Modeling and Analysis of Fasciola Hepatica Disease Transmission
Abstract
In this paper, a mathematical model for the transmission dynamics of Fasciola hepatica in cattle and snail populations is formulated and analyzed. The snail mortality rate (μs) is the most important factor that indirectly impacts the basic reproduction number (R0). A 50% change, either an increase or decrease, in the snail mortality rate will result in an approximate 50% change in the opposite direction in the value of R0. The model shows a forward bifurcation at R0 = 1, indicating that the disease dynamics undergo a critical transition at this threshold. This change signifies a transition from a disease-free state to a persistent infection, highlighting the possibility of a continuous disease presence given specific epidemiological conditions. Simulations show that reducing miracidia, metacercariae, and snail populations, improving treatment, and lowering pathogen transfer between cattle and snails significantly decrease disease prevalence in cattle. To control the disease, transmission rates for cattle and snails must be reduced below γc = 1.4338 × 10−7 and γs = 1.1473 × 10−8, respectively. Current treatments are insufficient, and a combination of improved treatments reduced transmission rates, and increased snail mortality is recommended for better disease control.
1. Introduction
Fasciololasis (known as “liver fluke”) is a zoonotic disease induced by two parasitic flatworms or trematodes called fasciola gigantica and fasciola hepatica, which primarily affect the liver [1, 2]. The World Health Organization (WHO) has prioritized it as a neglected tropical disease that can affect both humans and livestock [3, 4]. It is a worldwide disease that primarily affects domesticated pets like cattle, goats, and sheep, but it can also affect humans [5]. Animal fascioliasis is a major issue that has a global impact on livestock productivity [6] and a serious problem in the veterinary field all over the world [7]. The fasciola parasite is observed in the livers of infected remnants as well as the bile ducts. Fasciololasis is a waterborne and foodborne zoonosis that is commonly acquired by drinking and eating the parasite metacercaria that is encysted in water and food [1].
Fascioliasis is becoming more common worldwide, and there is a strong correlation with a high infection rate among ruminant definitive hosts. Except for Antarctica, fascioliasis is endemic worldwide [8]. Fasciola hepatica is found worldwide but is most common in temperate areas, whereas Fasciola gigantica is only found in certain parts of Asia and Africa [2]. Both of these liver flukes are extremely pathogenic [4].
Fasciolosis has already infected 600 million animals, and ~180 million people are in danger of infection globally [9]. It is a significant parasitic disease affecting large livestock, including over 700 million animals potentially infected [10]. The WHO estimates that more than 2.4 million people, primarily in developing countries, are infected with F. hepatica [3, 11, 12, 13]. Bovine fascioliasis is a worldwide zoonotic disease, it occurs in over 70 countries [14], especially where cattle and sheep are reared. Likewise, human fascioliasis is currently spreading geographically and reemerging in several nations, with an increase in prevalence and severity [15, 16]. Global animal economic output losses due to fasciolosis are estimated to be more than $3.2 billion USD per year [13], with a direct or indirect damaging influence on milk and meat gain of 8%–80% [17, 18].
Clinical signs, grazing history, seasonal occurrence, laboratory tests on the faces, postmortem examination, and the existence of juvenile and adult fluke inside the liver are used to diagnose bovine fasciolosis [12, 19]. The premature worms begin penetrating the gut wall during the acute phase, causing symptoms such as vomiting, intermittent fever, skin rash, itching, nausea, malaise, swollen liver, extreme abdominal pain, weight loss, diarrhea, and anemia [1]. Triclabendazole is the preferred treatment for remnants of fascioliasis [12], but there is not a vaccine capable of protecting against liver fluke disease [20]. The intermediate host population must be reduced to control bovine fasciolosis, or an anthelmintic must be used [19, 21].
F. hepatica does have an indirect life cycle that involves several larval developments, including egg, miracidium, cercaria, metacercaria, and adult fluke [16]. The life cycle of the hepatica liver fluke starts whenever a female produces offspring in the hepatic of the main host. Immature embryos are evacuated into the environment via the feces after being liberated from the bile duct. Eggs are immersed in water, then they develop into embryos that hatch into miracidia larvae. The miracidium swims and invades an amphibian snail (intermediate host of the parasite). It infiltrates snails and rapidly progresses to the next stage, called sporocyst. Then, it transforms into redia, which allows the production of cercariae a few months later [5]. The presence of numerous cercariae issuing from the snail ensures that the life cycle will continue [3]. The cercariae release and leave the snail to attach to the vegetation, becoming infectious metacercariae. A definite host consumes the freshwater plant that contains the cyst.
Understanding and managing the spread of infectious diseases requires mathematical models. Mathematical population modeling is frequently used to analyze the dynamic behavior of epidemic diseases [22]. Some mathematical models, such as [5, 23, 24, 25, 26] developed to investigate the transmission and control of fasciola spread. Turner et al. [23] developed a methodology for evaluating the effectiveness of vaccinations in controlling fasciola spread. The study considered a vaccination that only provides limited protection for fasciola hepatica disease. The findings indicate that vaccination can reduce the burden and egg output, but not prevent infections. It may also reduce fluke fertility, lengthen average fluke maturation time, and increase juvenile fluke mortality. Diaby et al. [5] examined a mathematical model for fasciola hepatica disease. The study suggested that combining quarantine with medical therapy could be a successful disease prevention strategy.
This paper presents a deterministic mathematical model to characterize the transmission dynamics of F. hepatica disease in the cattle population. The model incorporates different transmission pathways and life stages of the F. hepatica parasite, including its interactions with cattle, and snail populations. Additionally, the research performs sensitivity analysis to evaluate the influence of different parameters on disease prevalence, providing essential insights for developing targeted intervention strategies. The study highlights the importance of integrated control strategies that target both the parasite and hosts, including reducing transmission, improving treatment, and controlling snail populations to ensure sustainable disease management.
2. Model Formulation
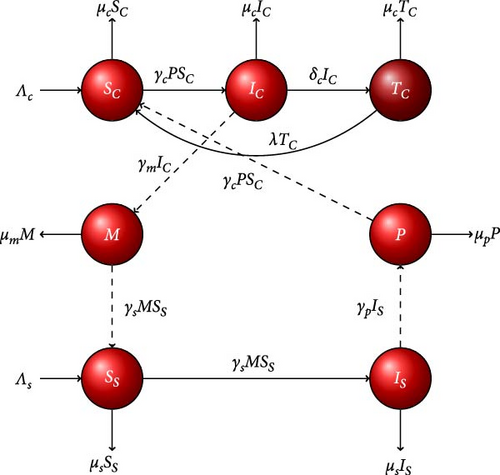
F. hepatica parasites live in three environments: free-living fasciola larvae, snails, and the cattle biological environment. The cattle population is split into three subpopulations: susceptible cattle population (SC(t)), infected cattle population (IC(t)), and treated cattle population (TC(t)) at any time. The snail population is divided into two classes: susceptible and infected snails, denoted by (SS(t)) and (IS(t)), respectively. Similarly, fasciola larvae have two subpopulations: miracidium (M(t)) and metacercariae (P(t)).
Susceptible cattle progress to infection at a rate γc, by consuming metacercaria on the pasture. The infected cattle are treated at rate δc and the treated cattle recover at a rate λ. All susceptible, infected, and treated cattle have a natural death rate of μc. The infected cattle release eggs and hatch into freely floating larvae known as miracidia at rate γm. The portion of the miracidia population penetrates the snail at rate γs. Figure 1 depicts the flowchart of the fasciola hepatica transmission model.
| Parameter | Epidemiological description | Value | Source |
|---|---|---|---|
| Λc | Cattle recruitment rate | 67/day | [27] |
| Λs | Snail recruitment rate | 3,000/day | [28] |
| μc | Cattle natural death rate | 0.0185/day | [27] |
| μs | Snails natural death rate | 0.001644/day | [27] |
| μm | Natural death rate for miracidia larvae | 0.9/day | [28, 29] |
| μp | Natural death rate for metacercariae larvae | 0.6452/day | [27] |
| γm | Rate of cattle produces miracidia | 6.96/cattle/day | [28] |
| γp | Rate of snail produces metacercariae | 2.6/snail/day | [28] |
| γc | Per capita infection rate of cattle | 0.0000005/cyst/day | [27] |
| γs | Per capita infection rate of snails | 0.00000004/miracidia/day | Assumed |
| δc | Treatment rate of infected cattle | 0.65/day | Assumed |
| λ | Treatment efficiency of the infected cattle | 0.9567/day | [27] |
3. Model Analysis
3.1. Feasible Region and the Disease-Free Equilibrium Point
3.2. Local Stability of the Disease-Free Equilibrium
Since Model (1) satisfies axioms (A1)–(A5) of Theorem 2 in van den Driessche and Watmough [30], we have the following result.
Lemma 1. The disease-free equilibrium point is locally asymptotically stable in Ω whenever R0 < 1 and unstable if R0 > 1.
3.3. Global Stability of Disease-Free Equilibrium
-
C1 When , U∗ is globally stable.
-
C2 for (U, V) ∈ Ω.
B = DVQ(U∗, 0) is Metzler-matrix (off-diagonal entries of B are non-negative), and Ω is the set at which the system becomes feasible (see the description of the method in Kadaleka et al.’s [33] study).
Theorem 1. The disease-free equilibrium, E0, is globally asymptotically stable if R0 < 1 and unstable otherwise.
Proof. From Models (1) and (7), we have the following equations:
Clearly, is the global asymptote of Equation (8) and hence the condition C1 is satisfied. Also, from Equation (12), for and . Thus, , ∀(U, V) ∈ Ω, and condition C2 holds. Since the nondiagonal entries are nonnegative, B is an M-matrix. This demonstrates that the DFE is globally stable whenever R0 < 1.
3.4. Existence of Endemic Equilibrium Point
Lemma 2. Model (1) has a unique endemic equilibrium for R0 > 1.
Thus, , , , M∗ > 0, , , and P∗ > 0 if R0 > 1. This proves that the disease becomes endemic if R0 > 1.
3.5. Local Stability of Endemic Equilibrium Point
Lemma 3. The endemic equilibrium point, , is locally asymptotically stable if R0 > 1; otherwise, it is unstable.
Applying Castillo-Chavez and Song’s [34] theorem, the model exhibits a forward bifurcation (refer to the proof of Theorem 2), which ensures the endemic equilibrium is locally asymptotically stable if R0 > 1.
3.6. Bifurcation Analysis
Bifurcation theory examines the qualitative changes in system behavior, particularly the stability or instability of fixed points and periodic orbits, as parameters vary. It is a useful tool for analyzing qualitative changes in steady-state solutions [35]. The occurrence of a forward bifurcation indicates that endemic equilibrium is locally stable if R0 > 1 [36].
Theorem 2. Model (1) has a forward bifurcation when R0 = 1.
Proof. We use the center manifold theorem in Appendix A to prove this theorem. For the sake of simplicity, the following change of variable is made for Model (1):
SC = y1, IC = y2, TC = y3, M = y4, SS = y5, IS = y6, and P = y7. Thus, Model (1) transforms into the following equation:
a1 = δcμm + μcμm + δcμp + μcμp + μmμp + δcμs + μcμs + μmμs + μpμs, and
a0 = δcμmμp + μcμmμp + δc μmμs + μcμmμs + δcμpμs + μcμpμs + μmμpμs.
Here a2, a1, and a0 are positive and a2a1 − a0 > 0. Thus, the Jacobian Jϕ(E0) has a simple zero eigenvalue, with all remaining eigenvalues having negative real components by the Routh–Hurwitz criterion. Therefore, the center manifold theory can be used to examine the behavior of the model. Let l = (l1, l2, l3, l4, l5, l6, l7) and h = (h1, h2, h3, h4, h5, h6, h7) are left and right eigenvectors of J(E0).
The left eigenvectors are obtained by multiplying l by J(E0) and equating with 0. So after evaluating, the left eigenvector is as follows:

3.7. Global Stability of Endemic Equilibrium
Theorem 3. If R0 > 1, then the endemic equilibrium point, , is globally asymptotically stable.
Proof. The following Lyapunov function can be designed:
At the endemic equilibrium point, , while it is positive for all other points in the set Ω, except for this specific equilibrium point. When differentiating the function V with respect to t, we obtain the following equation:
Substituting the derivative of each state variable from Equation (1) into Equation (23) yields the following equation:
Replacing the requirement for the existence of an endemic equilibrium point provides the following equation:
Rearranging Equation (24) yields the following equation:
If A ≤ B, then , satisfying the third condition of the Lyapunov criteria. As a result, the singleton , which represents the endemic equilibrium point, becomes the largest invariant set in Ω. Therefore, based on Lasalle’s invariance principle [38], it can be concluded that is globally asymptotically stable within Ω if A ≤ B. This condition holds if and only if R0 > 1.
4. Sensitivity Analysis
| Parameters | Sensitivity indices | |
|---|---|---|
| Λc | +0.5 | |
| Λs | +0.5 | |
| μc | −(δc + 2μc)/2(δc + μc)) | −0.5023 |
| μs | −1 | −1 |
| μm | −0.5 | |
| μp | −0.5 | |
| γm | +0.5 | |
| γp | +0.5 | |
| γc | +0.5 | |
| γs | +0.5 | |
| δc | −δc/2(δc + μc) | −0.497698 |
The results also show that the mortality rates of miracidia, metacercaria, and snail populations, as well as the treatment rate of infected cattle, indirectly impact the basic reproduction number. The snail mortality rate is the most significant parameter that has an indirect effect on R0. A 50% increase or decrease in the snail mortality rate (μs) will lead to an approximate 50% decrease or increase in the value of R0, respectively. Therefore, to prevent the spread of fasciola hepatica infection in cattle, stakeholders should increase treatment rates and the mortality rates of snail, miracidia, and metacercaria populations.

5. Numerical Simulations
This section conducts simulations using the parameter values listed in Table 1 to investigate the qualitative dynamics of fasciola hepatica disease spread. While many of these parameter values are sourced from existing studies, some are proposed specifically for the simulation. Numerical simulation has been performed using MATLAB ode45 code.
5.1. The Stability Behavior of Endemic Equilibrium
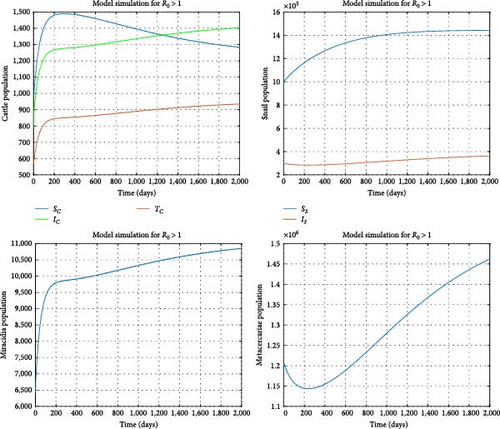
Simulations are performed for the first 2,000 days, with initial conditions set as . Using Table 1 parameter values in Model (1), we found the reproduction number, R0 = 1.93597 > 1. Indeed, using these parameter values in Model (1), we obtain a unique endemic equilibrium point . As shown in the graphical illustrations in Figure 4, all solution trajectories of the system eventually converge to the endemic equilibrium point, . As proved by the analytic solution, the endemic equilibrium is locally stable whenever R0 > 1. Biologically, the fasciola hepatica disease will continue to spread among susceptible hosts unless an external intervention mechanism is designed.
5.2. The Stability Behavior of Disease-Free Equilibrium
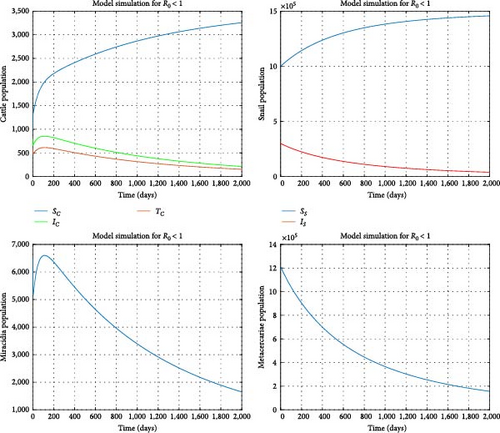
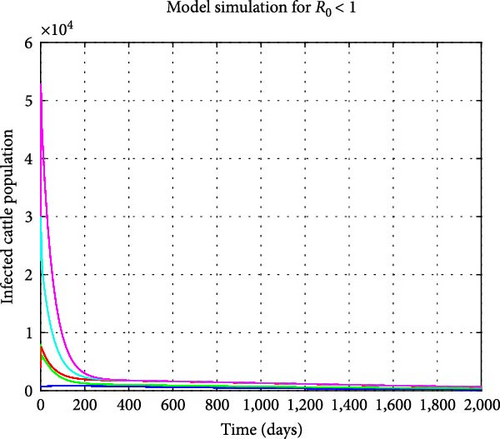
Assuming the transmission rates γc and γs are reduced to 0.0000003 and 0.00000002, respectively, the treatment rate δc is increased to 0.7, and the mortality rate of the snail population is elevated to 0.002. We find the reproduction number to be R0 = 0.840751 and the disease-free equilibrium to be . Figure 5 illustrates that all model solution trajectories converge to the disease-free equilibrium (DFE) over time, indicating its local stability. Additionally, Figure 6 indicates that the number of infected cattle declines to zero over time across various initial conditions, suggesting that the DFE is globally stable. This further demonstrates that the disease will eventually be eradicated in the cattle population. In contrast, the susceptible cattle and snail populations will reach their maximum values of and , respectively. From a biological perspective, it can be concluded that fasciola hepatica disease in cattle can be eradicated when R0 < 1. Stakeholders should aim to minimize the reproduction number as much as possible through interventions to expedite the eradication of the disease.
5.3. Contour and Surface Plots of R0 for Combination of Parameters
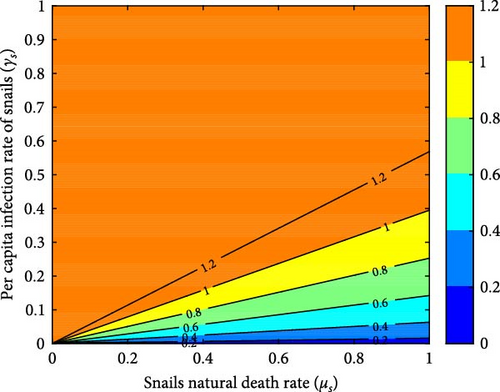
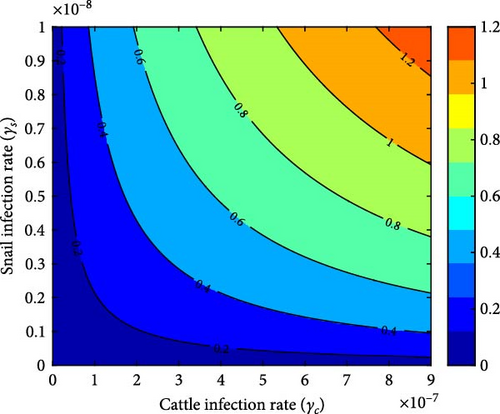
Figures 7(a), 7(b), 7(c), and 7(d) depict how the reproduction number is impacted by two control parameters, as demonstrated through the contour plots. Figure 7(a) clearly illustrates the necessity of giving utmost priority to the region below the yellow color when implementing snail control measures and proficiently managing the per capita infection rate of snails to accomplish successful eradication of the disease. When the snail death rate is low, there is an inverse relationship, causing the reproduction number to increase and the disease to persist. Figure 7(b) emphasizes the crucial role of snail and cattle infection rates in the transmission and impact of F. hepatica disease in cattle. It highlights that higher snail infection rates lead to a larger reservoir of parasites in the environment, which subsequently increases the risk of cattle exposure to the parasite. To effectively control and potentially eradicate the disease, policymakers should aim to minimize these infection rates to a level where the reproduction number is less than 1.
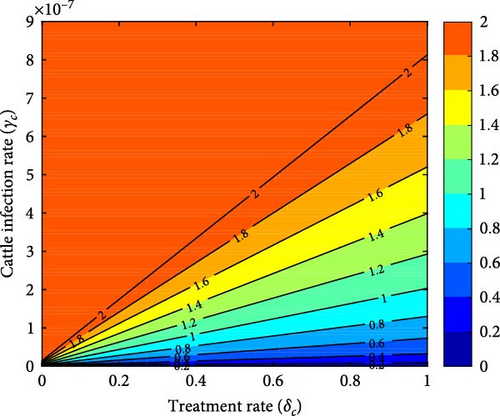
The contour plot and surface plot shown in Figures 7(c) and 8 suggest that reducing the treatment rate and cattle infection rate results in an R0 value below one, indicating a promising approach for controlling the disease. This finding suggests that interventions targeting the treatment rate and cattle infection rate can effectively limit the spread of the disease and potentially lead to its eradication.
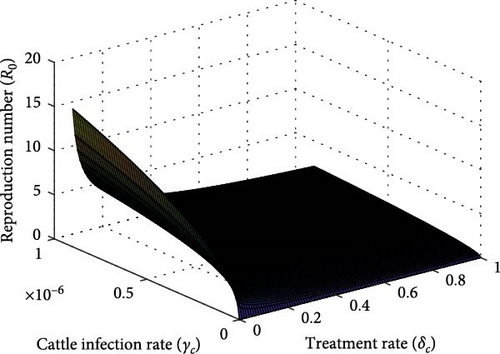
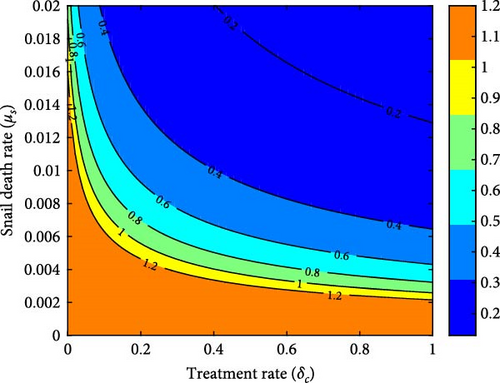
Policymakers and healthcare professionals might concentrate on establishing strategies that aim to reduce the treatment and cattle infection rates. These measures might include improving access to treatment options, implementing effective preventive measures for cattle, and adopting biosecurity protocols to minimize the risk of disease transmission. Finally, Figure 7(d) demonstrates that simultaneously improving the combined treatment rate for infected cattle and snail mortality rate leads to a lower reproduction number. Without adequate adjustments to these rates, the reproduction number remains high, indicating the persistence of the disease.
5.4. Parameter Impact on the Disease Dynamics
5.4.1. Effect of per Capita Infection Rates
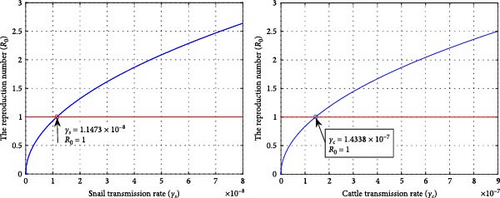
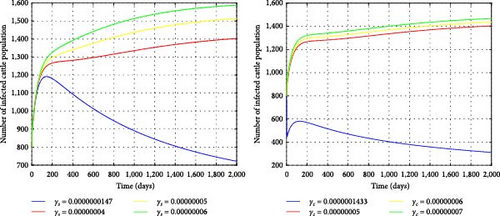
Figure 9 illustrates a graphical representation of the reproductive number in terms of disease transmission rates of cattle and snails based on parametric values in Table 1. It displays that, as the values of γc and γs increase, R0 also increases and becomes greater than one to the right of and . This shows that disease transmission between cattle and snails significantly contributes to the endemicity of fasciola hepatica. If we consider the transmission rates lower than and , the disease will die out unless it persists. Figure 10 depicts the effects of snail transmission rate on the infected cattle using various values of γs = 0.0000000147, 0.00000004, 0.00000005, 0.00000006. In the same manner, by considering different values of γc = 0.0000001433, 0.0000005, 0.0000006, 0.0000007, the impact of per capita infection rates on cattle has been investigated. Higher infection rates in both cattle and snails lead to an increase in infected cattle. Therefore, to control the spread of the disease in the cattle population, the transmission rates for cattle and snails should be reduced to below and , respectively.
5.4.2. Effect of the Cattle Treatment Rate
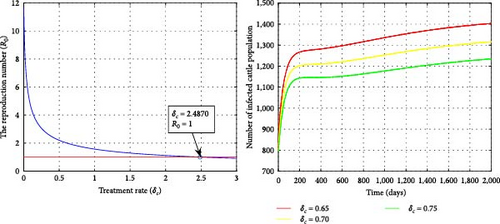
Figure 11 illustrates the impact of the treatment rate (δc) on R0 and the disease epidemiology. It has been shown that as the treatment intensity increases, both R0 and the number of infected cattle decrease. From an epidemiological perspective, improving treatment can reduce the number of infected cattle. For small values of the treatment rate, R0 remains greater than 1. As a result, the solution graphs of the system will converge to the endemic equilibrium point, allowing the infection to persist. Increasing the treatment rate and reducing disease transmission can help decrease the incidence of fasciola hepatica infection.
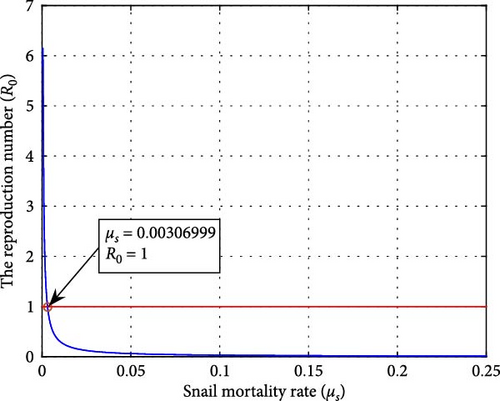
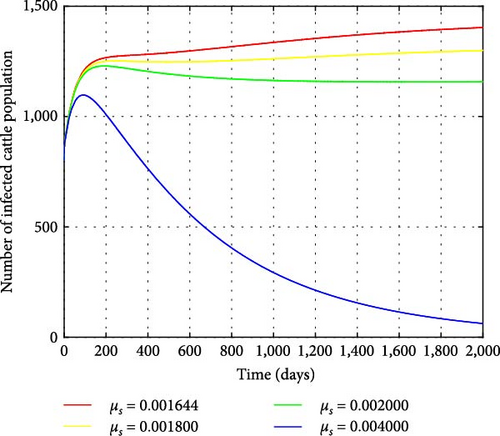
5.4.3. Effect of Snail Mortality Rate
Figures 12 and 13 illustrate the impacts of the snail mortality rates (μs) on the reproduction number and the infected cattle population, respectively. Figure 13 shows that the number of infected cattle correspondingly decreases as the snail mortality rate increases (with values of μs = 0.001644, 0.0018, 0.002, 0.004). When the snail mortality rate is below μs = 0.00306999, the reproduction number exceeds one, allowing the F. hepatica disease to persist. Conversely, when the snail mortality rate is above μs = 0.00306999, R0 falls below one, eradicating the disease. Controlling the snail population reduces cattle infection and the density of metacercariae in the ecosystem. Therefore, additional measures should be implemented to decrease the number of snails and eliminate the spread of F. hepatica in cattle.
6. Discussion
In this study, a deterministic mathematical model describing the transmission dynamics of F. hepatica disease is formulated and analyzed. The mathematical analysis and numerical simulation results show that the fasciola hepatica-free equilibrium point is locally and globally asymptotically stable if R0 < 1. It has also been demonstrated that the endemic equilibrium point is locally and globally stable when R0 > 1. The model exhibits a forward bifurcation. This situation happens when the fasciola hepatica-free equilibrium starts to lose its stability and a steady endemic equilibrium makes it appear while R0 tends to increase via one. In models exhibiting forward bifurcation, having R0 < 1 is a necessary and sufficient condition for fasciola hepatica disease eradication [40].
The sensitivity analysis is used to assess the impact of various parameters on fasciola hepatica. The results reveal that recruitment rates, transmission rates among cattle and snail populations, and shedding rates of miracidia and metacercariae directly affect the prevalence of fasciola hepatica in the cattle population. Furthermore, the mortality rates of miracidia, metacercariae, and snail populations, as well as the treatment rate of infected cattle, indirectly influence the spread of the F. hepatica parasite.
The numerical simulation results indicate that the current treatment is inadequate to prevent disease transmission within the cattle population, necessitating additional measures for disease control. Simulations also demonstrate that a strategy combining effective treatment, lower transmission rates, and higher snail mortality rates could greatly enhance disease management. This finding is consistent with a study by Diaby et al. [5], which found that the percentage of animals receiving medical treatment is not as important as combining quarantine with treatment for disease prevention. Additionally, a study by Kadaleka et al. [41] suggests that combinations of mollusciciding and therapy in a contaminated environment can effectively decrease the spread of disease, outperforming individual control methods. This integrated approach targeting both snail hosts and infected individuals could significantly reduce disease burden and improve public health outcomes, suggesting the importance of implementing such strategies in endemic regions for sustainable disease control and elimination. Therefore, stakeholders should reduce transmission rates, improve treatment rates, and increase snail population mortality rates to control this disease.
Ethical Approval
The authors agree to comply with all publication ethical standards.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Dagnaw Tantie Yihunie acknowledges the assistance provided by Debre Tabor University in conducting the research.
Appendix
A. Center Manifold Theory [34]
-
C1 Matrix M = DvG(0, 0) = (∂Gi/∂vj)(0, 0) is the linearization of Equation (A.1) around the equilibrium. The simple eigenvalue of A is zero, and the other eigenvalues have real negative parts.
-
C2 Let M has a left eigenvector l and a right eigenvector h that corresponding to the zero eigenvalue. Suppose Gk be the kth component of G, and we have the following relation:
()
- (i)
For both positive p and q. Zero is locally stable and there is a positive unstable equilibrium if π < 0 with |π| < 1; zero is unstable and there is a negative and locally stable equilibrium, if 0 < π < 1.
- (ii)
For both negative p and q. Zero is unstable, if π < 0 with |π| < 1; zero is locally stable, and a positive unstable equilibrium appears, if 0 < π < 1.
- (iii)
For positive p and negative q. Zero is unstable, and there is a locally stable negative equilibrium if π < 0 with |π| < 1; zero is stable, and a positive unstable equilibrium appears, when 0 < ϕ < 1.
- (iv)
For negative p and positive q. The stability of zero shifts from stable to instability, if π switches from negative to positive. A negative unstable equilibrium transforms into a positive and locally stable equilibrium.
Open Research
Data Availability
The research in this article used cited data sources and was not dependent on specific data or materials.




