Needle before Scalpel: Considering the Role of Intratumoral Therapy in Melanoma
Abstract
Advanced melanoma and nonmelanoma skin cancer or cutaneous metastases not amenable to surgery often require alternate therapy. Although surgery is first-line treatment for early-stage melanoma, it can be challenging with multifocal disease, sites with high morbidity, large lesions such as lentigo maligna on the head and neck, and patients with comorbidities that add surgical risk. Intratumoral therapy is a safe method of treating advanced melanoma which avoids the toxicities of systemic therapies. Our review examined the overall response rates and adverse effects of the following experimental and standard intralesional agents: ipilimumab, rose bengal (PV-10), cathelicidin LL37, SD-101, coxsackie A21 V937, and talimogene laherparepvec. Injection of oncolytic virus, immune-modulating drugs, cytotoxic agents, or studied combinations was well-tolerated and effective alternative treatments for advanced melanoma and cutaneous metastases. Response to treatment was observed in both injected and noninjected lesions demonstrating systemic antitumor effects of these intralesional therapies. Further utility of intralesional agents can be explored as neoadjuvant treatment of large lentigo maligna lesions or those in cosmetically sensitive areas. Intralesional therapy should be developed further for morbidity reduction in challenging melanoma cases.
1. Introduction
Melanoma, advanced NMSC, or cutaneous metastases not amenable to surgery or radiation often require alternate therapy. While surgery is first-line treatment for early-stage melanoma, it can be challenging with multifocal disease sites, body sites with high surgical or cosmetic morbidity, large area lesions such as lentigo maligna on the head and neck, and in cases where patient comorbidities add surgical risk. Loco-regionally advanced cases are often managed with systemic neoadjuvant therapy, which has variable efficacy and potentially significant adverse effects (AEs).
Intratumoral (IT) therapy, also referred to intralesional therapy, involves injecting therapeutic agents into a target lesion. Its goal is to achieve loco-regional disease control, through concentrating a drug in a small area, along with potentially achieving systemic disease control. Traditional systemic chemotherapy and immunotherapy often involve significant dose-limiting toxicity; however, IT injections can achieve higher local concentrations to maximize antitumor activity and maintain low systemic concentrations, minimizing AE [1]. While IT therapy has been used for solid tumors, dermatologists may have varying comfort levels with this approach. While IT therapy has some advantages, there are unique challenges as well. Novel IT agents such as oncolytic viruses can have cumbersome storage, preparation, and biosafety requirements that hinder frequent use [2]. There may be logistical barriers related to the time needed to inject lesions and biosafety precautions that can make administration difficult in busy clinics [2].
IT therapy is useful for anatomically difficult areas (such as periocular or genitals), elderly or frail patients, and when tumors are not readily resectable via surgery [3–5]. In addition, IT therapy could be offered to patients who failed systemic treatments, and in patients who cannot tolerate systemic therapy in stage III-IV melanoma.
The majority of melanoma patients present with local or loco-regional disease [6]. In these patients, local therapy could offer durable local and systemic disease control while limiting systemic toxicity. Toxicity from systemic immune therapy and BRAF-MEK inhibitor therapy is a major consideration in treatment. The severity of adverse effects is graded on a scale of 1 to 5 using the National Cancer Institute common terminology criteria. Grade 1 is mild symptoms, grade 2 is moderate, grade 3 is serious, grade 4 is life-threatening or disabling, and grade 5 is death. Patients receiving systemic immune checkpoint inhibitor therapy experienced grades 3-4 toxicity in 23% (single agent) to 59% (dual agents) of cases [7]. Similarly, with BRAF-MEK inhibitor therapy, approximately 50% of patients experienced grades 3-4 toxicity [8].
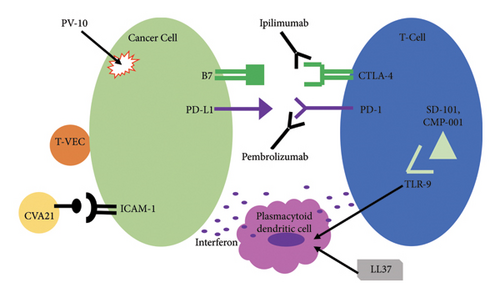
In this publication, we offer a review of current evidence on the response rates and adverse effects of novel IT agents in cutaneous melanoma. Specifically, we present the literature on a variety of injectable immune-enhancing, immune modulatory agents (IT pembrolizumab, ipilimumab, SD-101, and cathelicidin LL37); IT oncolytic viral therapy (coxsackie A21 V937 (CVA21) and talimogene laherparepvec (T-VEC)); and nonimmunologic agents such as IT rose bengal (PV-10). The mechanism of action of these agents is depicted in Figure 1.
2. Methods
The literature review was conducted via PubMed and clinicaltrials.gov databases for clinical trials and case studies using IT pembrolizumab, ipilimumab, cemiplimab, rose bengal (PV-10), cathelicidin LL37, SD-101, coxsackie A21 V937, and talimogene laherparepvec for the treatment of cutaneous tumors. Our search terms included all combinations of “intralesional,” “intratumoral,” and each agent listed above. Inclusion criteria included human studies with administration of IT therapy for the treatment of melanoma, published in English language, quantified response to therapy such as tumor regression or survival and quantified report of adverse events experienced. We excluded review articles, studies that did not quantify efficacy or response, did not report adverse effects, nonhuman trials, trials using IT therapy for diagnoses other than melanoma such as SCC and BCC, studies using only IV formulations, and studies which focused on visceral metastases only.
3. Results
3.1. Ipilimumab
Ipilimumab is a monoclonal antibody that inhibits the interaction of the cytotoxic T-lymphocyte antigen-4 (CTLA-4) receptor on T-cells with its corresponding inhibitory ligand on tumor cells [9]. Blockage of this checkpoint increases T-cells’ activation against tumor cells. Systemic ipilimumab (alone or in combination with nivolumab) is FDA approved for advanced melanoma. In phase III studies, systemic ipilimumab used in advanced melanoma yielded long-term, durable antitumor responses, and a significant improvement in overall survival (OS) from 6.4 months in the comparison group to 10 months in the ipilimumab group (P < 0.001) [10]. Systemic ipilimumab is also associated with immune-mediated toxicity. Toxicities grade 3 and higher have been reported with ipilimumab in 5–25% of patients and commonly include cutaneous toxicity and inflammatory enterocolitis [11]. To further explore the antitumor use of ipilimumab, it has been investigated as an IT agent.
In a phase I study, Ray et al. used IT ipilimumab (0.5 mg to 2.0 mg) with IT interleukin-2 (IL-2) (3 mIU) in 12 patients with stage III or IV melanoma. The patients had failed prior therapy, including 4 patients who failed systemic ipilimumab. IT ipilimumab was given weekly for 8 weeks and IT IL-2 was given two to three times weekly for 8 weeks. The overall response rate (ORR) was 40%. Of note, nine patients had multiple tumors (at loco-regional and distant sites). Of these nine, 8 patients (88.9%) demonstrated abscopal response, i.e., regression of noninjected tumors [12]. Common AEs were injection site reaction (7/12), fatigue (6/12), flu-like symptoms (5/12), pain (5/12), chills (4/12), and ulceration at the injection site (5/12) [12]. No patients experienced dose-limiting toxicity, and all participants completed the treatment phase as adverse effects were well tolerated [12].
In the phase I NIVIPIT trial, Tselikas et al. compared IT ipilimumab (0.3 mg/kg) with intravenous (IV) nivolumab (antibody targeting PD-1, 1 mg/kg) (n = 40) to IV ipilimumab (3 mg/kg) with IV nivolumab (1 mg/kg) (n = 21) in patients with stage III or IV melanoma [13]. Treatments were administered every three weeks for six months. The IT ipilimumab arm had lower rates of grade 3 or higher AE compared to the IV ipilimumab arm (30% vs. 57.1%). The ORR was 50% in the IT arm vs. 65% in the IV arm which was not a statistically significant difference. Of the tumors injected with ipilimumab, 65.7% showed a complete or partial response [13].
Schwarze et al. investigated the IT and IV administration of a multiple immune checkpoint inhibitors in combination with IT administration of autologous dendritic cells [14]. 9 patients with advanced solid malignancy refractory to other treatments received IT ipilimumab (10 mg), IT avelumab (40 mg) (avelumab is an antibody targeting programmed death-ligand 1 on tumor cells (PD-L1)), and IV nivolumab (10 mg) on day 1, followed by IT autologous myeloid dendritic cells on the following day. Ipilimumab (IT), avelumab (IT), and nivolumab (IV) were continued every 2 weeks. 4 of 9 patients had stage IV melanoma. Of these melanoma patients, there was a 25% ORR, and 2 patients had regression of injected metastases but overall disease progression. Local AE included pain, redness, and pruritus [14]. Taken together, IT ipilimumab with autologous dendritic cells can lead to both local and distant antitumor immunologic responses.
Tijtgat et al. preformed a phase 1 clinical trial with 8 patients with stages III-IV melanoma with disease progression on standard of care therapy [15]. Six patients were evaluable after IT treatment with adjuvant system 01b (AS01b, an immune stimulant commonly used in vaccines), autologous myeloid dendritic cells, ipilimumab (10 mg into one or multiple lesions), and IV nivolumab (10 mg) every two weeks. AS01b was coadministered as an immune adjuvant to stimulate adaptive immunity in dendritic cells [15]. The ORR was 37.5% (3/8 patients) consisting of 25% complete response and 12.5% partial response. The median overall survival was 42 weeks. The most common treatment-related AE included fatigue and injection site reactions. One patient experienced grade 3 AE of decreased lymphocyte count and one patient had grade 5 intracranial hemorrhage determined to be unrelated to treatment [15]. Overall treatment was well tolerated and AE were self-limiting [15].
3.2. T-VEC
Talimogene laherparepvec (T-VEC) is a genetically modified type-1 herpes simplex virus. T-VEC is FDA-approved for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent cutaneous melanoma after initial surgery [16]. T-VEC mediates antitumor responses through several mechanisms. The oncolytic virus preferentially “infects” tumor cells, causing virally mediated cell lysis [17]. The subsequent release of tumor antigens generates an adaptive immune response. In addition, genetic modification of the virus promotes local production of granulocyte macrophage colony stimulating factor (GM-CSF), which further enhances immune activation [18]. T-VEC has been investigated as monotherapy, and in combination with systemic immunotherapy agents for treatment of advanced melanoma.
In a randomized phase III trial, patients with unresectable stages III-IV melanoma received injections of T-VEC vs. GM-CSF. Among 436 patients, T-VEC demonstrated a significantly improved durable response rate compared to GM-CSF (16.3% vs. 2.1%) [19]. ORR was superior with T-VEC (26.4% vs. 5.7%). Median OS was 23.3 months with T-VEC and 18.9 months with GM-CSF. Treatment with T-VEC was well tolerated. Cellulitis was the only related grades 3-4 adverse event reported, which occurred in a minority of patients (2%). The most common adverse events with T-VEC were fatigue (50%), chills (49%), pyrexia (43%), nausea (36%), influenza-like illness (30%), and injection-site reaction (28%). Notably, vitiligo occurred in 5% of patients [19]. Following this study, T-VEC received FDA approval.
In a randomized phase II study, patients with stages IIIB to IV melanoma received T-VEC plus ipilimumab vs. ipilimumab alone [20]. IV ipilimumab was administered at 3 mg/kg, every 3 weeks, for a total of 4 doses. T-VEC was administered per standard dosing schedule (week 0, week 3, and subsequently every 2 weeks). Among 198 patients, the ORR in the combination arm was 39% compared to 18% for ipilimumab alone [20]. Interestingly, responses in visceral, noninjected lesions were also higher in the combination arm, indicative of an enhanced systemic antitumor response from T-VEC [20]. The combination group had a higher incidence of grade 3 or higher adverse events (45% vs. 35%). Adverse events attributed to T-VEC that were grade 3 or higher occurred in 15% of patients. This included influenza-like symptoms and lymphopenia [20].
MASTERKEY-265 was a randomized controlled study in which 692 patients with stages IIIB-IVM1c melanoma were randomized to receive T-VEC plus the anti PD-1 agent pembrolizumab vs. pembrolizumab with placebo [21]. The ORR for T-VEC-pembrolizumab vs. placebo-pembrolizumab was 48.6% and 41.3%, respectively. No improvement in progression-free survival (PFS) or OS was noted. However, improved PFS was noted in prespecified subgroups with limited disease burden. Although no improvement in PFS or OS was noted overall, subgroup analysis indicated more favorable response in patients with limited disease burden [21].
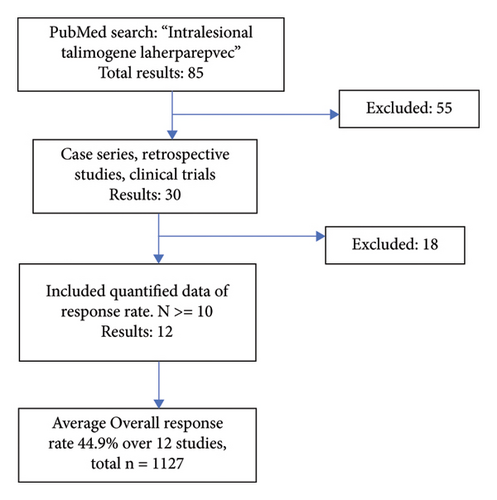
T-VEC has been investigated as monotherapy for the treatment of nonresectable melanoma in several studies. Twelve studies on T-VEC monotherapy in advanced melanoma (stages III-IV) with a quantified response to treatment were identified in our search as shown in Figure 2. The ORR from each study was weighted by the number of patients and a pooled ORR was calculated. The pooled ORR of the 12 clinical studies was 44.9% with a total patient population of 1127 patients [19, 22–32]. The reported ORR in the 12 studies reviewed ranged from 26.4% to 88.5% [19, 22]. T-VEC dosage ranged from 106 to 108 plaque-forming units/mL 1–4 mL injected per lesion, and injection frequency ranged from every two to every three weeks. There was no appreciable difference in ORR between the various dose ranges administered [19, 22–32].
3.3. Coxsackievirus
Coxsackievirus A21 is a virus that causes mild cold-like illness [33]. Oncolytic coxsackievirus A21 (CVA21) is a bio-selected strain that preferentially interacts with intercellular adhesion molecule-1 (ICAM-1 or CD54) and secondarily with decay-accelerating factor (DAF or CD55) [34, 35]. ICAM-1 is a cell surface glycoprotein expressed on various nonmalignant cells; however, it is expressed significantly more in melanoma [34–36]. Thus, oncolytic CVA21 preferentially lyses melanoma cells with elevated expression of ICAM-1/DAF [33].
In a phase II study (CALM study), 57 patients with stages IIIc-IV melanoma received IT CVA21 [37, 38]. IT CVA21 was administered up to a dose of 3 × 108 TCID50 (50% tissue culture infectious dose) on study days 1, 3, 5, 8, and 22, and then every three weeks for an additional 6 doses. An ORR of 28.1% was noted with 19.3% of the patients demonstrating a durable response rate of more than 6 months. The 1-year survival rate was 75.4%. Treatment was well tolerated, AE included grades 1-2 fatigue (30%), chills (26%), injection site reactions (32%), fever (12%), headaches (11%), myalgia (11%), and hyperhidrosis (9%) [38]. No grades 3-4 treatment-related adverse events were reported [37, 38]. Patients who had stable disease or responded continued to receive IT CVA21 [38]. Follow-up analysis demonstrated 6-month PFS rate of 38.6%. The best confirmed ORR was 28.1% [37, 38]. Interestingly, 26.7% of target noninjected lesions demonstrated regression, including visceral metastases [38].
In a single-arm, phase Ib clinical trial, IT CVA21 (3 × 108 TCID50), and IV pembrolizumab (2 mg/kg every 3 weeks) were administered to 36 patients with unresectable, stages IIIC-IVM1c melanoma. IT CVA21 was given on day 1, 3, 5, and 8, and then every three weeks for up to 19 sessions. The ORR was 47%, with a complete response in 22% of patients [39]. Common adverse effects from CVA21 were low-grade constitutional symptoms. Hypothyroidism occurred in 11% of all patients [39].
Another trial used IT CVA21 and IV ipilimumab to treat 50 patients with treated or untreated unresectable stages IIIB-IV melanoma. Patients received IT CVA21 (3 × 108 TCID50) on days 1, 3, 5, 8, and 22, and then every three weeks for 14 additional injections along with four cycles of IV ipilimumab (3 mg/kg) every 3 weeks starting on day 22 [40]. The ORR was 30% [40]. The ORR in the 33 patients with prior systemic anti-PD-1 therapy was 21% while the ORR in the 17 patients naïve to anti-PD-1 therapy was 47%. The common treatment-related adverse effects were pruritus (50%), fatigue (44%), diarrhea (32%), and nausea (22%), which are more typical of ipilimumab adverse effect profile. Of the 14% of patients that had grades 3-4 adverse effects, none were attributed to IT CVA21 [40].
3.4. Rose Bengal
Rose bengal was originally developed as a corneal stain due to its affinity for damaged epithelium [41]. Intracellular accumulation of rose bengal causes cytotoxic effects. Rose bengal has been formulated into a 10% weight over volume solution with saline called PV-10, which has antitumor effects with injection into melanoma [42]. The antitumor effect is due to the selectively higher uptake by tumor cells and accumulation in tumor cell lysosomes resulting in tumor cell autolysis [43]. Subsequent release of tumor antigens and inflammation propagates an antitumor response [43].
In a study by Thompson et al., eleven patients with recurrent stage III melanoma received PV-10 in one to three lesions (0.5 mL per cm3 of lesion). A total of 26 injected lesions and 28 noninjected lesions were evaluated. The ORR of injected lesions was 48% and was 27% for untreated lesions [44]. In a follow-up phase 2 study, 80 patients with stages III-IV melanoma received PV-10, injected at weeks 0, 8, 12, and 16. The ORR was 51.3% for injected and 33.3% for noninjected lesions. Common adverse effects included injection site reactions (80%), headache (16%), local photosensitivity (8%), and local ulceration (6%). Grade 3 treatment-related AE included injection site reactions (13%), cellulitis (1%), and photosensitivity (1%). No grade 4 or 5 AE occurred, and there were no dose-limiting toxicities [45].
Another study involved injecting all clinically apparent disease in 45 patients with stages III-IV melanoma with in-transit metastases. An ORR of 78.1% and a mean PFS of 9.8 months was reported [42]. Common adverse effects included injection site reactions. The only grade 3 or greater adverse events were local photosensitivity reaction, cellulitis, and ulceration [42].
Clinical trial NCT02557321 is actively enrolling patients to investigate the safety and tumor response to IT PV-10 plus IV pembrolizumab in patients with stages III-IV melanoma [46].
3.5. LL37
LL37 is a human peptide from the cathelicidin family which is a component of the innate immune system [47]. Cathelicidins are antimicrobial and immune-stimulating molecules. LL37 can have contradictory effects on tumors, depending on the tissue or tumor type and the receptor. For example, the interaction of LL37 with the formyl peptide receptor like-1 (FPRL-1) has been implicated in increased immune surveillance by natural killer cells and CD4+ T-cells in gastric cancer [47]. Also, LL37 induces caspase-independent apoptosis in colon cancers [47]. However, the interaction of LL37 with FPRL-1 has been linked to metastatic progression for ovarian cancer [47]. LL37 can also activate insulin-like growth factor-1 receptor (IGF-1R) and toll-like receptor 4 (TLR-4), which are linked to cell proliferation and tumor growth [47]. Therefore, LL37 may have protumor or antitumor effects in melanoma because melanoma frequently has increased TLR-4 expression [47].
LL37 also promotes antitumor immune modulation via interaction with plasmacytoid dendritic cells (pDCs) [48, 49]. pDCs normally release interferons when viral DNA activates their TLRs [49]. However, in the presence of LL37, TLR9 on pDCs is activated by self-DNA which induces an antitumor response [49]. Overall, the balance of antitumor and protumor effects of LL37 is not fully understood.
Our literature review revealed one completed study with results of IT LL37 (250 mcg to 500 mcg per injection) for three patients with metastatic melanoma to assess safety [49]. Injections were given weekly for 8 weeks. All three patients had an immune-related complete or partial response [49]. No serious adverse effects occurred. Reported AE (all, not just treatment-related) included anemia (2/3), decreased leukocytes (1/3), hypothyroidism (1/3), hypopigmentation (1/3), actinic keratosis (1/3), and SCC (1/3) [49]. This study size limits conclusions, although the three patients’ responses suggest LL37 may have efficacy against melanoma metastases.
3.6. TLR9 Agonist
Toll-like receptor 9 (TLR9) is an intracellular receptor that is found in a variety of immune cells [50]. TLR9 detects viral or bacterial DNA to stimulate an immune response [51, 52]. TLR9 agonists can enhance an antitumor response through several mechanisms. One mechanism is activation of pDCs to release interferon-alpha and maturation into antigen-presenting cells (APCs) [53, 54]. Several TLR9 agonists have been investigated in phases I and II studies.
SD-101 is a CpG-C oligodeoxynucleotide that agonizes TLR9. In a phase Ib study, IT SD-101 was administered into metastatic lesions, concurrently with IV pembrolizumab, in 22 patients with stages IIIc-IV melanoma [54]. Nine patients were naïve to anti-PD1 therapy, 13 patients had received prior anti-PD1 therapy. In the anti-PD-1 naïve group, the ORR was 78%. The estimated 12-month PFS rate was 88%. In the anti-PD1-treated group, ORR was 15% [54]. Common adverse effects attributed to SD-101 included malaise (77%), fatigue (77%), headache (77%), chills (77%), myalgia (73%), fever (36%), injection site reaction (32%), influenza-like illness (23%), and nausea (23%). The most common grade 3-4 AE were chills (14%), myalgia (14%), injection site pain (14%), fatigue (9%), and headache (9%) [54]. Biopsies of injected tumors revealed successful intratumoral immune modulation to an antitumor immunophenotype [54]. Adding TLR9 agonist to immune checkpoint inhibitors appears to enhance the efficacy of the antitumor response at the expense of minimal increment in toxicity. Furthermore, it has potential to convert immunologically “cold” tumors that are refractory to anti-PD1 therapy to immunologically “hot” tumors that are susceptible to immune targeting [55].
CMP-001 is a CpG-A oligodeoxynucleotide TLR9 agonist. An open-label, multicenter, phase 1b study was conducted by Milhem et al. involving patients with metastatic or unresectable melanoma, refractory to anti-PD1 therapy [56]. Part 1 treated patients (n = 159) with IT CMP-001 and IV pembrolizumab. In part 2, patients (n = 40) received IT CMP-001 monotherapy. Two dose-concentration levels of CMP-001 were tested in part 1, 0.01% and 0.00167%, respectively. A best ORR of 23.5% and 11.5% was reported for the higher and lower doses, respectively. The mean regression in injected and noninjected target lesions was 54.7% and 52.7%, respectively. Patients in part 2 were injected with the higher concentration dose of CMP-001. The ORR for higher dose CMP-001 monotherapy was 17.5% (n = 40). Common adverse effects included flu-like symptoms (>25%) and injection-site reactions (>25%). Grades 3-4 adverse events were reported in 36.5% (part 1) and 22.5% (part 2) of patients. The most common grades 3-4 adverse effect was hypotension (6.9% in part 1 and 5% in part 2) [56].
Another multicenter, phase 1b, dose-escalation trial by Ribas et al. studied IT vidutolimod (formerly termed CMP-001) in combination with pembrolizumab in 44 patients with metastatic or unresectable melanoma who failed anti-PD-1 treatment [57]. Injections were given weekly for seven weeks, then every three weeks with a median of 8 injections per patient. Both injected and noninjected lesions had similar volume reductions. The pooled ORR was 25% with no significant difference between dosage groups [57]. Grades 3-4 adverse events occurred in 45% of patients, the most common was hypotension. Biopsies of injected lesions showed increased CD8+ T-cell infiltration, PD-L1 expression, and interferon gene expression compared to baseline. IT vidutolimod has the potential to induce treatment response in melanoma previously resistant to anti-PD-1 therapy [57].
4. Discussion
Despite improvements in melanoma treatment, there is still an unmet need to develop new therapeutic strategies. Also, there is often high surgical morbidity when large tumors are resected, especially on the head and neck. About half of checkpoint inhibitor therapy patients and two thirds of BRAF-MEK inhibitor patients fail to achieve a durable response [58, 59]. Systemic therapy comes with adverse effects. For instance, combined checkpoint inhibitor therapy with ipilimumab and nivolumab was associated with treatment-related adverse events in over 95% of patients, with 53% of patients experiencing grades 3-4 toxicity. This led to discontinuation in more than 25% of the patients [58]. In addition to better-known immunotherapy agents, the less aggressive but effective therapies such as cathelicidins could be used as IT therapy and might be more appealing in the dermatologic setting for reduced morbidity, but this remains ripe for further study.
IT therapy broadens the landscape of melanoma treatment. It offers several advantages: (1) IT administration of immune-stimulating agents can produce antitumor responses in tumors unresponsive to systemic immune checkpoint therapy [57]. (2) IT immune therapy elicits local and distant antitumor responses [20, 57]. (3) More favorable adverse effect profile (Table 1). (4) IT immune therapy can potentially bypass some challenges limiting systemic immune therapy. One such challenge is a large tumor mass that might be less responsive to systemic therapy due to tumor burden immune-suppression [60]. IT therapy could potentially offer a robust local response that might not be achievable through systemic immune therapy. (5) IT therapy allows for the administration of novel therapeutic agents that might not be possible to administer intravenously due to safety or bioavailability.
| IT agent (IT dose) | Mechanism | Additional agents | ORR, n | AE of IT agent | Serious AE (grade ≥3) |
|---|---|---|---|---|---|
| T-VEC (1–4 mL) | Oncolytic virus |
|
|
Flu-like symptoms (50%), nausea (36%), and injection site reaction (28%) | Cellulitis (2.1%) |
| SD-101 (1–8 mg) | TLR-9 agonist | IV pembrolizumab | 34.8%, 230 | Flu-like symptoms (77%) and injection site reaction (32%) | Flu-like symptoms (14%) and injection site pain (14%) |
| CMP-001 (1–10 mg) | TLR-9 agonist |
|
|
Flu-like symptoms (>25%) and injection-site reaction (>25%) | Hypotension (5%) |
| CVA21 (3 × 108 TICD50) | Oncolytic virus |
|
|
Injection site reaction (32%), flu-like symptoms (30%), and hyperhidrosis (9%) | None |
| Rose bengal (PV-10) (0.5 mL/1 cm3) | Cytotoxic xanthine | None | 59.9%, 136 | Injection site reactions (80%), headache (16%), and local photosensitivity (8%) | Injection site reactions (13%) and cellulitis (1%) |
| Ipilimumab (0.5–10 mg) | CTLA-4 inhibitor |
|
|
Injection site reaction (58%) and flu-like symptoms (50%) | Local ulceration (42%) |
| LL37 (250–500 mcg) | Immune modulation | None | 100%, 3 | Anemia (2/3), leukopenia (1/3), hypopigmentation (1/3), SCC (1/3), and actinic keratosis (1/3) | Hypothyroidism (1/3) |
- Flu-like symptoms: fatigue, fever, chills, headache, arthralgia, congestion, and nasopharyngitis. Injection site reactions: pain, swelling, vesicles, erythema, and pruritus. All patients included had stage III-IV melanoma; however, prior treatments before enrollment were variable between studies.
However, barriers to implement IT therapy exist, including cumbersome storage, handling, lack of optimized injection techniques, access to tertiary care centers, training for administration, and cost. Oncolytic viruses such as T-VEC require storage at −70°C, thawing prior to administration, and can only be refrigerated in the thawed form for 12–48 hours before expiration [2]. This limited timeframe creates challenges with preparation, thawing time, and scheduling of patient injections. Missed appointments may result in costly expired medication. In terms of personnel, IT therapy can be administered by physicians and midlevel providers with appropriate training such as nurse practitioners and physician assistants [61]. There is no standardized injection technique. Several studies suggest a fanned administration where the clinician injects from a single point on the periphery of the lesion, repositions the needle without fully withdrawing, then injects at another angle [2, 61]. Multiple injection points can be used for larger lesions [2].
Cost may be an additional barrier for oncolytic viruses, immunotherapy, and novel agents. The oncolytic virus T-VEC costs approximately $65,000 for a treatment course [62]. Immunotherapy agents are in the range of >$100,000 for a full course, depending on the duration [62]. Alternatively, chemical IT agents such as PV-10 rose bengal are comparably cheap (25 g of purified powder for $64) [63] while retaining fair efficacy. In general, typical personal protective equipment, such as gloves, gown, and safety glasses are sufficient for handling IT agents, although package inserts indicate immunocompromised and pregnant providers should avoid handling oncolytic viruses [62].
Notably, some IT agents such as T-VEC and PV-10 have been studied as monotherapy and in combination with other agents, showing efficacy in both circumstances. Others, such as ipilimumab, have primarily been studied in combinations with adjuvants coinjected. Two studies combined myeloid dendritic cells with ipilimumab in hopes of enhancing the antitumor immune response [14, 15]. Myeloid dendritic cells were included because they play an important role in activating adaptive immune responses and relicensing antitumor T-cells [14, 15]. Another study used IL-2 as an adjuvant coinjected with ipilimumab to further stimulate cytotoxic T-cells activated against tumor cells [12]. It is not clear whether these adjuvants played a significant role in augmenting the efficacy of IT ipilimumab. Other studies comparing these combinations to IT ipilimumab monotherapy may be warranted.
An important highlight from the IT SD-101 study was the striking difference in ORR between the anti-PD-1 naïve and patients who previously received anti-PD-1 therapy (ORR 78% (7/9) vs. 15% (2/13), respectively) [54]. Notably, an IV anti-PD-1 agent (pembrolizumab) was part of the studied treatment. The significant difference could simply be the impact of pembrolizumab in the naïve population, or a marker of more resistant melanoma cases. Alternatively, prior exposure to PD-1 therapy may be a predictor of expected response to treatment.
An unexplored utility is IT treatment of melanomas in cosmetically sensitive areas. Another future direction for IT therapy includes possible neoadjuvant uses. For instance, IT therapy may be useful for shrinking large lentigo maligna lesions prior to surgery. IT therapy also has strong potential in patients who decline surgery, or are not surgical candidates. Our review highlights the potential of IT therapy to be explored and investigated in such patients. Specifically, T-VEC and PV-10 showed the strongest ORR as monotherapy agents in advanced melanoma (Table 1) and could be indicated for early melanoma treatment in these settings. This may further broaden the scope of IT therapy beyond patients with advanced disease, as a potential treatment option prior to surgery in select patients. There is a need to prospectively study the use of IT agents in these settings.
Limitations to our literature review include variability in patient and tumor characteristics, variable stage, prior treatment exposure, assessment of response, and publication bias. Several studies reported the efficacy of treatment in terms of tumor response categorized as complete or partial regression of tumors; however, there was a lack of data for long-term survival in many of the studies, partially attributable to small sample sizes. In addition, there were variations in treatment protocols among the studies reviewed, including variations in additional intratumoral agent coadministered with the therapy of interest, combinations with IV chemotherapy, heterogeneous injection techniques, and variable dosing protocols which could confound the observed response rates.
5. Conclusion
IT therapies offer a very favorable safety profile with moderate efficacy for treating advanced melanoma, and it is well tolerated. IT therapy can still be used in patients who failed systemic treatments, and in patients who cannot tolerate systemic therapy in stages III-IV melanoma. IT therapy should be considered in these patients. Although IT therapy has primarily been studied in later stage melanoma, the safety and efficacy suggest a potential role in treating earlier stage melanoma. IT therapy may be considered in early melanoma for patients not amenable to surgery. In addition, the use of IT agents as neoadjuvant treatments or alternative to surgery should be studied further.
IT treatment options include oncolytic viruses, immune modulating agents, and cytotoxic therapies which all can induce local and distant responses. Among monotherapy IT treatments studied in larger populations, T-VEC shows superior efficacy. Rose bengal monotherapy also shows strong efficacy in a smaller sample size. Overall, each of the agents reviewed show some efficacy and excellent safety in advanced melanoma. In addition, IT therapy is safe in combination with systemic immunotherapies to supplement the overall response to treatment.
Abbreviations
-
- AE:
-
- Adverse effect
-
- APC:
-
- Antigen presenting cell
-
- BCC:
-
- Basal cell carcinoma
-
- CR:
-
- Complete response
-
- CTLA-4:
-
- Cytotoxic T-lymphocyte antigen-4
-
- CVA21:
-
- Coxsackievirus A21
-
- DAF:
-
- Decay accelerating factor
-
- FPRL-1:
-
- Formyl peptide receptor like-1
-
- GM-CSF:
-
- Granulocyte macrophage colony stimulating factor
-
- ICAM-1:
-
- Intercellular adhesion molecule-1
-
- IL:
-
- Interleukin
-
- IT:
-
- Intratumoral
-
- IV:
-
- Intravenous
-
- NMSC:
-
- Nonmelanoma skin cancer
-
- ORR:
-
- Overall response rate
-
- OS:
-
- Overall survival
-
- pDC:
-
- Plasmacytoid dendritic cell
-
- PD-L1:
-
- Programmed death-ligand 1
-
- PD-1:
-
- Programmed death-1
-
- PFS:
-
- Progression-free survival
-
- PV-10:
-
- 10% weight over volume solution of rose bengal in saline
-
- SCC:
-
- Squamous cell carcinoma
-
- TCID50:
-
- 50% tissue culture infectious dose
-
- TLR:
-
- Toll-like receptor
-
- T-VEC:
-
- Talimogene laherparepvec
-
- 5-FU:
-
- 5-fluorouracil.
Disclosure
This research was conducted entirely in Iowa City, Iowa, the United Stated of America.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Open access funding was enabled and organized by BTAA 2023.
Open Research
Data Availability
Due to the nature of this review study, all of the objective information can be readily found in the references.