Challenges and Advances in Peripheral Nerve Tissue Engineering Critical Factors Affecting Nerve Regeneration
Abstract
Peripheral neuropathy is painful and can cause a considerable decline in quality of life. Surgery and autograft are the current approaches and clinical standards for restoring function after nerve damage. However, they usually result in unacceptable clinical results, so we need modern peripheral nerve defect treatment approaches. Tissue engineering techniques have been developed as a promising approach, but there are some considerations for translational application. Clinical application of novel tissue engineering methods is related to combining the appropriate cell and scaffold type to introduce safe and efficient bioscaffolds. Efficient nerve regeneration occurs by mimicking the extracellular matrix and combining topographical, biochemical, mechanical, and conductive signs via different cells, biomolecules, and polymers. In brief, ideal engineered biomaterial scaffolds will have to cover all characteristics of nerve tissue, such as nerve number, myelin, and axon thickness. Nerve regeneration has a highly sensitive response to its surrounding microenvironment. For designing a suitable construct, matching the regenerative potential of the autograft as the golden standard is essential. This review article examines the newest advancements in peripheral nerve tissue engineering. Specifically, the discussion will focus on incorporating innovative cues, biological modification, biomaterials, techniques, and concepts in this area of research.
1. Introduction
A peripheral nerve is injured due to different events such as diabetes, immunologic reactions, and physical damage, affecting millions worldwide and leading to social and economic burdens. An exciting feature of the peripheral nervous system (PNS) is the ability to regenerate after injury. The efficacy of spontaneous regeneration depends on the extent of the damage incurred and endogenous neuronal signals, and the lack of any external intervention is unlikely to result in an acceptable functional result [1].
In cases where the body cannot compensate for and repair severe nerve damage, the potential for improvement may be permanently limited. For designing alternative therapy in neuropathy, native architecture containing the surrounding microenvironment, surface topography, biochemical signals, electrical activity, axonal guidance, extension and neurotrophic factors (NFs), and extracellular matrix (ECM) proteins must be considered. Nerve regeneration is very complex, so many approaches have been used to solve this complexity [2].
The field of tissue engineering offers a promising path for developing complex structures that can be utilized in translational medicine [3]. An examined approach for neurotherapy involves using natural or synthetic nerve guidance conduits (NGCs). Engineered NGCs were varied in content (biomaterials, cell, growth factor, exosome [4], etc.), fabrication method, and especially in functional outcomes [2].
Autografts are still the most effective method for managing peripheral nerve injuries despite the need for donor tissue. Thus far, the implementation of biomaterials and manufacturing techniques has not matched autografts as the most effective method in treating critical-sized nerve defects [2, 5]. An engineered construct is needed to stimulate neural survival and facilitate axon extension and guidance to achieve optimal neurologic function, reduce recovery time compared to autografts, and facilitate axon extension and guidance [2]. However, guiding the proliferation, differentiation, and transplanted replacement or supporting cells is only a fraction of the challenges that must be overcome in tissue engineering-based design and evaluation. Here, we describe the methods for generating peripheral nerve constructs and strategies for cell, biomaterial, and other cue selection.
2. Neural Development Native Tissues
2.1. The Construct of Peripheral Neural Tissue
The peripheral nervous system consists of nerves (specifically, spinal, cranial, and visceral nerves) and ganglia, which act as conduits for transmitting nerve impulses and nutrients to neurons. The neuron, the structural unit of the nervous tissue, consists of a nerve cell body and several processes: dendrites, axons, and axon terminal.
The primary focus of experimental investigation in peripheral nerves is centered on the aggregation of axonal bundles, comprising a heterogeneous combination of myelinated and unmyelinated axons, that conduct efferent (motor) or afferent (sensory) electrical signals. Schwann cells (SCs), satellite cells, enteric glia, and olfactory ensheathing are the primary glial cells in the peripheral nerve tissue. SCs generate Bungner bands, which are elongated tubular fascicles [3]. A total of 43 nerves, including 12 pairs of cranial nerves, 31 pairs of spinal nerves, and autonomous nerves, form the foundation of the peripheral nervous system. Most studies and clinical trials on peripheral nervous repair focus on the brachial plexus and sciatic nerve [6].
2.2. Microenvironment
Improved understanding of nerve regeneration trends leads to the development of better approaches for regeneration. Native tissue features are closely related to the strategies employed for neural constructs. So, clarifying every tissue’s biological, physicochemical, and mechanical properties is critical for designing an appropriate scaffold. Any engineered structure must mimic the complicated native architecture and physiology. The biomimicking of the nervous tissue microenvironment requires neurons and diverse types of glial cells dispersed across multiple nervous system regions. This cell population’s density, diversity, and interactions determined tissue phenotypes. The 3D environment of cells is a critical factor that significantly affects the functionality of native tissues. The dynamic microenvironment, cell-cell, and cell-ECM connections are directly related to the developmental and physiological events of native neural tissue [6].
Nervous ECM differs from other organs as it supports cells and modulates their behavior. In nervous tissue ECM, lecticans, hyaluronan, and tenascins dominate; unlike other tissues, collagen, fibronectin, and laminin are rare [7]. ECM molecules consisting of growth factors, cytokines, and chemokines are the other critical neurotrophic factors that promote neural development and regenerate through concentration-dependent gradients [7, 8]; the spontaneous repair mechanism in the peripheral nerve results in poor reinnervation after a pathophysiological event. After a peripheral nerve injury, irreversible pathophysiological changes lead to an imbalance in the neuronal microenvironment, resulting in scar tissue formation and poor functional restoration [9]. Wallerian degeneration, a procedure that considered axon degeneration and myelin failure, is known to occur in the distal end. Concurrently, axonal degeneration occurs in a small distal zone to the proximal stump. Ranvier nodes are the origin of axonal sprouts that Schwann cells can myelinate [10]. Failure of the regenerative environment because of loss of trophic support and growth factors such as glial cell-derived growth factors (GDNF) and brain-derived neurotrophic factor (BDNF) and increase in growth-inhibiting molecules such as chondroitin sulfate proteoglycans in the distal axonal segments was causing the failure of reinnervation [3]. SCs are highly mobile and can migrate toward damaged tissue, which is crucial in regenerative tissue. Moreover, they secrete signaling molecules that attract macrophages, facilitating neuronal survival, promoting axon growth, activating resident mesenchymal stem cells, and interacting with various other cell types [11]. The support structures provide neurotrophic support to affected areas, restrict the penetration of fibrous tissue, and direct the growth of regenerating axons towards their appropriate targets [12].
After transplantation in the body, any tissue engineering construct would trigger a response from the body. The reaction to scaffolds is intricate since it involves the removal of migrated macrophages and monocytes from myelin and axon debris. On the other hand, activating inflammatory cells triggers the secretion of specific factors that promote creating a regenerative environment. The regeneration of peripheral nerves is influenced by several factors, such as the selection of biomaterial, the fabrication technique used, the rate and byproducts of degradation, and their effect on the regenerative microenvironment and their interaction with nerve cells. These factors can impact the neural microenvironment significantly [13]. Scaffolds and host cells/tissues are affected by each other. Therefore, it is desirable to have a construct that can change the direction of tissue repair.
The regeneration of peripheral nerves depends on a well-balanced local microenvironment. Degradation products of scaffolds, mechano- and electrotransduction properties, degree of tissue inflammation, and immune and cellular functions are crucial for regenerating peripheral nerves. Also, elements such as Schwann cells, polymorphonuclear cells, lymphocytes, plasma cells, giant cells, vascular endothelial cells, fibroblasts, macrophages, and biomaterials are involved in the local regenerative microenvironment and inflammatory and immune response. Overall, the microenvironment of neural regeneration is influenced by five crucial components: immune response, intraneural vascularization, bioenergetic metabolism, and biomechanical and bioelectrical conductions and their signaling pathways. Each of these components plays a crucial role in promoting the restoration of neural tissue [9, 13].
The polarization of macrophages from a proinflammatory state to an anti-inflammatory state, as well as changes in tissue structure such as necrosis, fibrosis, and fat infiltration, facilitate the promotion of peripheral nerve regeneration [13]. The support structures play a significant role in providing neurotrophic support to the affected regions while limiting the infiltration of fibrous tissue and directing the growth of regenerating axons towards their targets [11]. The conversion of mechanosensing stimuli into biochemical signals induces adaption in response to their surrounding microenvironment [14].
The microenvironment is crucial for determining and facilitating nerve repair cascade by integrating coordinated signals. The cell signaling activated by the extracellular matrix molecules functions to potentiate the role of the matrix in repairing extended peripheral nerve injury [15].
In the context of injury, SCs serve as myelinating cells. These cells can undergo dedifferentiation through the activation of TGFβ signaling, resulting in the gaining of mesenchymal characteristics and a transition to a progenitor-like state [16].
3. Engineering Approaches for Nerve Repair
Treatment approaches combining cell transplantation, molecule delivery, and biomaterial scaffolding will provide hope for tissue regeneration, repair, replacement, and efficient recovery. Developing and advancing NGC design, biomaterials, fabrication methods, cellular compounds, biomolecules, fillers, and topological cues will offer feasible neuropathy treatments.
3.1. Cell-Based Techniques
Cellular components are essential for combined therapy and scaffold efficiency in nerve injury healing involving multiple cell types. In cell-based therapy, selecting cell sources, administration routes, dosages, fates, and survival are significant challenges. It is worth noting that including SCs in autologous grafts plays a critical role in providing neurotrophic support and ultimately determining the positive outcomes of autografts in cases of motor nerve damage. Therefore, the significance of cellular selection is essential in cell-based therapy for nerve injury healing [3]. Previous studies have found cell selection for seeding sources very challenging. Regardless of the specific cell type, various autologous, allogeneic, and xenogeneic sources have been previously employed in the field of neural tissue engineering. The process of obtaining and collecting autologous neural cells and managing the immunological reactions of allogeneic cells has become challenging. Numerous factors must be considered when selecting a cell source, including limited resources, accessibility, purification, immune reactions, pathogen contamination, large-scale expansion, ease of harvesting, and tumorigenic and ethical issues.
Various cell populations play a vital role in the construction of tissue engineering for the peripheral nervous system. In particular, neuronal progenitor cells [17], neural stem cells [18], neurons, SCs [19], olfactory ensheathing cells (OECs) [20, 21], satellite glial cells (SGCs), and enteric glial cells (EGCs) were utilized (Table 1). Each cell type possesses unique characteristics that contribute to the tissue engineering process and are critical to successful outcomes. Research indicates that stem cells support axon regeneration using trophic mechanisms that influence SCs and increase angiogenesis [19].
| Stem cell therapy | Ref | |
|---|---|---|
| Source | Embryonic stem cells/bone marrow stromal cells/adipose-derived mesenchymal stem cells umbilical cord mesenchymal stem cells/fetal/adult/iPS/fibroblast immunomodulatory cell | [22] |
| Origin | Autograft/allograft/xenograft | |
| Administration routes | Intravascular/intrathecal/scaffold loaded/subcuticular/intramuscular/direct injection | [23–26] |
| Therapeutic actions | Angiogenesis/anti-inflammatory/antiapoptotic/antifibrosis/antioxidation/immunomodulatory/homing/renewal/differentiation/myelination/signaling/cytokine/exosome/neurotrophic factor release | [22, 27, 28] |
Different sources of stem cells have been used in regenerative therapy, including embryonic stem cells [17], bone marrow stromal cells (BMSCs) [18], adipose-derived mesenchymal stem cells (ADMSCs) [20, 29], umbilical cord mesenchymal stem cells [21], hair follicle stem cells [30–32], dental pulp stem cells [33], skin-derived precursor cells (SKPCs) [34], and induced pluripotent stem cells (iPSCs) [33, 35, 36] and even dorsal root ganglion (DRG) [37], fibroblast [38], and immunomodulatory cell have been used previously in regenerative therapy (Figure 1). The effectiveness and viability of supportive cell implantation in nerve conduits require further investigation to gain a comprehensive understanding. The current research indicates that there is still uncertainty in this regard [38]. Stem cells’ secretion of neurotrophic factors and exosomes suggests that they can differentiate into Schwann cell-like (SC-like) cells that facilitate peripheral nerve growth and myelination growth [39].
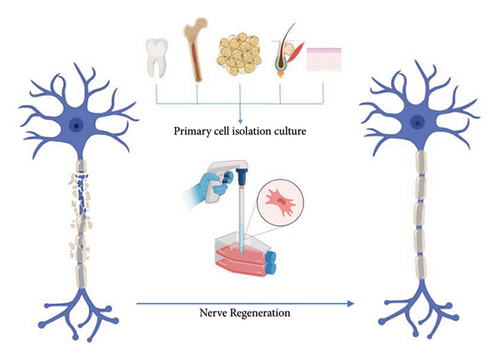
After cell source selection, cell coculture, gene-modified stem cells, engineered SCs [39], and suitable materials for 2D and 3D cell culture that can improve different cell populations’ viability and functional integrity are very discussable. Biological constructs were formed in vitro by coculturing DRGs and SCs of multiple types [10, 40]. The scaffold’s biological activity was improved in the different studies via gene-modified stem cells used for cell-based expression and delivery of nerve growth factor (NGF) [41], neurotrophin-3 (NT-3) [42], GDNF [43], and BDNF [44, 45].
SCs are an essential component of the healing process in the PNS. SCs are integral parts of regeneration that can differentiate from MSCs derived from bone marrow, adipose, and dental pulp. MSCs possess excellent differentiation capacity and proliferation potential, which enables them to transform into SC-like phenotypes and promote nerve remodeling [2, 12, 46, 47]. This ability has many clinical benefits and advantages. After MSCs differentiate into SC-like, these cells organize bands of Bungner, axon regeneration, and myelination. Growth factors then stimulate regeneration and ECM secretion via paracrine signaling. After selecting the appropriate target cell for nerve repair, the niche of those target cells will be provided via molecular or mechanical stimulation or both [6].
Although the precise mechanisms underlying the therapeutic benefits of MSCs remain fully elucidated, existing evidence suggests that these cells can promote anti-inflammatory and immune regulatory effects in the context of peripheral nerve injury. Furthermore, MSCs can attenuate muscle mass loss while altering the microenvironment towards a proregenerative phenotype [48]. The remarkable regenerative capacity of the peripheral nervous system is attributed to the inherent plasticity displayed by SCs. In the aftermath of PNS damage, SCs undergo a dedifferentiation process and transition into repair phenotypes. These phenotypes are instrumental in promoting axonal regeneration, facilitating myelin formation, and disposing of axonal and myelin debris. Supplementing necrotic regions with SCs via gene modification or stem cell transplantation is clinically valuable due to the restricted self-repair capacity of SCs for extended peripheral nerve defects. Furthermore, developing tissue-engineered nerves with bioactive scaffolds represents a promising strategy with great potential in addressing such tissue defects [39].
3.2. Bioactive Molecules Supplementation
The inclusion of biochemical components in neural scaffold architecture presents a significant challenge. Numerous barriers must be overcome to ensure such components’ successful integration and subsequent efficacy [6, 49]. Growth factors’ short half-life and rapid degradation within body fluids make administering them directly to the distal end challenging [50, 51]. So, adding exogenous growth-promoting agents within the ECM proteins of the interior of the NGC is vital for efficient deployment and continuous local delivery. These agents are comprised of a diverse range of small molecules, growth factors, cytokines, biochemical cues, adhesion molecules, signaling factors, inflammatory mediators, antioxidant reagents, bioactive peptides, and ECM protein that have specified enhancement effects on the axonal regeneration and functional outcomes [6].
After an injury, endogenous growth factors released from the distal end of the nerve can regulate axon regeneration. It is noteworthy, however, that exogenous growth factors may need to be present continuously to stimulate the repair process. Axonal regeneration is facilitated by aligned SCs and their ECM, which are made of laminin and fibronectin and provide vital pathways and growth factors [3].
Appropriate cell and extracellular matrix interactions are critical in tissue-engineered designs. Engineered microenvironments and scaffold modification using natural or non-native growth factors, biomolecules, or peptide gradients can potentially improve this interaction. Incorporating bioactive peptides into a scaffold through a biofunctional epitope significantly enhances cellular behavior, including adhesion, proliferation, migration, differentiation, and alignment. This process can be achieved by utilizing various biomolecules and methodologies.
Researchers have specifically explored sequences that are relevant to neural adhesion. These include the RGD peptide found in collagen, laminin, and fibronectin. Peptides derived from laminin consist of YIGSR, IKVAV, RNIAEIIKDI, and RYVVLPR. Polylysine and various proteins such as integrin, talin, vinculin, α-actinin, filamin, and paxillin are also included. Modifying biomaterial surfaces with cell adhesion molecules involves utilizing several methodologies such as blending, surface deposition, electrostatic, and covalent attachment. The selection of appropriate adhesion molecules, their coatings’ stability, and their optimal concentration are paramount in promoting axon repair [52–54].
Promoting axon repair through growth factors induces early regeneration, increasing nerve growth near the injury site. However, it is essential to note that the increased growth factors are not sustained indefinitely, as they eventually decrease to prevent the immune system from attacking the regenerating axon. Ultimately, successful innervation is achieved through this process [47]. Any of the cellular sources described previously can initiate growth factor generation.
Several growth factors are commonly used for neural tissue engineering, which can be classified into two categories: (1) neurotrophins and (2) neurotrophic action factors. The first category includes neurotrophin-3 (NT-3), BDNF, and NGF. The second category comprises GDNF, endothelial cell factors, fibroblast growth factors (FGFs), insulin-like growth factor (IGF), ciliary neurotrophic factor (CNTF), systemic growth hormone (GH), or a combination of these factors. Platelet-derived growth factors (PDGFs) have been used as filling agents for nerve conduits and as scaffolds for nerve stumps [55–57] (Figure 2).
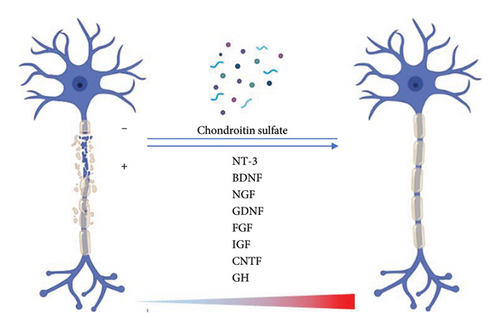
SCs, the critical factor in nerve regeneration, can secrete neurotrophic factors, N-cadherin, neurotrophins, gamma integrins, neural cell adhesion molecules (N-CAMs), and collagen and laminin [58]. The existence of ECM proteins, including collagen I, collagen IV, laminin, fibronectin, and neurite guidance proteins, strengthens the tissue regeneration process. Integrins play a crucial role in facilitating axonal regeneration through their ability to interact with key signaling ligands. Recent studies have shown that laminin and integrin’s “outside-in” signaling is critical in nerve regeneration. It has been determined that the laminin-binding domains (LBDs) are necessary components for proper BDNF and CNTF functioning [59].
The process of transdifferentiating stem cells into a Schwann cell-like phenotype involves exposure to various agents. These agents include β-mercaptoethanol, all-trans retinoic acid, fetal bovine serum, forskolin, recombinant human bFGF, recombinant human platelet-derived growth factor-AA, recombinant human heregulin β-1, and glial growth factor [60, 61]. The gradient of granulocyte colony-stimulating factor (G-CSF) displays significant potential for neurotrophic and angiogenic effects [62]. Bioactive agents’ precise delivery and control are crucial for repair processes and can affect the three-dimensional cell orientation. In this regard, a multitude of axone-promoting cue delivery systems is available, including distribution on scaffold surfaces, attachment to scaffold walls, incorporation, lumen fillings using hydrogel, microspheres, covalent immobilization, and osmotic minipump or injection device-supported continuous release. Bioactive three-dimensional scaffolds are considered a suitable substrate for administering axon-promoting cues that can effectively enhance the process of nerve regeneration. Gene-modified SCs or stem cells can express various growth factors, including FGF, BDNF, CNF, and GDNF, which can be used for cell-based delivery [45]. Previous research has demonstrated this approach through transduced SCs [41, 43]. Modification of neurotrophic factors is an approach that can be employed to sustain their activity during elongated release from the cellular environment. Immobilization techniques, such as crosslinking or photochemical reactions, as well as coaxial electrospinning, have been utilized for this purpose [63]. Furthermore, it has been determined that their capacity to bind with cells or scaffold biomaterials at a high-affinity level presents a feasible resolution.
Another method for enhancing nerve regeneration involves differential adsorption of growth factors into neural scaffolds [2]. Recent research has identified a significant biomolecule known as the stem cell recruitment factor, demonstrating the ability to enhance the functional regeneration capacity of host stem cells. Specifically, substance P (SP) has been found to attract stem cells and modify NGCs through a micropattern-based fabrication method. These findings highlight the potential of SP to play a critical role in neural tissue engineering [64].
Now that we have investigated the intricate world of bioactive molecules and their pivotal roles in cellular processes, it is critical to shift our attention to the structural scaffolds. The successful integration and sustained delivery of bioactive components within the neural guidance channels pose significant challenges, necessitating a thoughtful consideration of the scaffold’s structural design. The synergy between bioactive molecules and scaffold architecture is vital to coordinating precise cellular responses and tissue regeneration. Therefore, in the following, we investigate how different generations of scaffold designs, including different luminal fillers, affect the functionality and performance of these essential biomaterials in the field of nerve regeneration.
3.3. Scaffold Configuration
The configuration of scaffolds significantly impacts the topological properties and cell fate of neural guidance channels, ultimately influencing the performance of neural scaffolds [49]. Three generations have been established based on luminal fillers to classify NGCs. The architecture of peripheral nerves depends on the structure of elongated tubular fascicles composed of Bungner fibers. The first generation of these fibers is characterized by a simple hollow tubular duct without any topological indication. To accurately mimic the endoneurium in nerve structure, it is necessary to incorporate intraluminal microarchitecture and account for the frequently porous nature of the external wall [47, 65]. Thus, the next generations and more complex types of NGC were created. The fillers comprise fibers, filaments, microchannels [66], gels, sponges, and grooves [6, 65, 67]. A compact filler could potentially inhibit the development of neural pathways [5].
4. Structural Characteristics of Scaffolds
- (1)
Porous conduit and its pore size profoundly impact the exchange of nutrients and growth factors, cell viability, adverse cell invasion, and vascularization [69].
- (2)
The impact of the scaffold’s flexibility on the compression and mechanical strength of the conduit is notable, as it inhibits any harm to the regenerative tissue [2, 70].
- (3)
Speed of degradation has a significant impact on the processes of inflammation and swelling, as well as tissue compression. Furthermore, this velocity also regulates regeneration and prevents inflammation and compression [2, 6].
4.1. Porous NGCs
The three-dimensional porous structure has a significant impact on the NGC’s properties. The pores are designed to facilitate the migration of cells, ensure a sufficient supply of nutrients and oxygen, and remove metabolic byproducts. Regarding size, permeability, structure, and connectivity, the pore architecture within the scaffold needs to align with the pathophysiological requirements for repairing peripheral nerves by considering the synergistic effects of various cells and bioactive factors. Pore dimensions and structure impact cellular responses, such as adhesion, expansion, and migration. The permeability of the pore structure is closely associated with its beneficial function in facilitating the regeneration of peripheral nerves. Also, porosity is crucial in influencing the surface-to-volume ratio, mechanical characteristics, and biodegradation rate of NGCs. Various fabrication techniques exist for each NGC morphology to develop innovative pathways and create the most appropriate structure and topography conducive to optimal neural growth [71, 72].
4.2. Fabrication Techniques
Neural scaffolds can be fabricated through various conventional microfabrication techniques, such as immersion precipitation particulate leaching [73], extrusion [74], injection molding solvent evaporation [75], nonwoven or woven mesh rolling [76], centrifugal casting [76], spinning mandrel technology [77], electrostatic direct writing [78], cutting holes into the walls, film casting plus rolling, and molding plus freeze-drying [60]. In addition, advanced techniques such as electrospinning of fibers [61], phase separation [79], gas foaming [80], gradient freezing method, in situ polymerization [63], self-assembly [81], micropattern [82], computer-aided design-based fabrication techniques [66], and bioprinting can also be utilized (Table 2).
| Cells | Material | Fabrication | GF/nano | Animal | Defect | Time of study | Result | Ref |
|---|---|---|---|---|---|---|---|---|
| — | PCL | Electrospinning | Conductive GDY | Rat | 10-mm sciatic nerve defect model | 3 (m) | GDY/PCL NGCs significantly promoted myelination and axonal growth | [83] |
| SKP-derived Schwann cells (SKP-SCs) or nerve-derived SCs | — | — | — | Rat | 12 mm gap in the sciatic nerve | 8 (w) | Survival of both cell types’ early regeneration in SKP-seeded grafts | [84] |
| Hair follicle pluripotent stem cells | — | — | — | ICR nude (nu/nu) mice | Impinged sciatic nerve | — | Differentiated into glial fibrillary acidic protein-positive Schwann cells and promoted the recovery of pre-existing axons | [30] |
| The upper part of the hair follicle containing the hfPSA | — | — | — | Mice | Severed sciatic nerve | — | Implanted hfPS cells grew and promoted the joining of the severed nerve | [31] |
| Human hair follicle pluripotent stem (hfPS) cells | — | — | — | Mouse | Severed sciatic nerve | — | They differentiated into glial fibrillary-acidic-protein (GFAP)-positive Schwann cells and promoted the recovery of pre-existing axons, leading to nerve generation | [32] |
| Schwann cell (SC), olfactory ensheathing cell (OEC), or mixed SC/OEC | Poly (dl-lactide-co-glycolide) acid | Conduit | — | Rat | Sciatic nerve transection | — | OEC synergistically improves SC-mediated sciatic nerve repair | [85] |
| Rat Schwann cells (RSCs) | Porous PVDF/PCL composite | Cast/annealing-solvent displacement method | — | Rat | Sciatic nerve model for 4 months | 4 (m) | Piezoelectric scaffold | [86] |
| — | Collagen/PLA | Electrospun | — | — | — | — | — | [87] |
| Schwann cells | PCL and CNTs | Aligned electrospinning | BDNF | Rat | 10 mm sciatic nerve defect model | — | Promote sciatic nerve regeneration and functional recovery | [88] |
| Pure porcine decellularized nerve matrix/proanthocyanidins | Electrospinning | — | Rat | 8 mm sciatic nerve | 12 (w) | Excellent ability to transmit electrical signals | [82] | |
| — | Soft carbon nanotubes@gelatin methacryloyl/poly (L-lactic acid) (CNTs@GelMA/PLLA) | — | — | Rat | 10-mm sciatic nerve defect | 12 (w) | — | [89] |
| — |
|
|
— | Rat | Sciatic nerve defect | 12 (w) | — | [90] |
| — | (rGO with GelM) and (PCL) nerve conduits | Electrostatic spinning | BMSC-derived extracellular vesicles | Rat | 10 mm sciatic defect | 12 (w) | Promote peripheral nerve regeneration and neurological function recovery | [91] |
| — | Hyaluronic acid methacrylate | Photocrosslinked hydrogel | MSC-Exos | Rat | SNCI model | 1 and 2 (w) | Inhibit the infiltration of macrophages and the expression of the proinflammatory factors IL-1β and TNFα | [92] |
| — | Pyrrole electroconductive hydrogels | — | BMSC-Exos | Rat | Diabetic sciatic nerve crush injury | 4 (w) | Enhance myelinated axonal regrowth | [81] |
| — | Chitin conduits + injectable decellularized matrix hydrogel | Physical, chemical, and enzymatic digestions | ADSC | Rat | Sciatic nerve defects | — | Improved peripheral nerve regeneration | [93] |
Regardless of the method employed in construction, the characteristics of the structure hold considerable significance in ensuring favorable clinical outcomes. The construct’s intricate internal architecture and topology impact various factors, including cell adhesion, proliferation, and differentiation. This effect is observed both on the surface and within the scaffold. In addition, it influences biodegradability, biocompatibility, controlled degradation, reabsorption of metabolites, immunogenicity, and mechanotransduction [46, 94].
4.3. Biomaterial Selection
A scaffold is a substrate for the adhesion, proliferation, migration, and function of neural companion cells. A range of synthetic and natural polymers can create an ideal scaffold [2, 6]. The biomaterial selection for scaffold fabrication depends on the scaffolding’s expected function [1]. For ideal neural scaffold fabrication, selecting biomaterials that align with biological, biomechanical, and physicochemical considerations is crucial. Appropriate properties are usually obtained by combination or modification [6].
Biomaterials should meet specific criteria that ensure they are safe for use in the body. They should not be toxic, allergenic, mutagenic, or carcinogenic. In addition, they should minimize scar tissue formation and promote the growth and recovery of target cells. They should also be capable of controlling mass transport and providing biological factors that aid in cell adhesion, proliferation, survival, and differentiation. Selecting appropriate materials for neural tissue engineering techniques, particularly bioprinting, has become increasingly complicated. A limited selection of materials can provide the necessary characteristics of printability, cell support, and mechanical reinforcement. Generally, hydrogels have emerged as a suitable choice for bioprinting applications [1].
The position of exogenous or endogenous cells, cellular adhesion, the formation of the ECM, and the general improvement of repair are related to the type and characteristics of the material incorporated into the scaffold composition. Some biomaterials can be intentionally engineered to provide detectable microenvironmental cues, resulting in modifying the phenotype of a cell, for example, the changing polarization of invasive macrophages into a regenerative phenotype [95]. The mechanical strength and flexibility properties of synthetic materials, especially conductive polymers, have caused a significant increase in the extension and proliferation of neurites [46].
All aspects of scaffold fabrication strategies must be considered, including porosity, permeability, flexibility, stiffness, biological features, biocompatibility, biodegradability, conductivity, surface area, and properties [96]. A critical characteristic of a suitable scaffold is its ability to support cellular function and mimic the natural ECM [1]. The neural scaffold can distribute biochemical agents via diffusion [10]. The conduit’s porosity promotes angiogenesis and inhibits scar tissue [97]. The scaffold’s biocompatibility is critical in facilitating cell adhesion, proliferation, migration, and maturation.
It is worth noting that unwanted bodily reactions are often experienced, and inducing inflammation may produce positive and negative effects on the regeneration of the peripheral nervous system. As shown in Table 1, a wide range of scaffolds of different categories, including natural, synthetic, and nanofibrous variations, has been extensively evaluated for their potential use in the field of neural tissue engineering.
4.4. Conductive Biomaterials and Electrical Stimulation
The ability of neurons to produce electrochemical signals is a fundamental aspect of the nervous system. Several investigations have shown that electrical excitation has the potential to facilitate nerve regrowth [98, 99]. This method has typical clinical applications but has limitations for deep muscles, which the tissue engineering approach resolves. As previously detailed, a fundamental attribute of appropriate materials for producing nerve tissue scaffolds is their electrical conductivity. Materials that exhibit electrical conductivity and piezoelectric properties are categorized as electrically conductive materials. Examples of such conductive materials include polypyrrole (PPy), polyaniline (PANI), polythiophene, polyphenylene sulfide, conductive nanoparticles, such as gold and silver nanoparticles, graphene nanosheets, carbon nanotubes, poly(3,4-ethylenedioxythiophene) (PEDOT), and nanoparticle [100]. Also, piezoelectric materials include black phosphorus and piezoceramics [101].
The effects of electrical conductivity on cellular neurite outgrowth, cell division, cell polarity, differentiation, migration, and angiogenesis have been investigated regarding their patterns, strengths, and time courses [2]. Through electrical stimulation within conductive scaffolds, effector proteins such as BDNF, NGF, CNTF, vascular endothelial growth factor (VEGF), and calcium ion channels can be activated. This activation subsequently increases cAMP levels, leading to the extension of neurites, nerves, and myelinated fibers and, ultimately, an elevation in the repair of both motor and sensory nerves in the direction of the administered electrical current [102]. It is imperative to acknowledge that the critical function of neurons in generating electrochemical signals plays a pivotal role in the innate nervous system. Extensive research has demonstrated that electrical excitation can significantly enhance nerve regrowth [98]. As such, using conductive material becomes essential to stimulate a functional recovery of both motor and sensory pathways and reduce muscle atrophy [47].
Integrating conductive biomaterials and electrical stimulation presents significant potential for advancing nerve tissue engineering. Conductive NGCs can produce electrical energy by themselves, making them an excellent option for self-powered nerve scaffolds. This approach offers several advantages, including enhanced nerve regrowth, precise control of cellular processes, and functional recovery of both motor and sensory pathways. However, successfully translating this technology into clinical applications depends on addressing critical challenges related to biocompatibility, optimal stimulation parameters, and integration with other scaffold components [103, 104].
4.5. Traditional Biomaterials Selection
The utilization of autografts for nerve regeneration is subject to limitations, including the inconsistent thickness of regenerated nerves [78]. Using commercially available artificial NGCs is a viable solution to this issue, as well as for the other restriction of autograft (Table 3). Neural scaffolds have gained significant outcomes in numerous clinical applications. Multiple research studies have been published focusing on the commercially available items of neural scaffolds and their resultant clinical impact [5]. Table 4 shows that the FDA-approved product structure primarily comprises natural and resorbable materials, with some synthetic and nonresorbable elements included [3]. FDA-approved commercial NGCs are crucial in peripheral nerve repair and regeneration.
| Nerve graft/replacement | Inert | Immune rejection | Topographical cues |
|---|---|---|---|
| Autograft | + | − | + |
| Allograft | + | + | + |
| Processed acellular nerve allografts | + | − | + |
| Hollow tube synthetic conduits | + | − | − |
| Biomaterial | Product ∗ |
|---|---|
| Collagen type I | NeuraGen™ (K011168, 2001) |
| NeuroMatrix™ (K012814, 2001) | |
| NeuraWrap™ (K041620, 2004) | |
| NeuraFlex™ (K012814, 2001) | |
| NeuroMend™ (K060952, 2006) | |
| Nerve cuff (K132660) | |
| Flexible collagen nerve cuff (K131541) | |
| NeuroCap™ (K152648, 2016) | |
| Type I collagen and glycosaminoglycan (chondroitin-6-sulfate) | NeuraGen™ 3D (K130557, 2014) |
| NeuraGen®3D nerve guide matrix (K163457) | |
| Type I acellular porcine collagen membrane | Cova™ ORTHO-NERVE (K103081, 2012) |
| Collagen matrix derived from porcine pericardium | NervAlign® nerve cuff (K202234) |
| Collagen type I + absorbable polymeric suture filament | Reinforced flexible collagen nerve cuff (K170656, 2017) |
| Polyglycolic acid (PGA) | Neurotube™ (K983007, 1999) |
| NeuroFlex™ (K131541, 2014) | |
| Nerbridge™ (K152967, 2016) | |
| Polyglycolic acid (PGA) and type I and III collagen and porcine | Nerbridge™ (K152967) |
| Poly D, L-lactide-co-e-caprolactone (PLCL) | Neurolac™ (K032115, 2003; K050573, 2005; K112267, 2011) (K112267) |
| Nerve capping device (K152684) | |
| Polyvinyl alcohol (PVA) | Salu tunnel™ (2010, K100382) |
| Salubridge (K002098, 2001) | |
| Porcine small intestine submucosa | AxoGaurd™ (K031069, 2003) (K162741) |
| Axogen (K163446) | |
| Porcine small intestinal submucosa; primarily types I, III, IV, and VI collagen | Axoguard nerve connector (K162741) |
| Human nerve allograft | Avance |
| Chitosan |
|
| NeuroShield (K190246) | |
| Hyaluronic acid and alginate | VersaWrap nerve protecto (K201631) |
- ∗The products mentioned have been sourced from the FDA website.
Nonetheless, these conduits have certain limitations. The conduits approved by the FDA are unsuitable for the third-generation NGC and do not contain cells, growth factors, or internal patterns or structures. New advanced NGCs consist of multiple layers containing innovative materials supporting cell populations and releasing stimulating factors.
On the other hand, these products are intended for short-distance nerve injuries of less than 3 cm and can only restore nonessential sensory nerves. Up-to-date research is essential to address patient needs and intolerance cases, decrease costs, and develop success rates and industry needs. Researchers are investigating innovative strategies to improve performance, such as complex structures, intraluminal scaffolds, growth factor integration, and autologous and allogenic supporting cells to improve nerve regrowth. Commercial NGCs remain a valuable tool in peripheral nerve repair despite these limitations.
In the clinical usage of NGC, irrespective of nerve diameter and lesion location, choosing a particular conduit causes a challenge due to the lack of actual comparative data among different conduits [105].
5. Implantable Scaffolds
The commonly used scaffold in neural tissue engineering is known as NGC, which is typically found in tubular structures, either hollow or solid. The most common forms of these scaffolds are hydrogel and fibrous structures (Table 5).
| Scaffold morphologies | Fabrication | Advantages | Disadvantage | Prospective | Ref |
|---|---|---|---|---|---|
| Porous |
|
(i) Infiltration |
|
(i) Optimizing the size of pores | [71] |
| Hydrogels |
|
|
|
|
[106, 107] |
| Fibrous |
|
|
(i) Regulate fiber diameter and alignment |
|
[72] |
5.1. Hydrogels
Hydrogel-based scaffolds exhibit unique characteristics that make them suitable for regenerating soft tissue. These scaffolds have regulating and unprecedented physicochemical properties, which create 3D-supporting substrates for various cellular processes. Moreover, they establish a hydration network within a three-dimensional space, are injectable [108], and can adapt to irregular injury site patterns of different dimensions and shapes. The printability capacity of hydrogel presents an opportunity to establish hydration networks in three-dimensional space, which can be adapted to various sizes and forms of neural tissues and utilized as substrates for the adhesion, proliferation, and differentiation of neurogenic cells, particularly axons [47, 109, 110]. Furthermore, their application has facilitated the delivery of encapsulated cells, ions [111, 112], bioactive molecules, growth factors [108], nanoparticles [112], and drugs via controlled delivery vehicles in peripheral nerve tissue engineering. Also, hydrogels can transport medicines and growth factors to specific tissue areas with varying concentration gradients.
Among the various biomaterials employed in scaffolding, hydrogels based on naturally occurring biological macromolecules, particularly proteins, polysaccharides, and extracellular matrix hydrogel [113], have demonstrated remarkable outcomes due to their unique physicochemical properties and physiological relevance. The structural attributes of hydrogels can be significantly transformed by environmental factors such as pH, temperature, light intensity, and enzymes, leading to self-assembled peptide (SAP) hydrogels (Figure 3).
5.2. Nanofibrous Scaffolds
Advancements in nanomedicine have enabled the regeneration of peripheral nerves using fiber scaffolds at the micro- or nanoscale level, which have become valuable tools in tissue engineering. Fibrous scaffolds are a better alternative to nerve suturing and grafting [2]. However, nanofibers are more effective than microfibers as they promote better cell adhesion, expansion, and proliferation. Nanostructured neural scaffolds have demonstrated promising results in enhancing their bulk and surface properties [6, 114].
The nanoscale surface area, combined with electrical activity, creates an environment that promotes cell adhesion and regulates cell response through the controlled release of bioactive molecules. This property facilitates the regeneration of peripheral nerves through nanofibers and nanoparticles, which also aid in drug delivery [49, 115]. Scaffolds at the nanoscale have the potential to regulate cell cycle progression, cellular viability, and growth factor secretion through surface modifications. These findings significantly affect the field of tissue engineering [9, 115]. For example, electrospinning enables precise control over fiber mechanical properties, size, structural regularity, diversity, fiber orientation, and conduit shape while incorporating various sources such as materials, cells, drugs, and growth factors [116].
Despite the established toxicity of metallic nanoparticles, whether employed individually or in conjunction with scaffolds or conduits, they have demonstrated the ability to manipulate cellular behavior, modify the topography of scaffolds and conduits, increase the secretion of neurotrophic factors, improve ion flow, and regulate electrical signals [117].
5.3. Acellularized Scaffolds
Utilizing acellularized ECMs is an effective strategy for making nerve conduits, especially for commercially viable grafts [113, 118]. Acellularized natural tissue can provide a suitable environment for cell growth and proliferation by creating a cell niche [3]. Autologous, allogeneic [119–121], or xenogeneic [119, 122, 123] neural and non-neural tissues were acellularized recently. A notable benefit of this process is the prevention of immune reactions, particularly in xenograft tissue [84, 122, 124].
Utilizing an acellularized ECM has been shown to positively impact the continuous delivery of cells and NFs while inducing the release of NFs such as NGF, GDNF, and BDNF. Furthermore, it has been observed to increase the expression of markers associated with SCs and angiogenesis, specifically VEGF and CD34. Moreover, it promotes axonal regeneration and functional recovery and decreases inhibitory chondroitin sulfate proteoglycans [47, 125–127]. The acellular scaffold exhibits similarities to an autograft by its ability to preserve laminin. This preservation of laminin is a crucial factor in the scaffold’s ability to support tissue regeneration.
Acellular nerve grafts have significant drawbacks, such as loss of ECM components and natural ultrastructure. However, these issues can be addressed with optimized multichannel acellular nerve allografts and a modified protocol that preserves more ECM bioactive molecules and regenerative factors while eliminating cellular antigens [128].
Different acellularization techniques, including physical [129], chemical [45], enzymatic, and thermal [130] methods, have been employed to remove cellular components and immunogenic agents. The optimal acellularization protocol should preserve ECM proteins and native nerve architecture. Previous studies recommend implementing an acellularization protocol that prioritizes the preservation of ECM proteins, specifically collagen and laminin while maintaining the native nerve architecture to achieve the most favorable outcome [2, 47]. Multiple studies have demonstrated that using sodium dodecyl sulfate (SDS) [126], Triton X-100, and 3-[(3-cholamidopropyl) dimethylammonio]-propane-1-sulfonate (CHAPS) at the appropriate concentration and duration represents the optimal selection [122] (Figure 3).
6. Effect of Scaffolds on Angiogenesis
Improper vascularization frequently leads to tissue necrosis in numerous cases, and neovascularization is necessary for the survival and integration of scaffolds. So, this treatment, with insufficient neovascularization and poor nutrient supply, leads to incomplete functional recovery, especially for significant nerve defects. Research on peripheral nerve scaffolds focuses on enhancing neuronal and axonal repair rather than vascularization. The application of diverse cells (such as human umbilical vein endothelial cells (HUVECs), fibroblasts, or pericytes), growth factors (NO, VEGF, PDGF, and IKVAV), and biomaterial scaffolds (biogenic conduits, soft lithography, micropatterning, and photolithography) in tissue engineering offers a unique strategy for vascularizing long-gap nerve repair [131]. There is a lack of data on combining bioactive factors for improving nerve regrowth and vascularization [132]. Inspiration from analogous tissues is recommended to attain vascularized nerve tissue. In tissue engineering, the various molecules and factors and broad complex pathways are observed to have comprehensive utilization and an impact on the process of angiogenesis [6]. Immobilizing angiogenesis-related biofactors on scaffolds can potentially enhance endothelial cell tube formation, angiogenesis, and revascularization. Dual-biofunctionalized chitosan/collagen scaffolds with IKVAV/VEGF-enhanced neural processes such as neovascularization, axonal regeneration, myelination, and neuromuscular transmission. The designed scaffolds support the proliferation and differentiation of Schwann cells and endothelial cells, aiming to stimulate neovascularization and nerve regeneration. VEGF-related factors from SCs induce arterial differentiation and promote vascular patterns. Endothelial cells secrete nitric oxide to suppress Schwann cell dedifferentiation [132]. These pathways show the complex interaction between vessels and nerves. Controlled delivery of VEGF and rNGF on the surface and core of emulsion electrospun poly (l-lactic acid) (PLLA) nanofiber could affect the regeneration of vascularized nerve tissue [133].
Despite its short half-life, nitric oxide (NO) is a vital signaling molecule in capillary development, vascular growth, and axon regeneration. Three nitric oxide synthase (NOS) isoforms are present in the peripheral nerves. These isoforms include the inducible isoform (iNOS) in various cell types such as macrophages and glial cells. The endothelial isoform (eNOS) is present in the vascular endothelium. The third isoform is the neuronal isoform (nNOS) located in specific neuronal populations [6, 94, 134, 135].
7. Effect of Scaffold on Immune Response and Inflammation
PNI causes Wallerian degeneration, which leads to a complex and dynamic microenvironment. The cellular and molecular changes in the microenvironment lead to an imbalance in immune cells and their modulatory factors. The neuroinflammatory response to PNI causes tissue damage, repair processes, inflammation, and rejection postimplantation. The regeneration and inflammation processes consist of various stages and specific populations of cells, including SCs, resident and hematogenous macrophages, and T cells. The immune system, especially T cells, plays a crucial role in nerve regeneration. T cells exhibit both neuroprotective and neurodegenerative roles. In particular, Th2 and Treg cells secrete IL-4 and IL-10, which promote nerve repair. In contrast, Th1 and Th17 cells release IFN-γ or IL-17 for enhancing acute inflammatory responses. Modulating the immune response using various therapies that regulate T cells, such as biomaterials design, cell therapy, immunomodulators/cytokines/chemokines, or immunosuppressive drug delivery, is seen as a promising method to improve outcomes related to neuroinflammation and neurodegeneration.
Immunosuppressive therapy is a treatment option for severe traumatic injuries. However, it can be hindered by inflammation that affects multiple tissues. Biomaterial strategies should be considered to address graft rejection and inflammation in various tissues to improve future scaffold designs. The ideal scaffold should have an immunomodulation design that complements nerve regeneration promotion strategies. Material characteristics, such as chemistry, macroarchitecture, surface topography, surface coating, and stiffness, can influence the control of the immune response. Systemic anti-inflammatory or immunomodulation drugs may also enhance nerve regeneration [136–139].
8. Clinical Step: Advanced Surgical Technologies and System
Managing peripheral nerve injuries poses significant challenges due to their complex nature and vital functions. Surgeons employ a variety of systems and technologies, ranging from traditional surgical techniques to advanced regenerative medicine, to enhance nerve repair and functional recovery. Table 6 presents a compilation of several commercial products currently available. This section examines several critical methods and tools currently utilized in this field. Since complete explanations of nerve grafting, regenerative medicine, and tissue engineering were previously provided, this section skips those cases.
| Model | Product name and company | Material | Description |
|---|---|---|---|
| Nerve conduits (nerve guides) |
|
Porcine small intestine submucosa (SIS) |
|
|
Type I collagen | (i) A biodegradable scaffold that supports nerve regrowth | |
|
Polyglycolic acid (PGA) |
|
|
|
Type I collagen |
|
|
| Nerve Allografts |
|
Decellularized human nerve allograft |
|
| Reprocessed human nerve allografts | Cadaveric nerve |
|
|
| Nerve wraps |
|
Porcine small intestine submucosa (SIS) |
|
|
Type I collagen |
|
|
| Bioengineered nerve scaffolds | Reaxon nerve guide (Medovent GmbH) | Chitosan |
|
| NeuroRegen Scaffold (MedXpress) | Collagen | (i) Combining a physical scaffold with growth factors to enhance nerve repair | |
| Neurotube (Synovis Micro Companies Alliance) | Polyglycolic acid (PGA) |
|
|
| Neurolac nerve guide (Polyganics) | Poly (DL-lactide-ε-caprolactone) |
|
|
| Electrical stimulation devices (neurostimulation devices) | ActiGait (Neurodan) | — |
|
| ReActiv8x (Mainstay Medical) | — |
|
|
8.1. Microsurgical Techniques
- (i)
Operating microscope
-
This tool provides the necessary magnification for delicate nerve repair, enhancing visibility and precision.
- (ii)
Microinstruments
-
These specialized tools handle delicate nerve tissues, enabling meticulous manipulation and suturing.
- (iii)
Sutures and glues
-
Ultrafine sutures, as small as 10-0, and biological glues are utilized to reattach nerve ends with minimal trauma to surrounding tissues.
8.2. Neurostimulation and Electrostimulation
Neurostimulation techniques are increasingly pivotal in augmenting nerve regeneration and function. Key modalities encompass transcutaneous electrical nerve stimulation (TENS) and implantable nerve stimulators, demonstrating substantial potential in fostering nerve growth and facilitating functional recovery.
8.2.1. Transcutaneous Electrical Nerve Stimulation
TENS is a noninvasive technique that administers electrical impulses to nerves to alleviate pain. It has been widely used to manage various types of chronic pain by targeting sensory fibers to block pain signals. It has been widely used to manage various types of chronic pain by targeting sensory fibers to block pain signals. Research suggests that TENS helps in pain management and enhances neuroplasticity, aiding in the recovery of motor functions. The mechanism involves modulating inhibitory circuits and enhancing reciprocal inhibition, which can be particularly beneficial in conditions such as spasticity following spinal cord injuries [141, 142].
8.2.2. Implantable Nerve Stimulators
Implantable nerve stimulators provide consistent electrical stimulation near the nerve injury site. These devices promote nerve growth and improve functional recovery by directly stimulating the nerves. Studies have shown that using these stimulators can augment the natural regenerative processes of peripheral nerves and contribute to improved reinnervation outcomes. It is important to note that these stimulators are often utilized with other interventions, such as conduits, to guide nerve regrowth effectively [143, 144].
Neurostimulation techniques have the potential to significantly enhance the treatment of nerve injuries by improving synaptic plasticity and promoting axonal regeneration. This innovation promises improved outcomes for individuals with peripheral nerve damage. Further human studies must refine these techniques and facilitate their broader integration into clinical practice.
8.3. Robotic Surgical Systems
8.3.1. Robotic Systems
Robotic surgical systems such as da Vinci’s have significantly advanced nerve surgeries by elevating precision and overall surgical outcomes. These innovative systems have notably enhanced skill and accuracy in intricate microsurgeries. The da Vinci system, for instance, employs robotic arms to execute delicate operations, enabling meticulous manipulation of nerve tissues. This system offers substantial advantages, including enhanced visualization facilitated by a 3D endoscope, reduced impact of human tremors, and the capability to adjust movements for heightened precision [145, 146].
8.3.2. Laser Devices
Laser devices are increasingly employed in nerve surgeries due to their precision in cutting and sealing nerves, resulting in minimized collateral damage and improved healing outcomes. These devices enable surgeons to perform minimally invasive procedures with increased accuracy, leading to quicker patient recovery and fewer postoperative complications [147].
8.4. Exoskeletons and Rehabilitation Devices
8.4.1. Exoskeletons
Wearable robotic devices, or exoskeletons, are essential in postsurgical rehabilitation as they aid patients in movement and muscle-strengthening exercises. These devices play a crucial role in helping patients to regain mobility and promote nerve recovery. For instance, the “Float” upper-limb exoskeleton is specifically designed to enhance motor and functional recovery of the shoulder joint following injuries. It enables patients to perform various activities of daily living (ADLs) by providing support without bearing the weight of the exoskeleton itself [148]. In addition, research has shown that robotic exoskeletons can significantly improve rehabilitation outcomes by offering consistent, controlled assistance and resistance during exercises, which are vital for rebuilding strength and coordination [149].
8.4.2. Virtual Reality (VR) Systems
VR systems are more widely used in rehabilitation to involve patients in interactive exercises stimulating nerve function. Rehabilitation programs based on VR provide an immersive environment that encourages patients to participate in activities that resemble real-life tasks, thus assisting in the recovery of motor skills and cognitive functions. Research has shown that VR systems can improve patient motivation and adherence to rehabilitation protocols, resulting in better functional recovery outcomes [148, 149].
8.5. Advanced Prosthetics
Advanced prosthetics offer crucial functional support for cases where nerve injuries result in limb loss, mainly through myoelectric prosthetics and targeted muscle reinnervation (TMR). These advanced prosthetic technologies are crucial for improving amputees’ mobility and quality of life, with ongoing innovations to enhance their effectiveness and user experience [150].
8.5.1. Myoelectric Prosthetics
Myoelectric prosthetics are controlled by electrical signals from the remaining muscles, which provide precise and responsive control. These devices use surface electrodes to detect muscle electrical activity, allowing hand opening and closing movements. Despite advancements, challenges persist in achieving natural, intuitive control. The ongoing research is focused on improving the accuracy and usability of these systems.
8.5.2. Targeted Muscle Reinnervation (TMR)
The surgical procedure known as TMR enhances the control of prosthetic limbs by reassigning nerves to different muscle groups. This innovative technique involves transferring residual arm nerves to nearby muscles, which are then reinnervated to provide more natural control signals for the prosthetic. TMR not only improves the functionality of myoelectric prosthetics but also addresses issues such as phantom limb pain and neuromas, thus offering a comprehensive solution for individuals with limb amputations.
9. Conclusions
Despite the considerable advancements in medicine, the regeneration process of peripheral neural tissue still presents a challenge. Overcoming gaps more prominent than 30 millimeters is a critical barrier in the regeneration process. Nerve system modeling is challenging due to the diverse cellular structures and complexity of regulating cell-to-cell connections and interactions. Another hurdle is the proper utilization of topographical cues and the timely and spatial release of biochemical signals, which play an active role in regulating the rate of nerve regeneration progression. The complicated and multifaceted nature of the body’s response to scaffolds is another confusing factor, ultimately transforming the microenvironment.
The first measure in developing alternative interventions requires a broad understanding of the anatomy and physiology of the peripheral nervous tissue. By understanding the critical elements that lead to adverse functional outcomes following nerve restoration, we can identify opportunities for therapeutic intervention.
One of the critical challenges in the engineering of peripheral nerves pertains to the organization of various sensory and motor neurons within a nerve branch. This process involves simulating a complex mixture of biomaterials next to the need for distinct concentrations of growth factors. Consequently, achieving a universally applicable and fully operational neuron may be an unattainable objective. These elements make the construction process challenging and increase complexity in future assessments [151].
Combining biomaterials and fabrication techniques with cellular technology and tissue engineering is a successful approach to achieving an optimal scaffold that can enhance response rates and expedite regeneration. This multifaceted methodology is currently being utilized to advance the field of scaffold fabrication. The supplementation of biomolecules, transplantation of stem cells, and modulation of cell surfaces through neural scaffold engineering may yield further synergistic effects, potentially exceeding the efficacy of the current clinical method. The other concern is designing an ideal delivery system that effectively controls the spread of the vital cue or drug throughout the entire nervous system regeneration process until functional reinnervation. The interaction between scaffolds and host cells/tissues is a significant factor in nerve regeneration. It is worth noting that cells and neurotrophic factors enrich the scaffold, providing a conducive ECM and microenvironment for the successful regeneration of nerves. Despite extensive research, the optimal cell dose and fate remain ambiguous.
10. Future Directions
The current tissue engineering strategies, although helpful, are not perfect. Therefore, there is a need for new techniques that can help improve the regeneration and overall function of engineered neural tissues. In the future, it may be possible to achieve the physiological behavior and better functionality of engineered neural tissues by developing a better understanding of biomaterials and engineering methods and making advancements in cell technology and molecular gradients. The main goal is to increase the number of axons that can regenerate and move through the repair site while also speeding up their growth towards the distal stump.
The construction of engineered neural tissues requires complex interactions multifaced between physicochemical and biological cues, and it is essential that all structures, whether synthetic or natural, are matched in their structural, biological, mechanical, and biochemical compatibility with autografts. Thus, in the future, there is a requirement to develop materials that offer more advantages for reinnervation and more advanced methods for constructing guidance structures.
10.1. Data Selection
Relevant article selection was based on specific inclusion criteria. A thorough literature search (up to May 2023) was conducted using different online databases (PubMed, Google Scholar, and Web of Science).
Isolation criteria were based on terms such as “peripheral nerve regeneration,” “stem cells,” “scaffold,” “biomaterials,” and “tissue engineering” in different combinations. Experiment design, sample size, biomaterial selection, fabrication method, seeded cells or growth factors, application, and results were recorded for each study. Original research articles, selected review reports about the objectives, and articles published in English were included. We isolated 151 studies about advances in peripheral nerve tissue engineering.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
All authors made significant contributions in the following areas: conception and design of the study, acquisition of data, analysis and interpretation of data, and drafting of the article. They have also provided their final approval for the article version to be submitted and have committed to being personally accountable for their contributions. The specific author contributions are as follows: MJF and FK wrote the main manuscript. SA supervised and reviewed the manuscript. AR supervised, reviewed, edited and wrote the part of the manuscript with the insights of all other authors.
Acknowledgments
The authors acknowledge the use of AI tools for natural language processing, which has significantly enhanced the quality and efficiency of our research.
Open Research
Data Availability
The data supporting this review are from previously reported studies and datasets, which have been cited.




