Supplementation of Multistrain Probiotics Improves Milk Production, Blood Metabolites, Digestibility, and Rectal Microbiota during the Prepartum and Early Lactation Stages in Crossbred Cows
Abstract
The current study aimed to study the effects of administration with a multistrain probiotic formulation (MPF) initiated from the prepartum stage on lactation performance, digestibility, rectal microbiota, and blood metabolites of postpartum dairy cows. A total of 30 multiparous crossbred (Holstein Friesian × Balady) dairy cows were randomized into three groups: control (no MPF supplementation), MPF1 (0.5 g MPF/cow/d), and MPF2 (1 g MPF/cow/d). The administration of MPF started 28 days before calving and continued for 84 days after. The results revealed that cows in the MPFI and MPF2 groups had higher milk production, 3.5% FCM, and ECM than those in the control group. The MPF-supplemented cows had significantly greater milk energy output, DMI, and yields of solids-not-fat, lactose, total solids, ash, fat, and protein. The MPF supplementation significantly enhanced feed conversion and significantly interacted with milk solids-not-fat, lactose, total solids, and protein. Hematological indicators did not significantly alter between the MPF and control groups. However, cows receiving MPF supplements had higher levels of Hct. Serum biochemical measurements showed lower serum concentrations of LDL, total cholesterol, and VLDL in MPF-fed cows than in the control group. The digestibility of organic and dry matter tended to be higher in MPF-supplemented cows. The populations of lactobacillus, bacillus, and total bacterial count increased due to MPF administration, while the count of Clostridium, Salmonella, E. coli, and coliforms decreased in MPF-fed cows. In summary, administering MPF during late gestation and early lactation period improved milk production, fecal microbial composition, and overall health of dairy cows.
1. Introduction
The worldwide need for a sufficient and regular milk supply is predicted to rise by 2030 35%, owing primarily to growing global requirements [1, 2]. To meet this ever-increasing need, many countries have invested heavily in increasing their dairy production in recent decades [3, 4]. Milk production strategies face many socioeconomic and environmental challenges, including the hazards associated with the antibiotics and growth hormones used in animals [5]. These challenges have prompted a global quest for natural eco-friendly feed additives to increase cow milk supply [6–8].
Dairy cows’ gut microbiota is crucial in energy intake and nutrition. As a result, it should be considered a target for improving milk quality, milk yield, and dairy cow health [9]. Probiotics are live microorganisms that, when given appropriately, regulate the gut microbes’ balance, enhance the animals’ growth, and boost their resistance to diseases [10]. The commonly accepted goal in probiotics production is to raise the proportion of beneficial bacteria in the gut to promote effective nutrient utilization while leaving harmful bacteria behind, increasing dairy production [11]. The most frequently used probiotic organisms are Streptococcus thermophilus, Lactobacillus spp., Bifidobacterium spp., and fungal species [12].
Various factors determine the probiotic’s effectiveness, including the microbial species composition, the dose of each preparation, the delivery route, the kind of diet, and the feeding duration [13]. One of the most important factors is the dosage used in each given probiotic treatment and/or the specific combination of probiotic organisms as single- or multistrain [14]. Several recent reports verified the efficacy of multistrain probiotic formulation rather than a single strain in improving the health and growth of poultry and aquatic species [15]. Improved effectiveness with some multistrain preparations may be related to the synergistic and additive effects and symbiotic interaction between the organisms involved [16]. Despite extensive research on the benefits of probiotics in dairy products, commonly with single-strain preparations [17, 18], the results of the effects of multistrain on dairy cows are scanty in the literature.
Given the above data, we hypothesized that feeding a mixture of probiotics initiated from the prepartum stage could enhance dairy cow health and lactation performance. Consequently, the present study aimed to evaluate the influence of multistrain probiotics formulation (MPF) containing Lactobacillus spp., Streptococcus thermo phallus, Bifidobacterium spp., and some fungal species as a functional feed additive during the late gestation and early lactation period on the lactation performance of postpartum crossbred (Holstein Friesian × Balady) dairy cows. To further assess the safety of this MPF product, blood biochemical indices of kidney and liver functioning, hematological parameters, and protein profile were analyzed.
2. Materials and Methods
2.1. Cows, Trial Scheme, and Management
A total of 30 multiparous crossbred (Holstein Friesian × Balady) dairy cows were selected one month before the predictable parturition date and alienated into three groups (10 cows/group) in a completely randomized design based on their parity number (mean ± SD: 2.17 ± 1.12) and body weight (mean ± SD: 528 ± 61 kg). Before beginning their experimental treatments, all cows were exposed to a 14-day acclimation period. Dairy cows’ health was monitored at the start of the trial and regular intervals after that.
Cows in the control group (not administered with MPF) were fed a basal diet consisting of rice straw, a concentrate feed mixture (CFM), and Egyptian clover, with a roughage-to-concentrate ratio of 60 : 40 on a dry matter basis. The second group (MPF1) and third group (MPF2) received the same control diet supplemented with MPF (Guardizen-M®, Dong Bang Co., Gyeonggi-do, Republic of Korea) at a rate of 0.5 g/cow/d or 1 g/cow/d, respectively. The administration of MPF started 28 days before calving and continued for 84 days after. The MPF contains 1 × 1010 CFU probiotics mixture of Enterococcus faecium, Bifidobacterium bifidum, Lactobacillus acidophilus, Streptococcus thermophilus, L. rhamnosus, L. bulgaricus, and L. plantarum along with dextrose as a carrier. The dosages of the MPF utilized in this study were within the recommended range of the producing company. The cows in the study were offered experimental diets twice a day at 08:00 and 16:00 h. To ensure complete consumption of MPF, each cow in the supplemented groups received 100 g of concentrate DM containing the probiotic mixture prior to their morning feeding at 08:00. Throughout the experiment, the dry matter intake (DMI) was calculated by subtracting delivered feed from orts collected daily and weighed before the next feeding. The animal received a certain amount of CFM, Egyptian clover, and rice straw. The cows had free access to clean water. The control diet’s ingredients and proximate composition are listed in Table 1.
| Item | Concentrate feed mixture | Rice straw | Berseem clover | Control diet‡ |
|---|---|---|---|---|
| Dry matter, g/kg wet material | 895.7 | 888.3 | 176.6 | 606.6 |
| Organic matter | 957.3 | 897.5 | 875.2 | 912.5 |
| Crude protein | 173.7 | 39.3 | 154.4 | 139.1 |
| Ether extract | 37.6 | 20.3 | 35.5 | 33.3 |
| Ash | 42.7 | 102.5 | 124.8 | 87.5 |
| Neutral detergent fiber | 338.4 | 716.9 | 416.0 | 445.1 |
| Acid detergent fiber | 186.7 | 485.6 | 261.5 | 276.4 |
- The concentrate feed mixture contained (per kg DM): 440 g yellow corn, 200 g uncorticated cotton seed meal, 50 g soybean meal, 250 g wheat bran, 30 g molasses, 20 g dicalcium phosphate, 7 g sodium chloride, and 3 g vitamin and mineral mixture. ‡The control diet based on (per kg DM): 400 g of concentrate feed mixture, 400 g of berseem clover (Trifolium alexandrinum), and 200 g of rice straw.
2.2. Sampling and Chemical Analysis of Milk
2.3. Nutrient Digestibility and Chemical Analysis
Throughout the experiment, composite samples of Egyptian clover, rice straw, and CFM were collected, dried at 65°C for 48 hours, powdered, and stored until analysis. A digestibility trial was performed throughout the last week of the trial (days 78–84). Acid-insoluble ash (AIA) was utilized as an internal marker to estimate nutrient digestibility following the Van Keulen and Young [22] method. The feeds and feces were dried and ashed. Next, they were subjected to boiling in hydrochloric acid (HCl) with a concentration of 2N. The resulting mixture underwent filtration, washing, and a final reashing process to determine the amount of AIA. Twice a day, at 07:00 and 15:00, the feces were collected from five cows per group. The collected feces were then dried at 60°C for 48 hours and blended by the cow. Throughout the digestibility trial, orts, feed, and feces samples were milled to pass a 1-mm screen, and their chemical composition was estimated by AOAC [23] techniques. The contents of dry matter (DM; Procedure No. 925.10) and crude ash (Procedure No. 954.01) were determined in the collected samples. To determine the amount of crude protein (CP; Procedure No. 954.01), the Kjeldahl technique was used. Petroleum ether was used in a Soxhlet extractor to determine the content of ether extract (EE; Procedure No. 920.39). Acid detergent fiber (ADF; AOAC [23]; Procedure No. 973.18) and neutral detergent fiber (NDF; Van Soest et al. [24]) were quantified without the use of α-amylase.
2.4. Sampling and Serum Analysis
Blood samples (10 mL) (n = 5 cows/group) were collected 2 h following the morning feeding on days −7, 28, 56, and 84 relative to the parturition date. The blood was collected from the tail vein (vena coccygeal) using vacationer tubes. The blood sample taken from each animal was split into two equal parts. The first part, about 5 mL, was placed in K3-EDTA tubes for hematological examination. The second half was obtained without the use of anticoagulant (K3-EDTA) to enable the collection of serum for the assessment of biochemical and immunological parameters. After clotting, the blood samples underwent centrifugation at 4°C for 20 minutes with a force of 3000 × g. Then, the serum was harvested, transferred into 2-mL Eppendorf tubes, and preserved at a temperature of −20°C until the biochemical analysis was conducted.
In the aliquot contained anticoagulant, the red blood cell (RBC) count, hematocrit (Hct), platelet, hemoglobin (Hb), white blood cell (WBC) count, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) were determined according to Jain [25] methods. The serum samples were used to determine the total protein, globulin, albumin, total cholesterol (TC), urea, triglyceride (TG), and low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), high-density lipoprotein (HDL), and blood urea nitrogen contents using commercial kits (Diamond Diagnostic, Dokki, Giza, Egypt). To calculate the levels of globulin, the serum albumin levels were subtracted from the total protein levels. The serum concentration of immunoglobulin G (IgG) was determined by employing commercially available ELISA kits, following the guidelines provided by the manufacturer.
2.5. Enumeration of Bacterial Populations in Bovine Feces and Cloacal Swabs
The cattle feces were collected by rectal palpation during the last week of the experiment (days 78–84). To identify the total bacterial count (TBC), lactobacilli count (LC), spore-forming bacteria (SFB) such as Bacillus and Clostridium, and coliform count (CFC), as well as Escherichia coli and Salmonella in the samples obtained from animals, the cloacal swabs and cattle feces were combined with buffered peptone water (consisting of 8.5 g sodium chloride per liter and 1.0 g peptone per liter). Next, the mixture was incubated at a temperature of 35–37°C for 18–24 hours. TBC, CFC, SFB, Salmonella, and E. coli were enumerated utilizing specific media, following the methods outlined by Ayyat et al. [26].
2.6. Data Analysis
SAS software (SAS Institute, Cary, NC; version 9.0) was used to analyze the obtained data in a completely randomized design. Lactation performance, dry matter intake, and blood metabolites were analyzed by the PROC MIXED method by considering treatment, time, and the interaction of time × treatment as fixed effects. The random variable was the cow × treatment interaction. Parity was included as a term in the initial model, but it was found insignificant and removed from the final model. Data for nutrient digestibility and fecal microbial populations were analyzed by the GLM option of SAS with treatment as a fixed effect. As the somatic cell count and fecal bacterial count values were not normally distributed, the natural logarithm was normalized before analysis. The PDIFF procedure of least squares separated means statement exists in the case of significant effects. The significance level was set at P < 0.05, while trends were considered at 0.05 < P < 0.10.
3. Results
3.1. Lactation Performance and Feed Utilization
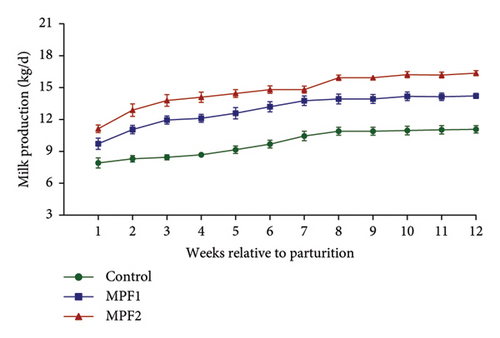
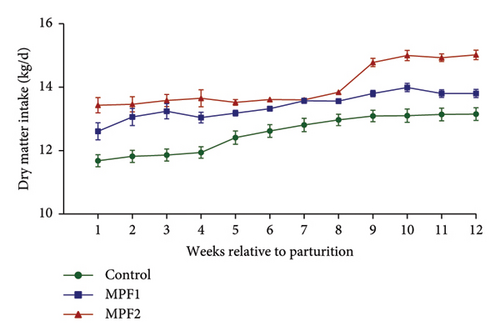
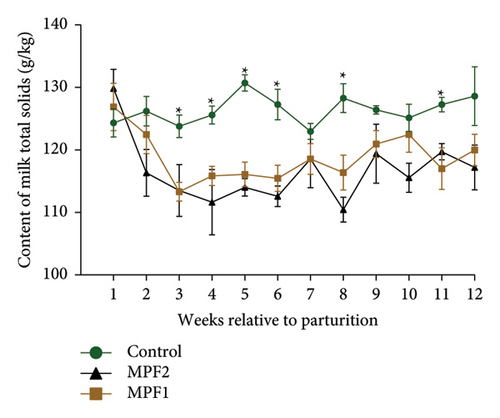
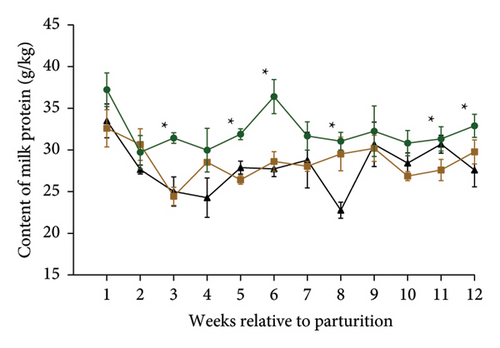
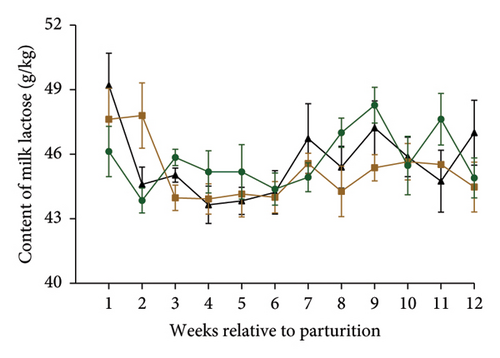
Table 2 and Figures 1(a) and 1(b) present the results for milk production, milk composition, DMI, and feed utilization of dairy cows that were supplemented with different doses of MPF. The cows’ daily milk production, 3.5% FCM, and ECM in the MPFI and MPF2 groups were higher (P < 0.001) than those of the control group. The MPF-supplemented cows also exhibited significantly greater milk energy output, DMI (expressed as a percentage of body weight or kg per day), and yields of solids-not-fat, lactose, ash, protein, total solids, and fat compared to the control group (P < 0.001). DMI (kg/d) significantly increased in dairy cows that consumed MPF from parturition to day 84 of the trial period (Figure 1(b)). However, lower concentrations of milk solids-not-fat, fat, total solids, and protein were observed in cows fed MPF-containing diets compared to the control group (P < 0.001). The supplementation of MPF significantly (P < 0.001) enhanced feed conversion, as indicated by kg of DMI per kg of milk and kg of DMI per kg of ECM (Table 2). Additionally, a significant interaction effect was observed regarding the concentrations of milk protein (P = 0.017) and solids-not-fat (P = 0.015), with a trend observed for total solids (P = 0.062) and lactose (P = 0.096; Figures 2(a)–2(d)).
| Treatment (Trt) | SEM | P value | |||||
|---|---|---|---|---|---|---|---|
| Control | MPF1 | MPF2 | Trt | Week | Trt × week | ||
| Milk production (kg/d) | |||||||
| Milk | 9.79c | 12.90b | 14.71a | 0.21 | <0.001 | <0.001 | 0.748 |
| 3.5% fat-corrected milk | 10.71c | 13.36b | 14.70a | 0.19 | <0.001 | <0.001 | 0.794 |
| Energy-corrected milk (ECM) | 9.64c | 11.75b | 12.98a | 0.16 | <0.001 | <0.001 | 0.971 |
| Milk composition (g/kg milk) | |||||||
| Total solids | 126.37a | 118.77b | 116.56b | 0.62 | <0.001 | 0.003 | 0.062 |
| Solids-not-fat | 86.13a | 82.05b | 81.82b | 0.42 | <0.001 | 0.002 | 0.015 |
| Fat | 40.24a | 36.72b | 34.74b | 0.39 | <0.001 | 0.790 | 0.815 |
| Protein | 32.05a | 28.61b | 27.92b | 0.35 | <0.001 | 0.019 | 0.017 |
| Lactose | 45.73 | 45.20 | 45.63 | 0.19 | 0.399 | 0.001 | 0.096 |
| Ash | 8.34 | 8.25 | 8.28 | 0.06 | 0.805 | 0.103 | 0.444 |
| Somatic cell count (×105) | 145.83 | 124.45 | 126.50 | 3.56 | 0.081 | 0.875 | 0.871 |
| Milk energy content (MJ/kg) | 3.06a | 2.84b | 2.76b | 0.04 | <0.001 | 0.027 | 0.266 |
| Milk yield (kg/d) | |||||||
| Total solids | 1.24c | 1.53b | 1.71a | 0.02 | <0.001 | <0.001 | 0.983 |
| Solids-not-fat | 0.85c | 1.06b | 1.20a | 0.02 | <0.001 | <0.001 | 0.955 |
| Fat | 0.39c | 0.47b | 0.51a | 0.04 | <0.001 | <0.001 | 0.848 |
| Protein | 0.32c | 0.37b | 0.41a | 0.04 | <0.001 | <0.001 | 0.338 |
| Lactose | 0.45c | 0.58b | 0.67a | 0.01 | <0.001 | <0.001 | 0.908 |
| Ash | 0.08c | 0.11b | 0.12a | 0.01 | <0.001 | <0.001 | 0.128 |
| Milk energy output (MJ/d) | 29.95c | 36.53b | 40.36a | 0.51 | <0.001 | <0.001 | 0.973 |
| Dry matter intake (kg/d) | 12.55c | 13.41b | 14.03a | 0.07 | <0.001 | <0.001 | 0.952 |
| Dry matter intake (% BW) | 2.70b | 2.94a | 2.86a | 0.02 | <0.001 | <0.001 | 0.927 |
| Feed conversion (kg/kg) | |||||||
| Kg DMI/kg milk | 1.30a | 1.05b | 0.96b | 0.09 | <0.001 | <0.001 | 0.842 |
| Kg DMI/kg ECM | 1.32a | 1.16b | 1.09b | 0.08 | <0.001 | <0.001 | 0.944 |
- a–cDifferent lowercase superscript letters in the same column indicate that the difference between groups is significant (P < 0.05). SEM: standard error of the mean. The experimental treatments were with no MPF supplementation (control group) or with the addition of MPF at 0.5 g/cow/d (MPF1 group) or 1 g/cow/d (MPF2 group).

3.2. Hematological and Serum Biochemical Measurements
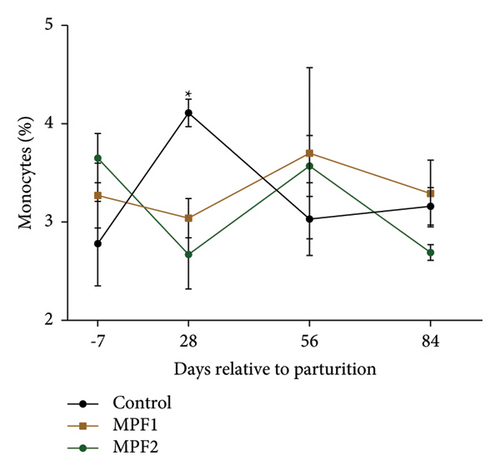
Most hematological indicators, including Hb, RBCs, MCHC, MCH, MCV, platelet count, and leukogram parameters, did not alter significantly (P > 0.05) between the two MPF groups and the control group (Table 3). However, cows receiving MPF supplements had higher levels of Hct (P = 0.011). Most of the hematological measurements had no statistically significant interactions between treatment and time. Nevertheless, the cows administered MPF had a slightly lower percentage of monocytes (T × W; P = 0.077) than the control group 28 days after calving (Figure 3(a)).
| Treatment (Trt) | SEM | P value | |||||
|---|---|---|---|---|---|---|---|
| Control | MPF1 | MPF2 | Trt | Day | Trt × day | ||
| Erythrogram | |||||||
| RBCs, 106 × µL | 3.05 | 3.28 | 3.32 | 0.07 | 0.216 | 0.064 | 0.376 |
| Hb (g/dL) | 9.19 | 9.85 | 10.02 | 0.16 | 0.075 | 0.240 | 0.471 |
| Hct (%) | 31.05b | 34.87a | 34.47a | 0.61 | 0.011 | 0.089 | 0.456 |
| MCV (fL) | 103.78 | 107.58 | 106.79 | 3.34 | 0.867 | 0.019 | 0.508 |
| MCH (pg/dL) | 30.34 | 30.21 | 30.69 | 0.45 | 0.894 | 0.047 | 0.567 |
| MCHC (g/dL) | 30.03 | 28.35 | 29.17 | 0.58 | 0.418 | 0.004 | 0.876 |
| Platelet count (103/µL) | 259.9 | 230.0 | 283.5 | 20.41 | 0.514 | 0.034 | 0.433 |
| Leukogram | |||||||
| WBCs (103/µL) | 12.82 | 13.80 | 11.70 | 1.25 | 0.794 | 0.236 | 0.509 |
| Lymphocyte (%) | 51.96 | 56.40 | 57.00 | 2.55 | 0.636 | 0.173 | 0.455 |
| Monocytes (%) | 3.27 | 3.32 | 3.14 | 0.12 | 0.788 | 0.658 | 0.077 |
| Neutrophils (%) | 43.83 | 39.38 | 38.88 | 2.61 | 0.695 | 0.228 | 0.439 |
| Eosinophils (%) | 0.58 | 0.57 | 0.63 | 0.06 | 0.847 | 0.000 | 0.996 |
| Basophils (%) | 0.29 | 0.27 | 0.29 | 0.02 | 0.956 | 0.010 | 0.950 |
- a–bDifferent lowercase superscript letters in the same column indicate that the difference between groups is significant (P < 0.05). SEM stands for the standard error of the mean; Hb, hemoglobin; RBCs, red blood cells; Hct, hematocrit; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; WBCs, white blood cells. The experimental treatments were with no MPF supplementation (control group) or with the addition of MPF at 0.5 g/cow/d (MPF1 group) or 1 g/cow/d (MPF2 group).
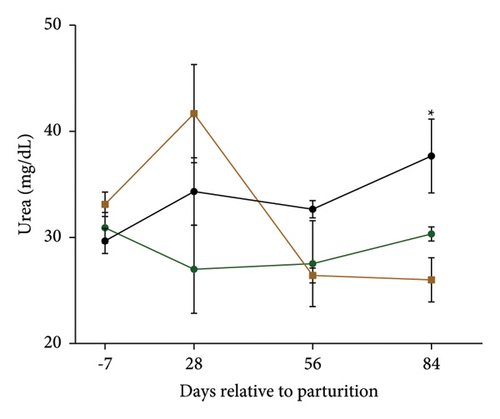
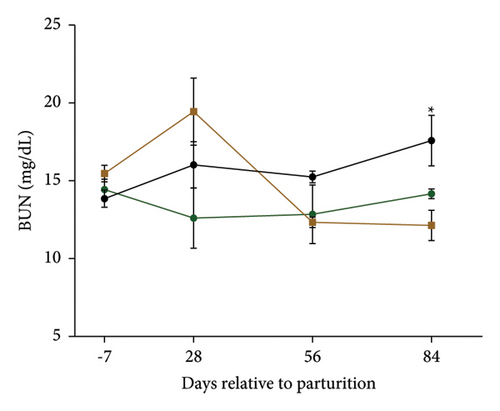
Serum biochemical measurements are shown in Table 4, and changes in urea and BUN are presented in Figures 3(b) and 3(c). Serum concentrations of total cholesterol (P = 0.041), LDL (P = 0.002), and VLDL (P = 0.001) were lower; and urea (P = 0.07) and BUN (P = 0.07) tended to be lower in the MPF cows than in the control cows. Yet, serum HDL concentrations (P = 0.055) tended to be higher than the control cows (Table 4). Furthermore, the concentrations of blood IgG tended (P = 0.096) to be increased in the blood of the MPF2 group. Cows that were given MPF supplements had a lower serum concentration of urea and BUN at 84 days of parturition (T × W; P = 0.01; Figures 3(b) and 3(c)).
| Treatment (Trt) | SEM | P value | |||||
|---|---|---|---|---|---|---|---|
| Control | MPF1 | MPF2 | Trt | Day | Trt × day | ||
| Total protein (g/dL) | 5.88 | 5.99 | 6.17 | 0.10 | 0.924 | 0.642 | 0.913 |
| Albumin (g/dL) | 3.09 | 3.08 | 3.24 | 0.07 | 0.769 | 0.108 | 0.478 |
| Globulin (g/dL) | 2.74 | 2.91 | 2.93 | 0.08 | 0.905 | 0.783 | 0.508 |
| Albumin/globulin | 1.20 | 1.08 | 1.12 | 0.05 | 0.723 | 0.159 | 0.350 |
| Total cholesterol (mg/dL) | 76.50a | 72.58ab | 67.00b | 1.54 | 0.041 | 0.072 | 0.777 |
| Total triglycerides (mg/dL) | 87.42 | 85.92 | 82.17 | 1.27 | 0.263 | 0.487 | 0.758 |
| High-density lipoprotein (mg/dL) | 40.75 | 45.58 | 44.41 | 0.81 | 0.055 | 0.635 | 0.810 |
| Low-density lipoprotein (mg/dL) | 21.52a | 16.31b | 15.11b | 0.90 | 0.002 | 0.006 | 0.661 |
| VLDL (mg/dL) | 14.23a | 10.70b | 9.15b | 0.59 | 0.001 | 0.260 | 0.124 |
| Urea (mg/dL) | 33.58 | 31.80 | 28.94 | 1.01 | 0.070 | 0.134 | 0.010 |
| Blood urea nitrogen (mg/dL) | 15.67 | 14.84 | 13.51 | 0.47 | 0.070 | 0.134 | 0.010 |
| Immunoglobulin G (mg/mL) | 29.38 | 27.99 | 35.56 | 1.26 | 0.096 | 0.864 | 0.649 |
- a–bDifferent lowercase superscript letters in the same column indicate that the difference between groups is significant (P < 0.05). SEM stands for the standard error of the mean; VLDL, very low-density lipoprotein. The experimental treatments were with no MPF supplementation (control group) or with the addition of MPF at 0.5 g/cow/d (MPF1 group) or 1 g/cow/d (MPF2 group).
3.3. Feed Digestibility and Fecal Bacterial Microbiota
An increasing trend was observed in DM digestibility (P = 0.079) and organic matter (P = 0.071) in cow groups supplemented with MPF, compared to the control group. There were no significant changes in the digestibility of CP and EE between the experimental groups (Table 5; P > 0.05). The populations of lactobacillus (P < 0.001), bacillus (P = 0.033), and total bacterial count (P < 0.001) were significantly increased due to MPF administration. In contrast, the count of coliforms, E. coli, Salmonella, and Clostridium was significantly (P < 0.001) decreased in the MPF-fed cows than in the control cows. The MPF2 group of cows showed the greatest suppression of Clostridium, Salmonella, coliforms, and E. coli populations compared to the control group (Table 5).
| Treatment | SEM | P value | |||
|---|---|---|---|---|---|
| Control | MPF1 | MPF2 | |||
| Digestibility (g/kg DM (kg/d)) | |||||
| Dry matter | 584.7 | 602.7 | 613.9 | 5.52 | 0.079 |
| Organic matter | 625.1 | 637.2 | 654.6 | 5.47 | 0.071 |
| Crude protein | 618.9 | 658.2 | 655.6 | 9.65 | 0.183 |
| Ether extract | 698.9 | 716.1 | 743.8 | 10.59 | 0.231 |
| Fecal bacterial microbiota (log cfu/g) | |||||
| Total bacterial count | 8.21c | 9.73b | 10.56a | 0.30 | <0.001 |
| Coliforms | 6.81a | 5.23b | 4.55c | 0.29 | <0.001 |
| Lactobacillus | 5.83b | 7.77a | 7.95a | 0.29 | <0.001 |
| Bacillus | 6.32b | 6.87ab | 6.93a | 0.11 | 0.033 |
| E. coli | 5.96a | 4.46b | 4.19b | 0.24 | <0.001 |
| Salmonella spp. | 4.86a | 4.55a | 3.65b | 0.18 | 0.001 |
| Clostridium | 4.93a | 4.59b | 4.47b | 0.07 | 0.001 |
- a–cDifferent lowercase superscript letters in the same column indicate that the difference between groups is significant (P < 0.05). SEM: standard error of the mean. The experimental treatments were with no MPF supplementation (control group) or with the addition of MPF at 0.5 g/cow/d (MPF1 group) or 1 g/cow/d (MPF2 group).
4. Discussion
In the current work, the MPF use considerably increased the milk yield and the yield of major milk components, including total solids, protein, solids-not-fat, lactose, fat, and ash, while slightly suppressing the SCC and blood monocytes. The favorable effects are most likely due to an enhanced ruminal microbiota due to MPF probiotic addition. For example, some ruminal bacteria such as L. plantarum [27], Bifidobacterium bifidum [28], and S. thermophilus [29] have been described as strong proteolytic bacteria, and microbially derived amino acids can account for as much as two-thirds of the amino acids absorbed by the small intestine of a ruminant. To that end, these microorganisms in the intestine produce the microbial crude protein, which greatly affects the amount and quality of metabolizable protein absorbed in the small intestine. As a result of its conversion to milk protein, the metabolizable protein ultimately affects the milk production yield [30]. These strains are the main components of the used MPF. Additionally, the populations of lactobacillus, bacillus, and total bacterial count were significantly increased due to MPF administration. The milk output observed in our study (ranging from 9.79 to 14.71 kg/d) reflects the productivity levels typical of crossbred cows in our region [31].
It is worth noting that in our study, there was a decrease in the concentration (g/kg milk) of major milk components, including total solids, protein, solids-not-fat, and fat, despite an increase in their yield. This phenomenon can be attributed to a combination of factors, such as cattle genotype, diet composition, the dilution effect resulting from increased milk production associated with MPF supplementation [32], and potentially a reduction in the mammary blood flow rate [33]. The higher DMI observed in our study with MPF supplementation is in line with previous findings. Nocek et al. [34] reported an increased DMI of 2.6 kg/day in dairy cows supplemented with a combination of E. faecium and Saccharomyces cerevisiae probiotics from 3 weeks prepartum to 10 weeks postpartum. Similarly, Kembabazi et al. [35] found that supplementing lactating dairy cows with mixed strain probiotics during the early stage increased DMI. The ability of probiotics to improve DMI in ruminants can be attributed to several factors. One possible mechanism is the enhanced proliferation and growth of ruminal cellulolytic bacteria, which leads to improved fiber degradation and nutrient utilization. Probiotics can also help prevent ruminal acidosis, a condition that negatively impacts feed intake and overall rumen function [36]. Additionally, the supplementation of dairy cattle with probiotics has been shown to increase the frequency of feed intake, fiber degradation, and feed digestibility in the rumen [37].
The slight reduction in SCC and blood monocytes in cows subjected to MPF administration suggests that this probiotic product can decrease inflammation in the mammary glands of dairy cows, leading to an increase in the milk production, as evident in our findings. In this regard, a relationship was recorded between the composition of blood monocyte types and postpartum infectious diseases in dairy cows like mastitis [38]. Besides, Eger et al. [39] reported that the blood cell counts of the different monocyte subgroups were higher in cows with postpartum mastitis. Additionally, numerous research proposed that low SCC values are associated with higher milk production [40, 41], a finding we observed in the present study.
The fecal bacterial composition of cows fed MPF demonstrated a considerable increase in the beneficial populations of lactobacillus and bacillus, whereas a significant reduction in the count of Clostridium, Salmonella, coliforms, and E. coli proposing that these probiotic supplements reinforced an improved defense mechanism of the rumen and intestine, possibly decreasing the hazard of pathogens’ colonization. Comparably, Guo et al. [42] demonstrated that using MPF-containing B. animalis, L. casei, B. cerevisiae, and S. faecalis did not disturb the overall fecal microflora at the phylum level but increased the beneficial Prevotella abundance while decreased the opportunistic pathogens Dorea in Holstein’s calves. In this regard, many of the Lactobacillus probiotic strains inhibit the growth of pathogens and aid in the body’s natural defenses against infections by producing metabolites such as diacetyl, hydrogen peroxide, acetoin, and bacteriocins [43, 44]. Because of MPF’s beneficial effect on commensal microbial community composition, rumen fermentation efficiency may be increased, leading to maximized milk production [45]. Besides, several studies have demonstrated that probiotics play an indirect influence in increasing milk production by raising the digestive efficiency and feed conversion ratio of dairy animals [46, 47]. This role was evident in the results of the current study, as the MPF supplementation significantly enhanced the feed conversion and also indicated a trend toward increased digestibility coefficients of DM and OM.
Marinating the dairy cow’s health status is needed for good milk production and quality during lactation. In the current experiments, the safety of MPF as a feed supplement in dairy cows has been determined using multiple biochemical indicators in the animals’ blood. Serum urea and BUN levels were thought to predict renal function in dairy cows accurately [48]. Total protein, globulin, albumin, and BUN levels in the serum of animals have been utilized extensively to indicate the amount of energy and protein required for production [49]. The recorded normal range of altogether shows the conditions for optimal production. BUN might increase the following water deprivation [50]: thirst, diarrhea [51], urinary diseases [52], pregnancy toxemia [53], and acidosis [54], which were not the case for these cows. Yet, compared with the cows fed nonsupplemented diets, the cows that were fed MPF-supplemented diets had lower urea and BUN, indicating a better kidney function [48] and a positive impact on nitrogen proficiency and energy metabolism [55].
Probiotics serve as immunological adjuvants, stimulating IgG synthesis [56]. In the current study, MPF-fed cows had an increasing tendency of the serum IgG level. Extensive evidence supports the significant impact of the rumen microbiome on the development and functioning of both innate and systemic immunological components [57]. Hence, improving the fecal bacterial composition recorded in the current study could be responsible for enhancing immunoglobulin levels [57, 58].
Lipid metabolism and alterations in serum lipid and lipoprotein profiles, especially HDL, constitute a fundamental component of dairy cows’ energy metabolism and physiology [59]. HDL-C is the chief lipoprotein for transferring cholesterol in cows [60]. Energy balance is maintained, and oxidized fatty acids are kept to a minimum by keeping HDL-C-rich lipoprotein profiles in dairy cows [61]. HDL-C may effectively predict energy balance for distinguishing healthy cows from diseased ones. Cows suffering from fatty liver and milk fever exhibited lower mean HDL-C levels than healthy dairy cows [62]. In the current experiment, serum HDL concentrations in MPF-fed cows tended to be higher than that of the control cows. VLDL, LDL, and total cholesterol serum concentrations were lower in the MPF-fed cows than in the control cows. The different probiotic strains in MPF could be responsible for this improved lipid profile. For instance, numerous Lactobacillus strains have reduced TC and TG concentrations in rats [63, 64]. Likewise, B. bifidum significantly reduced serum total cholesterol and LDL levels and slightly increased serum HDL in rats [65].
5. Conclusions
The overall results showed that supplementing lactating cows with 0.5 g or 1 g of MPF/cow/day during late gestation and early lactation improved milk production and feed efficiency. Both MPF doses increased blood Hct levels and reduced LDL, total cholesterol, and VLDL levels without affecting other blood parameters. MPF supplementation also led to higher feed digestibility and favorable shifts in bacterial populations, promoting beneficial microbes while reducing harmful ones. The cows supplemented with 1 g/day of MPF demonstrated the highest milk production and the most significant reduction in harmful bacterial populations in the feces. Utilizing these advantageous microbes presents a natural and consequence-free strategy for enhancing dairy production. More research is needed to understand how MPF affects the metabolic and physiological processes of dairy cows throughout the transition period.
Ethical Approval
The animal study protocol was approved by the Ethics Committee of Institutional Animal Care and Use Committee of Zagazig University (approval code: ZU-IACUC/2/F/320/2022).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.




