Brain Morphometry Differences Between Children With Autism Spectrum Disorder and Healthy Individuals in a Middle Eastern Population: A Cross-Sectional Retrospective Study
Abstract
This study is aimed at investigating brain morphometry differences between children with ASD and healthy controls (HCs) in Saudi Arabia and exploring the association between IQ levels and brain volumes within the ASD group. Participants (N = 29, 31.1% females and 68.9% males) ranging in age from 6 to 17 years were scanned by MRI at the King Faisal Specialist Hospital and Research Center (KFSHRC). Brain volumes were analyzed while correcting for age, sex, and total intracranial volume (TICV). The analysis revealed that individuals with ASD exhibited larger volumes compared to HCs in the left caudate (p < 0.001), right caudate (p < 0.001), total caudate (p < 0.001), and total hippocampus (p = 0.014). These findings provide evidence for anatomical brain abnormalities in individuals with ASD and highlight the heterogeneity of these differences across brain regions. Furthermore, the analysis revealed that higher IQ levels were negatively associated with the volumes of the left thalamus, left pallidum, left accumbens area, right thalamus, right hippocampus, and total thalamus but positively correlated with the third lateral ventricle volume in HCs, p < 0.05. These results suggest a meaningful relationship between cognitive abilities, as measured by IQ, and variations in brain volumes in HCs. The study significantly contributes to the understanding of the neurobiology of ASD in a Middle Eastern population and emphasizes the importance of considering cognitive functioning in relation to brain morphology in ASD research and clinical practice.
1. Introduction
Autism, also referred to as “autism spectrum disorder (ASD),” is a heterogeneous, neurodevelopmental condition characterized by deficits in social communication (verbal and non-verbal) and interaction, as well as restricted and repetitive patterns of behavior, interests, or activities [1]. Patients with ASD may also exhibit co-occurring disorders such as cognitive deficits, intellectual impairment, epileptic episodes, and heightened levels of anxiousness. ASD typically manifests with early onset of behavioral symptoms, usually between the ages of 1 and 2 years [2]. These symptoms primarily involve atypical social attention, language development, and emotional reactivity [3]. The prevalence of ASD has been estimated at approximately 1.5% among children, and this rate has been steadily rising globally, with current estimates suggesting it affects around 1 in 100 individuals worldwide [4–6]. In the Middle Eastern region specifically, epidemiological studies have reported ASD prevalence rates ranging from 1.4% to 3.0% across different countries [7–9]. These rates are largely consistent with global averages, indicating that ASD represents a significant public health concern for populations in this geographic area as well. In Saudi Arabia, the prevalence of ASD was found to be lower than the global average. A previous study [10] involving 1023 children with ASD found prevalence rates of 2.618 per 1000 children in Jeddah, 3.68 per 1000 children in Makkah, and 2.81 per 1000 children for both cities combined.
Courchesne et al. [2] have defined ASD as a neurodevelopmental disorder with a genetic basis, characterized by the onset of behavioral symptoms in early childhood. They further suggested that the degree of heritability is higher compared to breast cancer, Alzheimer’s disease, or schizophrenia [11]. Significant variations in the genetic pathways could exist between autistic children from multiplex families and those from singleton families without any prior autism cases [11]. Although ASD may also be influenced by nongenetic factors, such factors have yet to be identified. Developmental delay and impairment are believed to affect higher-order social, emotional, language, and communication functions [12].
Several studies have emphasized the significance of anatomical brain abnormalities in individuals with ASD, both in children and adults [2, 13–15]. However, previous studies have shown a significant degree of heterogeneity in the direction and magnitude of morphometric brain differences [16, 17] using magnetic resonance imaging (MRI). ASD has been associated with both increased and decreased volumes of striatal structures, elevated intracranial volume, increased total gray matter volume (GMV), and cortical thickness [14, 18–20]. Smaller hippocampal volumes and larger amygdala volumes during childhood have also been reported in individuals with ASD. These structural abnormalities in the frontal lobes, amygdala, and cerebellum, as evidenced by postmortem and structural MRI studies, suggest their pathological implications in ASD [13]. However, a definitive and uniform pathology for ASD has not yet been established. These previous studies indicate inconclusive results and a lack of clarity regarding the mechanisms underlying the development of variations throughout an individual’s lifetime. The current study was designed as an exploratory analysis to identify potential differences in brain morphometry between individuals with ASD and healthy controls (HCs) in a Middle Eastern population, rather than testing a specific hypothesis.
This exploratory study presents a contribution to ASD research by focusing exclusively on Saudi native Arabic-speaking children with ASD, considering the potential impact of cultural and linguistic factors. By investigating this specific population, the study is aimed at unveiling potential variations in brain morphology within unique cultural contexts, providing invaluable insights into the fundamental neurobiological mechanisms underlying ASD. The findings derived from this study have the potential to significantly advance our comprehension of ASD, facilitating the development of culturally attuned diagnostic and therapeutic interventions specifically tailored to Saudi native Arabic-speaking children diagnosed with ASD. Given the diverse morphometric brain differences observed in individuals with ASD, as well as the possibility of genetic variations within autism-associated pathways across families worldwide, this study is aimed at comparing total brain volume (TBV), regional gray and white matter (WM) volumes, ventricles, and cerebrospinal fluid (CSF) between ASD patients and HCs in the Middle East, specifically Saudi Arabia. This study also investigated the association of intelligence quotient (IQ) levels with brain volumes within ASD patients.
2. Methods
2.1. Subjects
A total of 32 children participated in this study, consisting of 16 diagnosed with ASD and 16 matched HCs. All participants were Saudi native Arabic speakers recruited from King Faisal Specialist Hospital and Research Center (KFSHRC) in Riyadh, Saudi Arabia. Each child underwent a routine brain MRI scan as part of the assessment. The study was reviewed and approved by the institutional review board (IRB) at KFSHRC (ORA # 2130009), and informed consent requirements were waived due to its retrospective nature. Participants were excluded if they had a history of any neurologic disorders such as seizure disorder, neurodegenerative disorder, traumatic brain, or any general medical diseases that would affect brain functioning, such as endocrine diseases, vascular risk factors (Type 1 and 2 diabetes, hypertension, any cardiac function abnormalities, myocardial infarction, cerebrovascular accident, and migraine headaches), and smoking (yes, no) known or suspected to influence brain morphology. Exclusion criteria for both groups included MRI scanning contraindications and full-scale IQ scores > 1 standard deviation below the mean on the KBIT-2. Parents of all participants confirmed that their children did not have any other medical conditions affecting the study outcomes.
2.2. Clinical Assessment
All children with ASD were diagnosed based on the Autism Diagnostic Observation Schedule-second edition (ADOS-2) [21] by an expert clinician. To confirm the accuracy of the diagnosis of ASD, the parents of both cohorts of children completed the Autism Spectrum Quotient: Children’s Version, AQ, in its Arabic version [22]. The patients were taking medications specific for the treatment of ASD. However, they were instructed to stop taking these medications 24 h prior to the brain imaging scan, and all participants confirmed that they had complied with this requirement. The measurements of IQ, as measured by the Kaufman Brief Intelligence Test-2 (KBIT-second edition) [23], were obtained for all participants. Other factors such as the ASD severity and the information about medication usage at the time of scanning (i.e., current use of psychiatric treatment for ASD or comorbid conditions), ADOS scores, and the presence or absence of at least one comorbid condition (i.e., attention-deficit/hyperactivity disorder (ADHD), obsessive–compulsive disorder, depression, anxiety, and/or Tourette’s syndrome) were not available for ASD patients in the current study.
2.3. Image Acquisition
MRI was conducted at the KFSHRC using a high-field 3 T Siemens TIM Trio system, equipped with an advanced eight-channel head coil. Both children with ASD and HC subjects were instructed to stay relaxed and maintain stillness throughout the scanning procedure. T1-weighted three-dimensional (3D) magnetization-prepared rapid gradient echo (MPRAGE) sequences were obtained in the sagittal orientation within a duration of 4 min and 59 s. The imaging parameters included a voxel resolution of 1 × 1 × 1 mm, with a repetition time (TR) of 2200 ms, an inversion time (TI) of 900 ms, an echo time (TE) of 2.88 ms, a flip angle of 8°, and a field of view measuring 208 × 250 × 250 mm, utilizing an acceleration factor of 2.
2.4. MRI Analysis
FreeSurfer (http://surfer.nmr.mgh.harvard.edu) was selected for this study due to its established longitudinal reproducibility in both cortical and subcortical segmentations [24–29] and its high reliability in morphometric measurements across various MRI scanners and field strengths [24, 30]. It demonstrates superior reproducibility in subcortical segmentation compared to other automated techniques [31]. Additionally, FreeSurfer has been widely utilized in previous research examining brain morphometric abnormalities in individuals with ASD [15, 32–34]. For processing the MRI data, all scans were converted to NIFTI (neuroimaging informatics technology initiative) format. Each T1-weighted MRI was carefully reviewed to identify motion artifacts and ensure overall quality. FreeSurfer operates on the principle of assigning neuroanatomical labels to each voxel in the MRI volume, utilizing probabilistic information derived from a manually labeled training set [35]. The labeling for each voxel is determined through algorithms that analyze the probabilities of image intensities and spatial structure, employing a Bayesian approach based on the maximum a posteriori probability. The processing pipeline in FreeSurfer includes several steps: averaging volumetric T1-weighted images, skull stripping, brain extraction (removing the skull, extracerebral tissues, and cerebellum), motion correction, transformation into Talairach space, intensity normalization to correct for inhomogeneity, and segmentation of subcortical structures, including WM and deep gray matter (GM) [36–38]. This pipeline resulted in the generation of 68 cortical volumes (34 from each hemisphere) and 46 subcortical volumes (measured in cubic millimeter). Volumes related to WM hyperintensities, optic chiasm, and major vessels were excluded from further analysis. The volumes of the third and fourth lateral ventricles were combined for this analysis in order to accurately reflect the distinct volumetric differences between the left and right hemispheres; cortical and subcortical volumetric measures from both sides were not averaged. The final analysis utilized 48 variables representing regional cortical and subcortical volumes, alongside summations across specific brain regions. To account for individual variations in head size, these volumes were controlled for total intracranial volume (TICV) [39]. FreeSurfer also calculated TBV as the sum of GM and WM volumes, along with TICV, which includes the total volumes of GM, WM, and CSF [40]. The segmentation utilized default settings with the command “recon-all,” and all volumes were extracted from the statistical files generated by FreeSurfer using the “asegstats2table” command. All processing steps, including parcellation and segmentation, were visually inspected for accuracy, and no manual corrections were made. No outliers were identified in the data.
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
Thirty-two Saudi children participated in this study: 16 children with ASD (9 males, age range 6–16, mean age = 10.4, SD = 2.96) and 16 age- and sex-matched HCs (11 males, age range 7–17, mean age = 11.1, SD = 3.16). The average IQ level for children diagnosed with ASD was found to be 83.5, whereas the average IQ level for HCs was 96.9. 3D T1-weighted images were collected from all participants. Out of 32 participants, 29 participants were included in the current cross-sectional study. Three ASD participants were excluded due to issues of their T1 MRI images during recon-all automated processing. There were no significant differences between ASD patients and HCs on covariables including age (all p > 0.05). There was a significant difference in IQ level (even when corrected for age and sex) between ASD patients and HCs as determined by the t-test (p = 0.015) (see Table 1). Demographic characteristics for each group are presented in Table 1. Characteristics of brain volumes for both patients with ASD and HCs are described in Table S2.
ASD (mean ± SD) N = 12 |
HCs (mean ± SD) N = 17 |
p value | |
|---|---|---|---|
| Age | 12.0 ± 9.41 | 11.1 ± 3.15 | 0.155 |
| Gender (M/F) | 3M/9F | 11M/6F | 0.571 |
| IQ | 83.5 ± 18.5 | 96.9 ± 8.79 | 0.01 |
- Abbreviations: ASD, autism spectrum disorder; F, female; HCs, healthy controls; IQ, intelligence quotient; M, male; N, number of the sample; SD, standard deviation.
3.2. Comparisons of Brain Region Volumes Between ASD Patients and HCs
Before correcting for multiple comparisons, there were significant differences in certain brain volumes corrected for age, sex, and TICV between ASD patients and HCs, p < 0.05. Results showed significant effects for ASD for the left caudate (p < 0.001), right caudate (p < 0.001), total caudate (p < 0.001), and total hippocampus (p = 0.042) (Figure 1). The full set of noncorrected statistical values, including p values, is provided in Table S1. Follow-up t-tests indicated that ASD patients had significantly larger volumes than HCs (see Figure 1 with ES). The percentage increases in volume for ASD patients compared to HCs were 15.66% for the left caudate, 15.71% for the right caudate, 15.68% for the total caudate, and 8.83% for the total hippocampus. However, after Bonferroni correction for multiple comparisons (0.05/45 = 0.001), only the comparisons of the left caudate (F (4) = 12.95, partial eta-square = 0.376, corrected p = <0.001), right caudate (F (4) = 14.37, partial eta-square = 0.38, corrected p = <0.001), and total caudate (F (4) = 14.98, partial eta-square = 0.398, corrected p = <0.001) volumes remained significant (Figure 1). The total hippocampal volume was no longer significant after the Bonferroni correction (p = 0.042). There were no other significant differences in brain volumes between ASD patients and HCs.
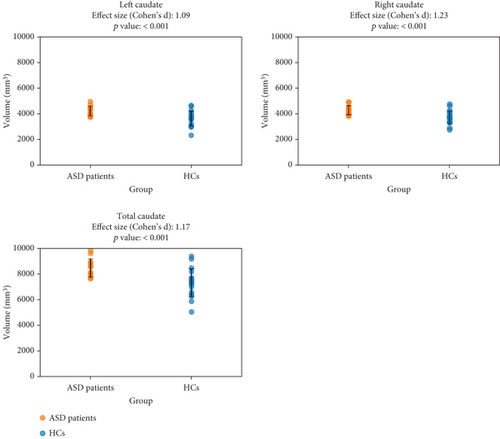
3.3. Associations of IQ Levels With Brain Volume in ASD Patients and HCs
Among ASD patients, there were no significant linear correlations of IQ levels with brain volumes corrected for age, sex, and TICV, p > 0.05 (see Table S3). However, in HCs, the analysis of the relationship between IQ scores and brain volumes revealed several significant linear correlations. Specifically, IQ was negatively correlated with the volume of the left thalamus (r = −0.726, p = 0.005), left pallidum (r = −0.538, p = 0.021), left accumbens area (r = −0.559, p = 0.045), right thalamus (r = −0.513, p = 0.044), right hippocampus (r = −0.555, p = 0.036), and total thalamus (r = −0.638, p = 0.012) but positively correlated with the third lateral ventricle volume (r = 0.562, p = 0.034), before correcting for multiple comparisons. The full set of noncorrected statistical values, including p values, is provided in Table S3. However, after Bonferroni correction for multiple comparisons (0.05/45 = 0.001), all the correlations did not remain significant.
4. Discussion
The present study is aimed at investigating cortical and subcortical brain morphometry differences between patients with ASD and HCs in Saudi Arabia. The results revealed significant differences in specific brain volumes, including the caudate and hippocampus, when adjusted for age, sex, and TICV. These findings support the presence of anatomical brain abnormalities in individuals with ASD in this population and contribute to a growing body of evidence highlighting the role of basal ganglia abnormalities in ASD. Additionally, associations between IQ levels and cortical brain volumes were explored within the HC group.
Consistent with previous research, our findings demonstrate that individuals with ASD exhibit variations in specific brain volumes compared to typically developing individuals [13–15, 33, 41–43]. Specifically, we observed significant differences in the volumes of the left caudate, right caudate, total caudate, and total hippocampus between ASD patients and HCs and that ASD patients showed larger brain volumes compared to HCs. The caudate nucleus is involved in various functions such as motor control and learning processes, and its abnormal volume may relate to the repetitive behaviors and restricted interest’s characteristic of ASD [44]. Interestingly, while initial analyses suggested significant differences in total hippocampal volume, these did not remain significant after correcting for multiple comparisons. This highlights the importance of stringent statistical corrections to avoid false positives and suggests that hippocampal differences may not be as robust in this population. Our findings are consistent with the heterogeneous nature of ASD, as previous research has reported both increases and decreases in the volumes of various brain structures in individuals with the disorder. For example, several studies [14, 45–47] have documented increased brain volumes, while others [15, 48] have observed reductions in different structures. Additionally, MRI studies focusing on regional or TBVs have shown that young children diagnosed with ASD, aged between 18 months and 4 years, exhibit an abnormal enlargement of approximately 5%–10% in brain volumes compared to typically developing control subjects [2, 13, 49, 50]. These observed differences indicate that the neurobiological basis of ASD may involve structural abnormalities in regions such as the caudate and hippocampus. However, it is essential to acknowledge that there is no definitive or uniform pathology for ASD, as its etiology and neuropathological mechanisms are complex and multifaceted.
Recent studies have specifically examined changes in the caudate and hippocampus volumes in children with ASD over time. For instance, Langen et al. [51] found that caudate volume was significantly larger in children with ASD compared to controls and that this enlargement persisted into adolescence. This study also noted a correlation between larger caudate volume and greater repetitive behaviors but did not find a significant relationship with IQ. Similarly, Qiu et al. [52] reported that hippocampal volume was larger in preschool children with ASD and that this enlargement correlated with age, but again, there was no significant correlation with IQ. In contrast, Schumann et al. [49] found that while the hippocampus was larger in younger children with ASD, this difference diminished with age, suggesting that abnormal growth trajectories may normalize over time. This study also highlighted the complexity of hippocampal volume changes and their potential impact on cognitive functions such as memory, which are often impaired in ASD but did not directly correlate these changes with IQ. Additionally, a longitudinal study [41] tracking caudate and hippocampus volumes from infancy to early adulthood in ASD found divergent trajectories compared to typically developing controls. Initial enlargement in early childhood did not mirror typical developmental patterns, with implications explored for cognitive functions including IQ and social functioning. These findings collectively highlight the dynamic nature of brain morphology in ASD and its nuanced relationship with cognitive outcomes over developmental stages.
These studies underscore the variability in findings related to brain volume changes in ASD, influenced by factors such as age, specific brain regions examined, and methodologies used. Our study aligns with these findings, showing significant enlargement in the caudate and hippocampus in children with ASD. However, unlike some studies, we found that these volumetric changes did not remain significant after correcting for multiple comparisons, highlighting the need for further research with larger sample sizes and more refined methods.
It is worth noting that brain volume differences in ASD could result from various underlying neurobiological mechanisms. For instance, increased brain volume in certain regions could be attributed to abnormal cellular proliferation, altered patterns of synaptic connectivity, or changes in neuroinflammation [53, 54]. It is postulated that an excess of neurons might play a role in causing premature brain enlargement, leading to disruptions in neural organization and connectivity [2]. This could manifest as increased local and proximal cortical activity, potentially impeding the effectiveness of distal, interregional neural communication. Further research is needed to better understand the underlying mechanisms and to identify potential subgroups within the ASD population that may exhibit distinct brain volume profiles.
Within the ASD group, no significant linear correlations were found between IQ levels and brain volumes when corrected for age, sex, and TICV. This lack of association aligns with some previous studies [55], suggesting that IQ and brain volume may be independently affected in ASD [13]. It indicates that the cognitive impairments in ASD may not be directly related to the volumetric changes observed in this study. This could suggest that other neurobiological or environmental factors may play a more significant role in influencing IQ levels in individuals with ASD. Conversely, in the HC group, several significant correlations between IQ and brain volumes were identified before correction for multiple comparisons. These included negative correlations with the volumes of the left thalamus, left pallidum, left accumbens area, right thalamus, right hippocampus, and total thalamus and a positive correlation with the third lateral ventricle volume. However, none of these correlations remained significant after Bonferroni correction, indicating that these findings should be interpreted with caution. The thalamus is known to play a crucial role in sensory processing, attention, and cognitive functions [2]. The hippocampus, pallidum, and accumbens area, which are part of the basal ganglia, are involved in motor control, habit formation, memory spatial, and reward processing. The third lateral ventricle is situated within the diencephalon and is involved in the circulation of CSF. The negative correlations between IQ and thalamic, pallidal, accumbens, and hippocampal volumes may indicate that individuals with higher cognitive abilities exhibit more preserved or optimized thalamic development in the context of ASD. In contrast, the positive correlations with the third lateral ventricle volume could suggest that larger volumes in this region are associated with better cognitive performance. The relationship between the larger third lateral ventricle volume and higher cognitive abilities is not well understood and requires additional research to elucidate the functional significance of this association. These findings align with previous studies highlighting the link between cognitive functioning and brain morphology in HCs [56, 57]. The relationship between IQ and cortical measures suggests a complex interplay between cognitive abilities and brain morphology in HCs. Additional research is needed to further elucidate the complex relationship between IQ, brain volumes, and cognitive abilities in HCs.
In addition to the findings related to brain morphometry, our study’s strength lies in its focus on a unique patient population from the Middle East, specifically Saudi Arabia. This region presents a distinct combination of genetic and environmental factors that may influence the neurodevelopmental profiles of individuals with ASD. Studies have shown that populations in the Middle East, including Saudi Arabia, have higher rates of consanguinity, which can increase the prevalence of recessive genetic disorders. This may contribute to a distinct genetic landscape for neurodevelopmental conditions like ASD in this region [58, 59]. Additionally, variations in autism-associated genetic pathways may differ from those observed in Western populations, possibly affecting the presentation and progression of ASD [11, 60]. Environmental factors unique to the Middle Eastern context, such as prenatal exposure to high temperatures, limited access to early intervention services, and cultural factors affecting the diagnosis and treatment of ASD, may also play a role in shaping the neurodevelopmental outcomes in this population [5, 8]. For instance, cultural attitudes toward mental health and developmental disorders could influence the age at which ASD is diagnosed and the types of interventions that are pursued [61, 62]. By considering these genetic and environmental factors, our study provides valuable insights into the neurobiological underpinnings of ASD in a Middle Eastern context. Future research should be aimed at further exploring these factors to better understand their impact on the etiology and expression of ASD in this unique population.
The current study contributes to the existing literature by providing insights into cortical brain morphometry differences in individuals with ASD compared to HCs in a Middle Eastern population, specifically in Saudi Arabia. However, several limitations should be acknowledged. First, the sample size was relatively small, which may limit the generalizability of the findings [63]. Future studies with larger sample sizes are warranted to enhance the robustness and generalizability of the results. Second, the cross-sectional design of the study precludes causal interpretations and longitudinal assessments of brain development. Longitudinal studies are needed to investigate the dynamic changes in brain volumes over time and their relationship with clinical outcomes in individuals with ASD. Additionally, future studies could consider incorporating other neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), to examine the functional connectivity patterns associated with the observed structural differences. Further, it is possible that brain morphology of ASD patients may be influenced by the duration between the diagnosis or initiation of ASD medication and the MRI scan, even if this interval is less than or for 1 year. Therefore, it is imperative to closely monitor patients undergoing ASD treatment for potential neurological or psychiatric symptoms. Future studies should be aimed at recruiting unmedicated ASD samples, or accounting for medication history in the analysis, to more accurately characterize the intrinsic neuroanatomical features associated with the disorder. Finally, this study lacks ADOS scores, as this data was not available from the KFSHRC. These scores would have enabled a deeper investigation into the relationship between brain structure and the core symptoms of autism. Future studies incorporating comprehensive clinical assessments, including the ADOS, will be crucial in elucidating the neural underpinnings of the full spectrum of autism symptoms.
5. Conclusion
In conclusion, our study provides evidence for cortical brain morphometry differences between individuals with ASD and HCs in Saudi Arabia. The findings support the presence of anatomical brain abnormalities in individuals with ASD and highlight the heterogeneity of these differences across brain regions. Moreover, our results suggest a positive association between IQ levels and thalamic volumes within the HC group but not the ASD group. These findings contribute to the growing body of literature on the neurobiology of ASD and emphasize the need for further research to better understand the underlying mechanisms and potential clinical implications of cortical brain morphometry differences in individuals with ASD.
Ethics Statement
This retrospective study received approval from the KFSHRC institutional review board. Informed consent was waived as the study involved only the retrieval of imaging findings and demographic data from the PACS and medical records.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Naif A. Majrashi: conceptualization, data curation, formal analysis, validation, writing–original draft, writing–review and editing, funding. Rafat Mohtasib: conceptualization, formal analysis, writing–original draft. Ahmed Masawi: data curation, formal analysis, writing–original draft. Abdullah Almujally: conceptualization, data curation, formal analysis, software. Ali S. Alyami: data curation, formal analysis, methodology, software. Yahia Madkhali: data curation, formal analysis, methodology, software. Ali Hendi: formal analysis, writing–original draft, writing–review and editing. Bandar Alwadani: data curation, formal analysis, methodology, writing–review and editing. Wael Ageeli: data curation, methodology, writing–review and editing. Turkey Refaee: conceptualization, data curation, formal analysis, methodology, supervision, writing–original draft.
Funding
The authors gratefully acknowledge the funding of the Deanship of Graduate Studies and Scientific Research, Jazan University, Saudi Arabia, through Project Number RG24-S0113.
Acknowledgments
We would like to thank the data collector who collected the MRI images and cognitive measures for each participant and the local committee at KFSHRC in Saudi Arabia for giving us their approval to conduct this study. The authors also would like to thank the Deanship of Graduate Studies and Scientific Research, Jazan University, Saudi Arabia, for funding this research project (RG24-S0113).
Open Research
Data Availability Statement
The data used in this study were obtained after obtaining approval from the Ethical Committee of KFSHRC. However, due to privacy and confidentiality concerns, the authors do not have permission to share the data used in this research.




