chinese scholars, which was 9.45 (6.83, 13.70). Our investigation revealed that the hourly wage of pharmacists in China surpassed previous estimations, and the adoption of the “zero-markup” policy additionally influenced the cost-effectiveness of pharmacy services. Moreover, the study’s limited sample size led to negligible disparities in drug expenses between the cohorts. Consequently, augmenting the sample size becomes imperative for a more comprehensive comprehension of the economic worth and financial advantages of hospital pharmacy services, as well as for investigating the enduring economic repercussions of graded pharmacy services provided by clinical pharmacists.
The reform of China’s healthcare system has driven a shift in the hospital pharmacy service model from drug centred to patient centred, as well as a shift from a focus on ensuring drug supply to a professional pharmacy service that places equal emphasis on drug supply and clinical use. Pharmaceutical care clinical research yields crucial data supporting policy initiatives aimed at optimizing pharmacy services for public benefit, showcasing the pivotal role of clinical pharmacists, and enriching research endeavors. A graded pharmaceutical care model for managing intrahepatic cholestasis in pregnancy was established, addressing a deficiency in obstetric disease management overseen by clinical pharmacists. Nonetheless, this study has limitations, notably in China where standardized criteria are lacking to govern the implementation of the graded pharmaceutical care model. Future endeavors could encompass a multicenter, multi-disease study and enlarging sample sizes via big data platforms to procure superior clinical evidence for a comprehensive evaluation of graded pharmacological care by clinical pharmacists. Moreover, broadening the assessment criteria and content of pharmacy oversight, along with instituting standardized oversight protocols, are crucial for ensuring study comparability and augmenting the universality and practical applicability of pharmacy services. The issue of triage for critically ill patients, particularly considering the heightened risk of adverse outcomes during pregnancy, remains largely unexplored. Subsequent investigations should delve into finer categorizations of critically ill patients and devise tailored treatment modalities. Lastly, pruritus serves as a significant clinical feature of intrahepatic cholestasis in pregnancy [6, 7, 38]; however, investigations into its correlation with pregnancy outcomes among affected women have been lacking, primarily due to the notable underrepresentation of women with pruritus scores.
5. Conclusion
In conclusion, employing a graded pharmaceutical care model that customizes monitoring measures based on various clinical indicators, gestational weeks, age, comorbidities, drug use, and disease exposure has demonstrated significant benefits for women with ICP. This approach aids in improving TBA management, lowering the risk of adverse pregnancy outcomes, and enhancing cost-effectiveness.
Ethical Approval
This study was approved by the Medical Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School (approval number 2023-312-01).
Consent
Informed consent was obtained from all individual participants included in the study. The participant has consented to the submission of the case report to the journal.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Hong-Liang Mei and Xiao-Hui Guo designed the study, and Xiao-Hui Guo performed all analyses and wrote the paper. Yuan Zhang, Yi-Ke Shen, and Meng-Di Sheng completed the data collection. Hai-Xia Zhang reviewed the entire framework of the manuscript and the results. All the authors contributed to the interpretation of the results and read and approved the final version submitted for publication.
Acknowledgments
This study was funded by Jiangsu Pharmaceutical Association—HENGRUI Hospital Pharmacy Fund Scientific Research Project (no: H202107); Nanjing Pharmaceutical Association—CHANGZHOU SIYAO Hospital Pharmacy Scientific Research Fund Grant Project (no: 2021YX004); and the National Natural Science Foundation of China (no: 81903870).
Open Research
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.




