Efficacy and Safety of Benvitimod in Patients With Palmoplantar Pustulosis: An Open-Label, Multicenter, Prospective Study
Abstract
Palmoplantar pustulosis (PPP) is often considered as pustular psoriasis of extremities. Benvitimod has been approved for mild to moderate psoriasis. We thus designed this study to evaluate the efficacy and safety of benvitimod for treating PPP. Of 47 PPP patients recruited from 4 hospitals, 40 finished 8 weeks visit and completed 4 weeks safety follow-up visit. The Palmoplantar Pustular Psoriasis Area and Severity Index (PPPASI) and Dermatology Life Quality Index (DLQI) scores fell continuously. At week 8, mean ± SD PPPASI and DLQI were 3.39 ± 3.86 (p < 0.0001) and 2.49 ± 3.34 (p < 0.0001), respectively. At week 8, 70% (28/40) achieved PPPASI-50 response, 35% (14/40) achieved PPPASI-75 response, and 17.5% (7/40) achieved PPPASI-90 response. Relapse occurred in 7.5% (3/40) of the patients. Of 47 patients enrolled, self-reported compliance was 12.77% (6/47). Common drug-related adverse events (5/47) included xerosis cutis. Only one patient’s disease progressed during treatment. Our study demonstrated the efficacy and safety of benvitimod.
1. Introduction
Palmoplantar pustulosis (PPP) is an idiopathic chronic disorder characterized by recalcitrant sterile pustules, crusts, and erythemas predominantly on the palms and soles. PPP is generally considered as pustular psoriasis of extremities [1]. The burden of palmoplantar psoriasis on patients is substantial and mostly due to functional disability, pain, and visibility of the lesion. Although various therapeutic modalities for the management of PPP exist, it is often challenging and unsatisfactory for refractory PPP treatment [2]. New emerging biologics such as secukinumab [3], guselkumab, bimekizumab, and adalimumab may bring short-term clearence [4], but they are expensive and result in increased risk of systemic side effects such as infection.
Benvitimod (3,5-dihydroxy-4-isopropylstibene, also known as tapinarof, GSK2894512, and WBI-1001) is a nonsteroid small molecule derived from the metabolites of a group of bacterial symbionts of entomopathogenic nematodes. It plays multiple anti-inflammatory roles via Aryl hydrocarbon receptor (AhR) modulation in different skin cells [5, 6]. In 2019, 1% benvitimod cream (Symbiox, Guangdong Zhonghao Pharmaceutical Co., LTD) was approved by CFDA (China Food and Drug Administration) for psoriasis treatment. We observed favorable results with this topical drug as single regimen in several PPP cases prior to this study. Thus, we designed and performed the open-label, uncontrolled, multicenter, prospective study to observe safety and efficacy of single topical regimen of 1% benvitimod cream for PPP treatment (March 2021–October 2021). The study was approved by Ethics Committee of West China Hospital, Yuanxin Technology Group Co., LTD (YXKJ-RWS-2020005).
2. Methods
Forty seven patients with PPP were enrolled. Each patient has been clinically diagnosed with PPP by two dermatologists independently. 1% benvitimod cream was applied externally twice a day for 8 weeks. Clinical assessments were performed at week 0, week 2, week 4, week 6, and week 8. Relapse will be evaluated at week 12. If complete remission was achieved, the drug would be discontinued for another 4 weeks safety observation. Pregnant and breastfeeding women, patients who did not follow instructions, and patients who were receiving concomitant treatments during the study were excluded.
3. Results
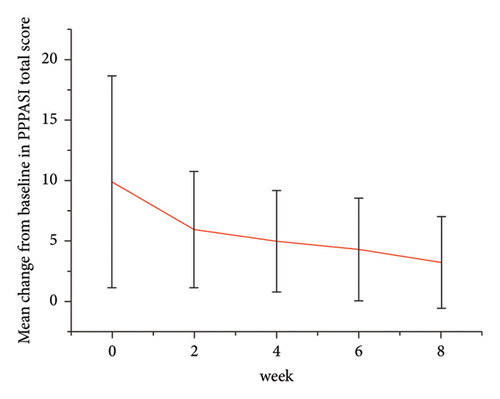
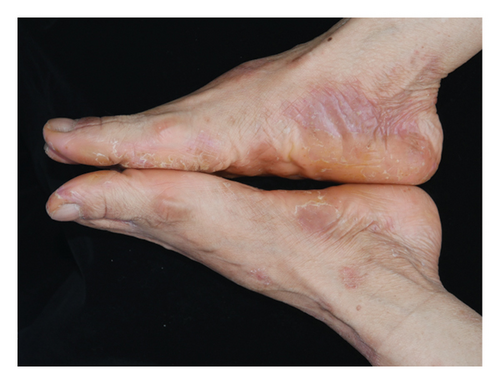
Forty patients completed week 8 visit and safety follow-up through week 12. Mean age was 45 (16–76) years old. 60% were females (24/40). Mean ± standard deviation (SD) duration of PPP was 1.18 ± 2.44 years. At week 0, mean ± SD PPPASI and DLQI were 9.9 ± 8.73 and 9 ± 5.85, respectively. The PPPASI (Figure 1) and DLQI scores fell continuously. The change was significant in the first 2 weeks (Figure 2), indicating its rapid action. At week 8, mean ± SD PPPASI and DLQI were 3.39 ± 3.86 (p < 0.0001) and 2.49 ± 3.34 (p < 0.0001), respectively. At week 8, 70% (28/40) achieved PPPASI-50 response, 35% (14/40) achieved PPPASI-75 response, and 17.5% (7/40) achieved PPPASI-90 response (Figures 3 and 4). Relapse occurred in 7.5% (3/40) patients. Of 47 patients enrolled, self-reported compliance was 12.77% (6/47). Common drug-related adverse events (5/47) included xerosis cutis and desquamation. Only one patient’s disease progressed during treatment, with pustules extending to bilateral legs. This patient dropped out of the study.
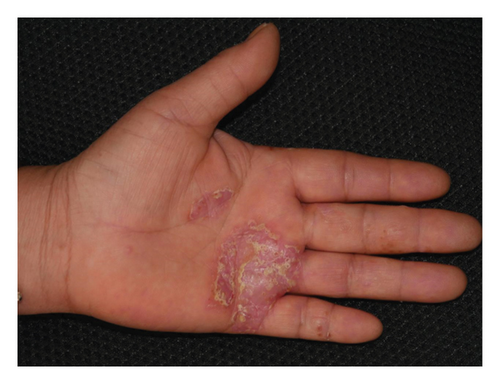
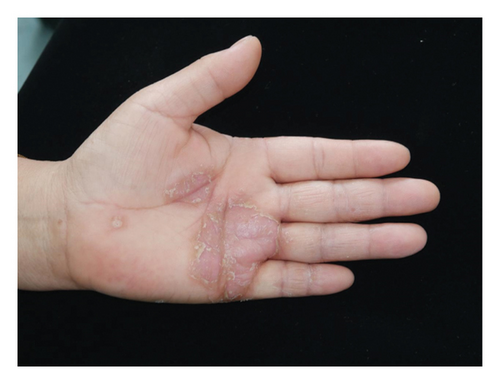
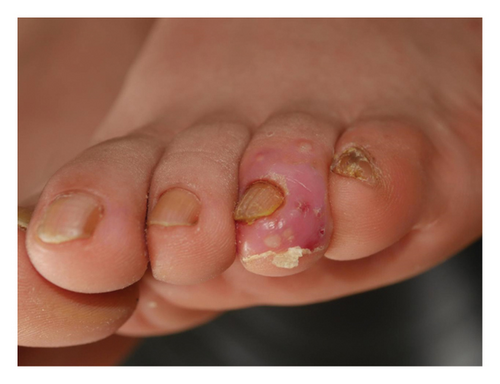
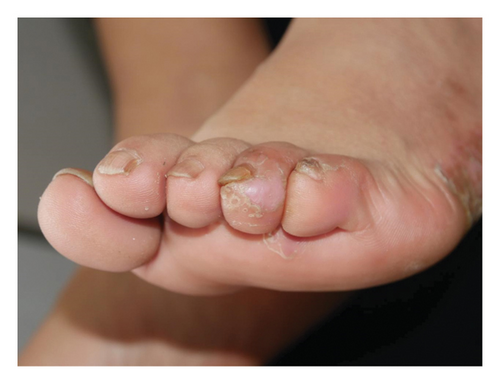
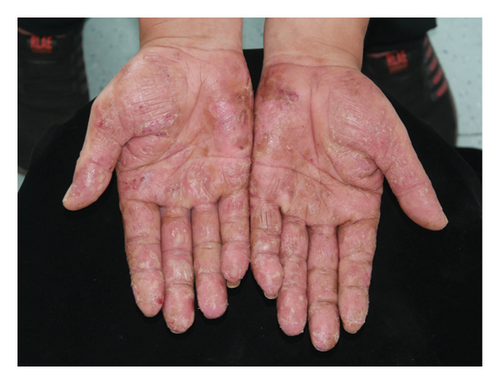
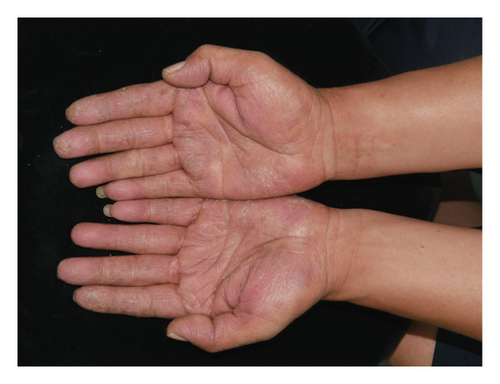

4. Conclusion
Our study demonstrated a good responses and tolerability of 1% benvitimod cream in PPP patients. Studies at larger scales are needed to further evaluate its efficacy and safety. Owing to its excellent treatment outcomes and mild adverse events in psoriasis and atopic dermatitis [7], benvitimod has the potential to become a new nonsteroidal topical therapy in more inflammatory skin diseases and chronic skin diseases for a long-term management.
Ethics Statement
At all stages of the project, the basic principles of the Declaration of Helsinki were taken into account, and the implementation protocol was investigated and approved by the Ethics Committee of West China Hospital, Yuanxin Technology Group Co., LTD (YXKJ-RWS-2020005).
Consent
All patients were informed of the purpose of the study and signed a detailed informed consent form describing the study design and possible adverse effects of benvitimod. All patients gave consent with the understanding that this information may be publicly available.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Guangping Wang, Shuai Zhang, Huimin Yan, Bingxin Zhang, Hongmei Wang, and Liming Ji contributed to the study conception and design. Patients’ data were collected by all the authors. Material preparation and analysis were performed by Guangping Wang and Liming Ji. The first draft of the manuscript was written by Guangping Wang and Shuai Zhang. All authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript. Guangping Wang and Shuai Zhang are co-first authors.
Funding
The research did not receive funding from any resources.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.